A Multistate Continuous Time-Inhomogeneous Markov Model for Assessing the CD4 Count Dynamics of HIV/AIDS Patients Undergoing Antiretroviral Therapy in KwaZulu-Natal, South Africa
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Study Population
2.3. Data Collection and Preparation
2.4. Statistical Analysis
2.5. Statistical Model
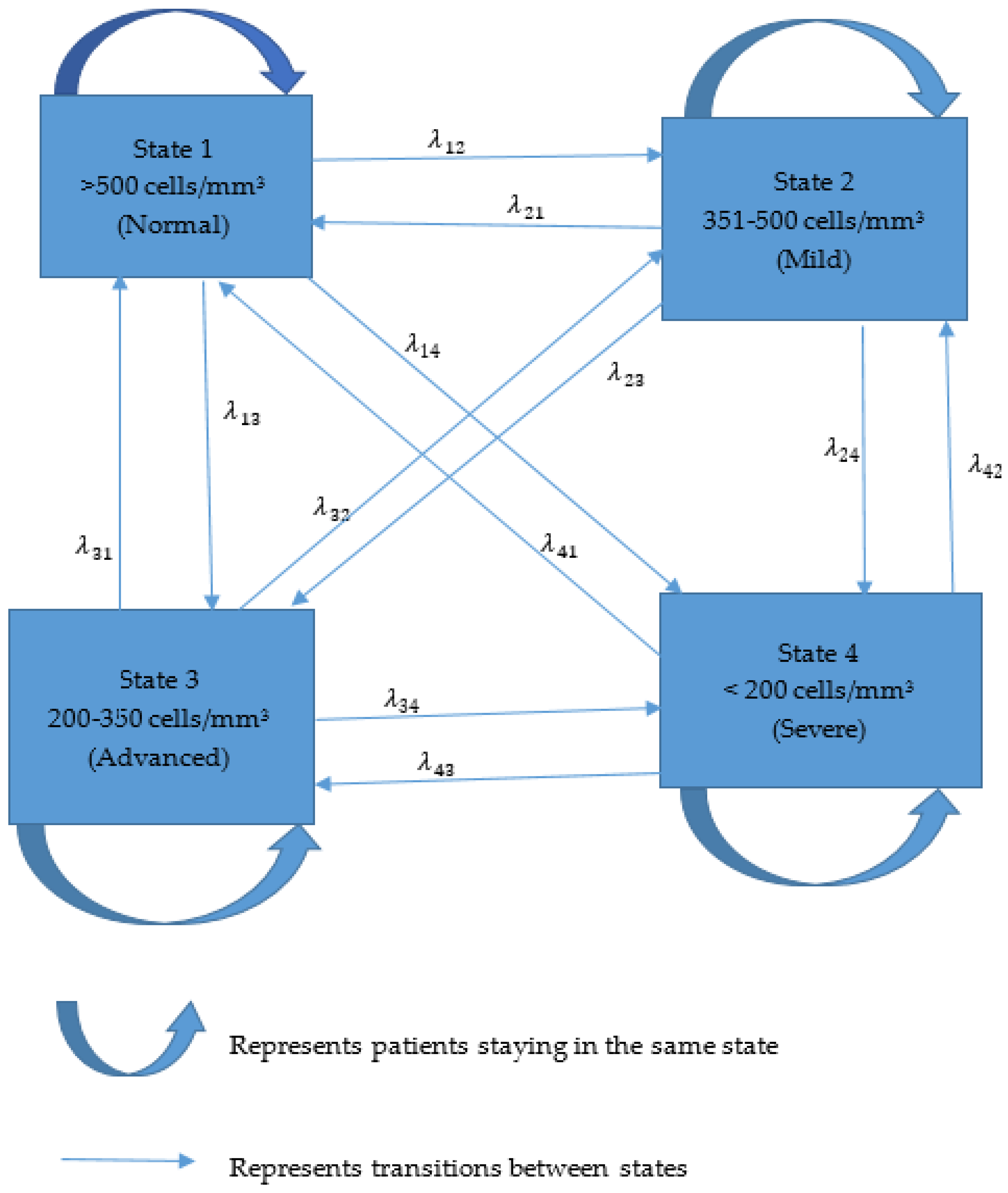
2.5.1. Multistate Markov Model
2.5.2. Properties of Transition Intensity Matrix
- 1.
- Non-negativity: For all and , for all .
- 2.
- Row Sums: The sum of each row must equal zero: for each state .
2.5.3. Assumptions of Multistate Markov Model in Statistical Formulation
- The future state depends only on the present state, not on the past.
- Transitions between states can occur at any time, not just at discrete intervals.
- The probability of transitioning from one state to another change over time.
- The model considers a finite number of possible states.
- The probability of a transition depends only on the current state and not on the history of states.
2.5.4. Chapman–Kolmogorov Equations
2.5.5. Mean Sojourn Time
2.5.6. Modelling the Intensity Function Using the Cox Proportional Hazard Function
2.6. Model Diagnostics
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Bekker, L.G.; Ratevosian, J.; Spencer, J.; Piot, P.; Beyrer, C. Governance for health: The HIV response and general global health. Bull. World Health Organ. 2019, 97, 170. [Google Scholar] [CrossRef] [PubMed]
- UNAIDS. National Commitments and Policy Instrument 2022; Joint United Nations Programme on HIV/AIDS: Geneva, Switzerland, 2022; Available online: https://lawsandpolicies.unaids.org/ (accessed on 6 July 2023).
- Maartens, G.; Celum, C.; Lewin, S.R. HIV infection: Epidemiology, pathogenesis, treatment, and prevention. Lancet 2014, 384, 258–271. [Google Scholar] [CrossRef]
- Abdullahi, H. Hematological Profile of HIV ART Naïve and HIV ART Experienced Individuals at the Aminu Kano Teaching Hospital North Western Nigeria. NISEB 2016, 1693–1698. [Google Scholar]
- Fauci, A.S.; Lane, F.C. Human immunodeficiency virus (HIV) disease: AIDS and related disorders. In Harrisons Principles of Internal Medicine, 16th ed.; Fauci, B., Hauer, K., Jamesons, L., Eds.; McGraw Hill: New York, NY, USA, 2005; pp. 1076–1139. [Google Scholar]
- Andualem, B.D. Progression of HIV Disease Among Patients on ART in Ethiopia: Application of Longitudinal Count Models. Front. Public Health 2020, 7, 415. [Google Scholar] [CrossRef]
- Mair, C.; Hawes, S.E.; Agne, H.D.; Sow, P.S.; N’Doye, I.; Manhart, L.E.; Fu, P.L.; Gottlieb, G.S.; Kiviat, N.B. Factors associated with CD4 lymphocyte counts in HIV-negative Senegalese individuals. Clin. Exp. Immunol. 2008, 151, 432–440. [Google Scholar] [CrossRef]
- Ford, N.; Meintjes, G.; Vitoria, M.; Greene, G.; Chiller, T. The evolving role of CD4 cell counts in HIV care. Curr. Opin. HIV AIDS 2017, 12, 123–128. [Google Scholar] [CrossRef]
- Temesgen, A. Application of Poisson mixed combined models for identifying correlations of CD4 count progression in HIV-infected TB patients during the ART treatment period. Int. J. Stat. Probab. 2017, 6, 42–52. [Google Scholar] [CrossRef]
- ’Joanna Lewis, A.; Walker, S.; Castro, H.; De Rossi, A.; Gibb, D.M.; Giaquinto, C.; Klein, N.; Callard, R. Age and CD4 Count at Initiation of Antiretroviral Therapy in HIV-Infected Children: Effects on Long-term T-Cell Reconstitution. J. Infect. Dis. 2011, 205, 548–556. [Google Scholar] [CrossRef] [PubMed]
- Dessie, Z.G.; Zewotir, T.; Mwambi, H.; North, D. Modelling of viral load dynamics and CD4 cell count progression in an antiretroviral naive cohort: Using a joint linear mixed and multistate Markov model. BMC Infect. Dis. 2020, 20, 246. [Google Scholar] [CrossRef]
- Matsena Zingoni, Z.; Chirwa, T.F.; Todd, J.; Musenge, E. Hiv disease progression among antiretroviral therapy patients in Zimbabwe: A multistate Markov model. Front. Public Health 2019, 7, 326. [Google Scholar] [CrossRef]
- Dessie, Z.G.; Zewotir, T.; Mwambi, H.; North, D. Modelling immune deterioration, immune recovery and state-specific duration of HIV-infected women with viral load adjustment: Using parametric multistate model. BMC Public Health 2020, 20, 416. [Google Scholar] [CrossRef] [PubMed]
- Miller, V.; Mocroft, A.; Reiss, P.; Katlama, C.; Papadopoulos, A.I.; Katzenstein, T.; van Lunzen, J.; Antunes, F.; Phillips, A.N.; Lundgren, J.D. Relations among CD4 lymphocyte count nadir, antiretroviral therapy, and HIV-1 disease progression: Results from the EuroSIDA study. Ann. Intern. Med. 1999, 130, 570–577. [Google Scholar] [CrossRef] [PubMed]
- Shoko, C.; Chikobvu, D. A superiority of viral load over CD4 cell count when predicting mortality in HIV patients on therapy. BMC Infect. Dis. 2019, 19, 169. [Google Scholar] [CrossRef] [PubMed]
- Kufa, T.; Shubber, Z.; MacLeod, W.; Takuva, S.; Carmona, S.; Bor, J.; Gorgens, M.; Pillay, Y.; Puren, A.; Eaton, J.W.; et al. CD4 count recovery and associated factors among individuals enrolled in the South African antiretroviral therapy programme: An analysis of national laboratoryBased data. PLoS ONE 2019, 14, e0217742. [Google Scholar] [CrossRef] [PubMed]
- Handoko, R.; Colby, D.J.; Kroon, E.; Sacdalan, C.; de Souza, M.; Pinyakorn, S.; Prueksakaew, P.; Munkong, C.; Ubolyam, S.; Akapirat, S.; et al. Determinants of suboptimal CD4+ T cell recovery after antiretroviral therapy initiation in a prospective cohort of acute HIV-1 infection. J. Int. AIDS Soc. 2020, 23, e25585. [Google Scholar] [CrossRef]
- Pantazis, N.; Papastamopoulos, V.; Paparizos, V.; Metallidis, S.; Adamis, G.; Antoniadou, A.; Psichogiou, M.; Chini, M.; Sambatakou, H.; Sipsas, N.V.; et al. Long-term evolution of CD4+ cell count in patients under combined antiretroviral therapy. AIDS 2019, 33, 1645–1655. [Google Scholar] [CrossRef]
- Liu, J.; Wang, L.; Hou, Y.; Zhao, Y.; Dou, Z.; Ma, Y.; Zhang, D.; Wu, Y.; Zhao, D.; Liu, Z.; et al. Immune restoration in HIV-1-infected patients after 12 years of antiretroviral therapy: A real-world observational study. Emerg. Microbes Infect. 2020, 9, 2550–2561. [Google Scholar] [CrossRef]
- García-Samaniego, J.; Soriano, V.; Miró, J.; Romero, J.; Bruguera, M.; Castilla, J.; Esteban, J.; Gonźlez, J.; Lissen, E.; Moreno, A.; et al. Management of chronic viral hepatitis in HIV-infected patients: Spanish Consensus Conference. HIV Clin. Trials 2002, 3, 99–114. [Google Scholar] [CrossRef]
- Jose, S. Assessing Outcomes of People Living with HIV in the UK in Relation to the Continuum of Care Framework. Ph.D. Thesis, UCL (University College London), London, UK, 2018. [Google Scholar]
- Opoku, S.; Sakyi, S.A.; Ayisi-Boateng, N.K.; Enimil, A.K.; Senu, E.; Ansah, R.O.; Aning, B.D.; Ojuang, D.A.; Wekesa, D.N.; Ahmed, F.O.; et al. Factors associated with viral suppression and rebound among adult HIV patients on treatment: A retrospective study in Ghana. AIDS Res. Ther. 2022, 19, 21. [Google Scholar] [CrossRef]
- Saag, M.S.; Benson, C.A.; Gandhi, R.T.; Hoy, J.F.; Landovitz, R.J.; Mugavero, M.J.; Sax, P.E.; Smith, D.M.; Thompson, M.A.; Buchbinder, S.P.; et al. Antiretroviral drugs for treatment and prevention of HIV infection in adults: 2018 recommendations of the International Antiviral Society–USA Panel. JAMA 2018, 320, 379–396. [Google Scholar] [CrossRef]
- Landovitz, R.J.; Scott, H.; Deeks, S.G. Prevention, Treatment and cure of HIV infection. Nat. Rev. Microbiol. 2023, 21, 657–670. [Google Scholar] [CrossRef] [PubMed]
- Boyd, M.A.; Boffito, M.; Castagna, A.; Estrada, V. Rapid initiation of antiretroviral therapy at HIV diagnosis: Definition, process, knowledge gaps. HIV Med. 2019, 20, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Ghidei, L.; Simone, M.J.; Salow, M.J.; Zimmerman, K.M.; Paquin, A.M.; Skarf, L.M.; Kostas, T.R.; Rudolph, J.L. Aging, antiretrovirals, and adherence: A meta analysis of adherence among older HIV-infected individuals. Drugs Aging 2013, 30, 809–819. [Google Scholar] [CrossRef] [PubMed]
- Wutoh, A.K.; Elekwachi, O.; Clarke-Tasker, V.; Daftary, M.; Powell, N.J.; Campusano, G. Assessment and predictors of antiretroviral adherence in older HIV-infected patients. JAIDS J. Acquir. Immune Defic. Syndr. 2003, 33, S106–S114. [Google Scholar] [CrossRef]
- Katirayi, L.; Maphosa, T.; Chilikutali, L.; Chamanga, R.K.; Petersson, J.; Khatib, S.; Munthali, B.; Nyirenda, R.; Matiya, E.; Nyirenda, L.; et al. Understanding gender differences of people with HIV newly diagnosed or returning to care with advanced HIV disease in Malawi: A qualitative study. BMC Public Health 2023, 23, 2382. [Google Scholar] [CrossRef]
- Takarinda, K.C.; Harries, A.D.; Shiraishi, R.W.; Mutasa-Apollo, T.; Abdul-Quader, A.; Mugurungi, O. Gender-related differences in outcomes and attrition on antiretroviral treatment among an HIV-infected patient cohort in Zimbabwe: 2007–2010. Int. J. Infect. Dis. 2015, 30, 98–105. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shoko, C.; Chikobvu, D. Time-homogeneous Markov process for HIV/AIDS progression under a combination treatment therapy: Cohort study, South Africa. Theor. Biol. Med. Model. 2018, 15, 3. [Google Scholar] [CrossRef]
- Kabudula, C.W.; Houle, B.; Collinson, M.A.; Kahn, K.; Gómez-Olivé, F.X.; Clark, S.J.; Tollman, S. Progression of the epidemiological transition in a rural South African setting: Findings from population surveillance in Agincourt, 1993–2013. BMC Public Health 2017, 17, 424. [Google Scholar] [CrossRef]
- Syed, S.T.; Gerber, B.S.; Sharp, L.K. Traveling towards disease: Transportation barriers to health care access. J. Community Health 2013, 38, 976–993. [Google Scholar] [CrossRef]
- Graves, J.M.; Abshire, D.A.; Alejandro, A.G. System- and Individual-Level Barriers to Accessing Medical Care Services Across the Rural-Urban Spectrum, Washington State. Health Serv. Insights 2022, 15, 11786329221104667. [Google Scholar] [CrossRef]
- Pope, C.N.; Stavrinos, D.; Fazeli, P.L.; Vance, D.E. Transportation barriers and health-related quality of life in a sample of middle-aged and older adults living with HIV in the deep south. AIDS Behav. 2022, 26, 2148–2158. [Google Scholar] [CrossRef]
- Fauk, N.K.; Gesesew, H.A.; Mwanri, L.; Hawke, K.; Ward, P.R. Understanding the quality of life of people living with HIV in rural and urban areas in Indonesia. PLoS ONE 2023, 18, e0280087. [Google Scholar] [CrossRef] [PubMed]
- Zimmer, Z.; Kaneda, T.; Tang, Z.; Fang, X. Explaining late life urban vs. rural health discrepancies in Beijing. Soc. Forces 2010, 88, 1885–1908. [Google Scholar] [CrossRef]
- Bedert, M.; Davidovich, U.; de Bree, G.; van Bilsen, W.; van Sighem, A.; Zuilhof, W.; Brinkman, K.; van der Valk, M.; de Wit, J. Understanding Reasons for HIV Late Diagnosis: A Qualitative Study Among HIV-Positive Individuals in Amsterdam, The Netherlands. AIDS Behav. 2021, 25, 2898–2906. [Google Scholar] [CrossRef] [PubMed]
- Ali, H.; Zakar, R.; Junaid, K.; Khan, A.; Fischer, F. Frequency and reasons for delayed treatment initiation after HIV diagnosis: Cross-sectional study in Lahore, Pakistan. BMC Public Health 2021, 21, 1000. [Google Scholar] [CrossRef]
- Pellowski, J.A. Barriers to care for rural people living with HIV: A review of domestic research and health care models. J. Assoc. Nurses AIDS Care 2013, 24, 422–437. [Google Scholar] [CrossRef] [PubMed]
- Birlie, B.; Braekers, R.; Awoke, T.; Kasim, A.; Shkedy, Z. Multi-state models for the analysis of time-to-treatment modification among HIV patients under highly active antiretroviral therapy in Southwest Ethiopia. BMC Infect. Dis. 2017, 17, 453. [Google Scholar] [CrossRef]
- Bousmah, M.a.Q.; Nishimwe, M.L.; Tovar-Sanchez, T.; Wandji, M.L.; Mpoudi-Etame, M.; Maradan, G.; Bassega, P.O.; Varloteaux, M.; Montoyo, A.; Kouanfack, C.; et al. Cost-Utility Analysis of a Dolutegravir-Based Versus Low-Dose Efavirenz-Based Regimen for the Initial Treatment of HIV-Infected Patients in Cameroon (NAMSAL ANRS 12313 Trial). PharmacoEconomics 2021, 39, 331–343. [Google Scholar] [CrossRef]
| Variables | Category | Number (%) |
|---|---|---|
| Age | ≤44 | 2880 (87%) |
| >44 | 445 (13%) | |
| Tuberculosis | Initiated ART with TB | 810 (24%) |
| Initiated ART without TB | 2515 (76%) | |
| Site | Eswatini (Urban) | 1555 (47%) |
| Vulindlela (Rural) | 1770 (53%) | |
| Gender | Female | 2148 (65%) |
| Male | 1177 (35%) | |
| Initial State | 1 | 57 (1%) |
| 2 | 126 (4%) | |
| 3 | 723 (22%) | |
| 4 | 2419 (73%) |
| To From | Normal | Mild | Advanced | Severe |
|---|---|---|---|---|
| Normal | 2058 | 444 | 122 | 48 |
| Mild | 967 | 1252 | 527 | 113 |
| Advanced | 363 | 1444 | 2821 | 667 |
| Severe | 98 | 383 | 2045 | 2896 |
| States | Normal Est (95% CI) | Mild Est (95% CI) | Advanced Est (95% CI) | Severe Est (95% CI) |
|---|---|---|---|---|
| Normal | −0.708 (−0.772, −0.650) | 0.595 (0.535, 0.661) | 0.078 (0.055, 0.112) | 0.036 (0.024, 0.053) |
| Mild | 1.215 (1.143, 1.292) | −2.105 (−2.218, −1.997) | 0.781 (0.704, 0.866) | 0.109 (0.082, 0.145) |
| Advanced | 0.003 (0, 0.073) | 1.170 (1.113, 1.230) | −1.738 (−1.817, −1.663) | 0.566 (0.518, 0.619) |
| Severe | 0.006 (0.002, 0.019) | 0.002 (0, 0.038) | 1.572 (1.499, 1.648) | −1.580 (−1.655, −1.508) |
| States | 1 (Normal) | 2 (Mild) | 3 (Advanced) | 4 (Severe) |
|---|---|---|---|---|
| 1 year | ||||
| 1 (Normal) | 0.63110667 | 0.2152040 | 0.1110817 | 0.04260763 |
| 2 (Mild) | 0.40843882 | 0.2978107 | 0.2128788 | 0.08087167 |
| 3 (Advanced) | 0.19980597 | 0.2688711 | 0.3726544 | 0.15866850 |
| 4 (Severe) | 0.09675155 | 0.1903095 | 0.3986161 | 0.31432282 |
| 5 years | ||||
| 1 (Normal) | 0.4302635 | 0.2443956 | 0.2211111 | 0.1042298 |
| 2 (Mild) | 0.4228654 | 0.2253476 | 0.2253476 | 0.1069569 |
| 3 (Advanced) | 0.4128209 | 0.2454068 | 0.2311008 | 0.1106715 |
| 4 (Severe) | 0.4042744 | 0.2458729 | 0.2359979 | 0.1138548 |
| 10 years | ||||
| 1 (Normal) | 0.4218898 | 0.2448793 | 0.2259069 | 0.1073239 |
| 2 (Mild) | 0.4217419 8 | 0.244887 | 0.2259917 | 0.1073786 |
| 3 (Advanced) | 0.4215407 | 0.2448994 | 0.2261069 | 0.1074530 |
| 4 (Severe) | 0.4213691 | 0.2449093 | 0.2262052 | 0.1075164 |
| State | Mean Sojourn Time | |
|---|---|---|
| Estimate | SE (95% CI) | |
| Normal | 1.4118 | 0.0620 (1.2954, 1.5387) |
| Mild | 0.4753 | 0.0127 (0.4510, 0.5010) |
| Advanced | 0.5751 | 0.0129 (0.5504, 0.6010) |
| Severe | 0.6330 | 0.0151 (0.6042, 0.6633) |
| Covariates | “−“2”∗(logLikelihood) | LR Test | DF | p-Value |
|---|---|---|---|---|
| No covariates | 33,844 | |||
| Site | 33,562 | 282 | 24 | <0.0001 |
| Tuberculosis (TB) | 33,604 | 240 | 24 | <0.0001 |
| Gender | 33,686 | 158 | 24 | <0.0001 |
| Age | 33,772 | 72 | 24 | <0.0001 |
| Regimen | 33,782 | 62 | 24 | <0.0001 |
| TB + Age | 33,532 | 312 | 36 | <0.0001 |
| TB + Age + Gender | 33,220 | 624 | 48 | <0.0001 |
| Regimen + Site + Age + Gender | 33,246 | 598 | 60 | <0.0001 |
| Variables | ||||
|---|---|---|---|---|
| Regimen (Ref: First-Line Treatment) Second-Line Treatment | Site (Ref: Urban) Rural | Age (Ref: ≤44) >44 | Gender (Ref: Female) Male | |
| Transitions | HR (95%CI) | HR (95%CI) | HR (95%CI) | HR (95%CI) |
| Normal–Mild | 1.310 (1.031, 1.664) | 1.079 (0.821, 1.420) | 1.062 (0.906, 1.246) | 1.614 (1.281, 2.034) |
| Normal–Advanced | 0.022 (0, 238.67) | 0.0009 (0, 5.786) | 1.648 (0.856, 3.174) | 3.125 (1.583, 6.185) |
| Normal–Severe | 0.121 (0.013, 1.066) | 0.071 (0.025, 0.200) | 0.743 (0.358, 1.543) | 0.916 (0.311, 2.696) |
| Mild–Normal | 1.180 (0.997, 1.40) | 1.142 (0.974, 1.338) | 0.957 (0.865, 1.059) | 0.909 (0.780, 1.060) |
| Mild–Advanced | 1.174 (0.093, 1.482) | 0.805 (0.641, 1.009) | 1.089 (0.933, 1.059) | 1.126 (0.922, 1.376) |
| Mild–Severe | 0.423 (0.179, 0.996) | 0.030 (0.002, 0.427) | 0.750 (0.447, 1.259) | 2.528 (1.354, 4.719) |
| Advanced–Normal | 0.078 (0, 324.064) | 0.011 (0, 20.422) | 0.428 (0.074, 2.495) | 0.301 (0, 292.98) |
| Advanced–Mild | 0.942 (0.818, 1.084) | 0.909 (0.808, 1.021) | 0.842 (0.774, 0.916) | 0.804 (0.718, 0.900) |
| Advanced–Severe | 1.373 (1.117, 1.688) | 0.596 (0.494, 0.719) | 0.926 (0.798, 1.075) | 1.358 (1.136, 1.623) |
| Severe–Normal | 1.005 (0.094, 10.741) | 0.093 (0, 98.023) | 5.150 (0.080, 332.34) | 1.322 (0.114, 15.384) |
| Severe–Mild | 7 (0.038, 1280.472) | 0.192 (0.006, 6.071) | 0.009 (0, 2.591) | 0.084 (0, 1062.727) |
| Severe–Advanced | 0.743 (0.655, 0.844) | 0.730 (0.657, 0.811) | 0.915 (0.841, 0.995) | 0.863 (0.779, 0.956) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mashiri, C.E.; Batidzirai, J.M.; Chifurira, R.; Chinhamu, K. A Multistate Continuous Time-Inhomogeneous Markov Model for Assessing the CD4 Count Dynamics of HIV/AIDS Patients Undergoing Antiretroviral Therapy in KwaZulu-Natal, South Africa. Int. J. Environ. Res. Public Health 2025, 22, 848. https://doi.org/10.3390/ijerph22060848
Mashiri CE, Batidzirai JM, Chifurira R, Chinhamu K. A Multistate Continuous Time-Inhomogeneous Markov Model for Assessing the CD4 Count Dynamics of HIV/AIDS Patients Undergoing Antiretroviral Therapy in KwaZulu-Natal, South Africa. International Journal of Environmental Research and Public Health. 2025; 22(6):848. https://doi.org/10.3390/ijerph22060848
Chicago/Turabian StyleMashiri, Chiedza Elvina, Jesca Mercy Batidzirai, Retius Chifurira, and Knowledge Chinhamu. 2025. "A Multistate Continuous Time-Inhomogeneous Markov Model for Assessing the CD4 Count Dynamics of HIV/AIDS Patients Undergoing Antiretroviral Therapy in KwaZulu-Natal, South Africa" International Journal of Environmental Research and Public Health 22, no. 6: 848. https://doi.org/10.3390/ijerph22060848
APA StyleMashiri, C. E., Batidzirai, J. M., Chifurira, R., & Chinhamu, K. (2025). A Multistate Continuous Time-Inhomogeneous Markov Model for Assessing the CD4 Count Dynamics of HIV/AIDS Patients Undergoing Antiretroviral Therapy in KwaZulu-Natal, South Africa. International Journal of Environmental Research and Public Health, 22(6), 848. https://doi.org/10.3390/ijerph22060848






