Abstract
Background: Tobacco use is responsible for eight million preventable deaths annually, making it a major modifiable risk factor for chronic conditions such as cardiovascular diseases, respiratory illnesses, and over 20 types of cancers. Objective: This study aimed to systematically review the barriers and facilitators of tobacco cessation interventions at both the population and healthcare system levels in the U.S. Understanding these determinants is critical for narrowing health disparities, optimizing resource allocation, and ultimately, enhancing tobacco cessation success rates across all demographic groups. Methods: A comprehensive literature search was conducted across the PubMed, Embase, and Web of Science databases, guided by the population, intervention, comparison, and outcome framework and quality assessment guided by PRISMA guidelines. Data extraction focused on study characteristics, intervention types, barriers, facilitators, and cessation outcomes at both the population and health system levels. The random effects forest plots were graphed to estimate pooled effect sizes for both medical and non-medical interventions. Results: A total of 35 studies met the inclusion criteria from an initial pool of 1555 identified records. Socioeconomic disadvantages, digital inequities, and low motivation constitute primary barriers at the individual level, while systemic factors such as healthcare access limitations, inadequate provider engagement, and lack of financial support further hinder cessation efforts. Financial incentives, culturally tailored interventions, and digital engagement strategies significantly improve tobacco cessation outcomes. Public health implications: as identified by the study, tailored interventions, the expansion of health coverage policies to include intervention, digital solutions, and healthcare resource workforce training will help improve tobacco cessation intervention outcomes.
1. Introduction
Globally, disparities in tobacco consumption exist among certain countries, with a few accounting for a significant proportion of consumers when compared to other developed countries. Tobacco is ingested by about 1.3 billion consumers globally; among these, 80% of consumers live in low- and middle-income countries [1]. Addictive tobacco consumption behavior is one of the causes of household poverty, as consumers prioritize its purchase over other essential needs like food or shelter. A total global health expenditure of about USD 1.4 trillion per year, along with productivity losses, are attributed to tobacco use, causing a significant economic burden, of which 40% is contributed by developing countries. In addition to the economic burden, there are significant preventable health risks related to tobacco consumption, leading to 8 million deaths annually. It is recognized as one of the preventable risk factors for chronic conditions such as cardiovascular and respiratory diseases, as well as 20 different types of cancers [1]. Cigarette smoking is one of the leading causes of preventable deaths in the U.S., killing about 480,000 of its consumers per year. In 2018, the U.S. economic burden due to tobacco consumption and its effect on health and productivity losses was estimated to be USD 600 billion [2].
Effective tobacco cessation interventions, including pharmacotherapy, behavioral counseling, and digital health solutions, are available; however, they remain underutilized and demonstrate less effectiveness in real-world settings compared to the results of randomized controlled trials (RCTs) [3]. Disparities in tobacco use are present among socioeconomically disadvantaged populations, racial minorities, and individuals facing adverse social determinants of health [4,5]. Disparities in utilization affect cessation treatment outcomes. Any treatment, while often tested in strict clinical environments, may be significantly influenced by real-world confounders when scaled for broad implementation. Understanding such contextual factors and whether they limit or support effectiveness can strengthen both causal analysis and the design of interventions aimed at narrowing tobacco-related health disparities. This review aims to establish the empirical foundation necessary to inform such evaluations and guide more equitable public health strategies. Therefore, it is important to identify these specific barriers [6,7]. At the population level, effective tobacco cessation interventions, i.e., behavioral counseling, alone or combine with pharmacotherapy, are available [8,9,10]. At the health system infrastructure level, various sources, such as face-to-face interactions, virtual messages, web-based platforms, or telephone interventions, exist to reach diverse populations and overcome challenges in implementation or population-level barriers. Several barriers are identified at both the individual and systemic levels, such as financial constraints, digital inequities, low motivation, limitations in healthcare access, and gaps in provider engagement, while identified facilitators, such as financial incentives, tailored interventions, social support, and improved healthcare infrastructure, play a crucial role in enhancing interventions’ effectiveness [11,12,13,14].
There are limited studies that comprehensively identify the barriers and facilitators of these extensive interventions and their implementation infrastructure [15,16,17]. However, these studies only focus on specific subpopulations or intervention modalities, which limits the generalizability of findings or the assessment of effectiveness in real-world settings. Furthermore, systematic evaluations of both medical (e.g., pharmacotherapy, nicotine replacement therapy) and non-medical (e.g., behavioral counseling, digital interventions) methods for overcoming cessation barriers are scarce. Understanding these factors is crucial for designing feasible and effective interventions in tackling tobacco use disparities. This review aims to synthesize the outcomes of medical and non-medical cessation interventions, while considering the population and health system level barriers and facilitators across diverse populations, settings, and implementation modalities in the U.S. While limited recent studies have explored the applications of evidence synthesis for constructing directed acyclic graphs (ESC-DAG) in other public health contexts [18], no work has leveraged systematic literature reviews to construct causally informed DAGs specific to tobacco cessation interventions. Additionally, as per a search across PubMed, Embase, Web of Science, and Google databases, there is no existing systematic literature review that assesses treatment effectiveness in the presence of barriers or facilitators across both the population and health system infrastructure level. Any treatment before implementation is tested in a strict clinical environment; however, its real-world implementation and effectiveness is affected by several confounders outside of a strict clinical environment. Identifying those confounders, i.e., barriers and facilitators, at both the receiving and implementation/facilitation levels can help strengthen future causal analysis. By systematically synthesizing barriers and facilitators across various intervention settings, this review aims to build the empirical foundation for developing ESC-DAGs that can enhance causal inference and statistical modeling in tobacco cessation research similar to the effect of limited existing research work in public health [18,19]. This is the first systematic review using a search across the previously mentioned databases to identify some of these confounding influences across the varied contextual factors of tobacco cessation treatment intervention receipt and implementation. Additionally, the review’s findings aim to guide public health policies and interventions regarding the real-world effectiveness of tobacco cessation interventions within the context of barriers and facilitators. For the same reason, this review does not focus on meta-regression analysis due to the presence of varied interventional modalities, along with the study design, study setting, and target population.
To clarify the scope of our review, we define “population level” as referring to barriers and facilitators experienced by individuals or groups receiving smoking cessation interventions, rather than to system-level implementation or provider-level factors. These include socio-demographic, behavioral, motivational, and contextual characteristics of target populations, especially among subgroups such as those with low socioeconomic status, racial and ethnic minorities, or individuals with mental health conditions. This distinction allows us to analyze how intervention effectiveness varies across end-user contexts, while differentiating between healthcare infrastructure or delivery issues.
Our review addresses a critical gap in the existing literature, where most studies assess barriers and facilitators within narrowly defined subpopulations or single intervention modalities. By synthesizing findings across both medical and non-medical interventions and examining these factors at both the individual and system levels, we aim to offer a broader and more actionable understanding of what influences tobacco cessation outcomes in real-world settings. This integrative approach allows for the identification of common barriers (e.g., digital inequity, mental health challenges) and facilitators (e.g., culturally tailored content, financial incentives) that recur across different implementation contexts.
2. Materials and Methods
2.1. PICO Framework
This systematic review followed the widely used population, intervention, comparison, and outcome (PICO) framework to guide study selection and synthesis [20]. The review focused on smokers residing in the U.S., evaluating tobacco cessation interventions that include behavioral therapies, pharmacotherapies, smoking cessation programs, and non-medical interventions. The primary outcome of interest was smoking cessation. PRISMA guidelines informed the reporting of study results.
2.2. Search Strategy and Database
A comprehensive literature search was conducted using the PubMed, Embase, and Web of Science databases on 1 December 2024. The gray literature source comprised connected papers [21], with manual bibliography searching. The citation management software used was Mendeley, version 1.19.5, released on 2018 [22], which helped identify duplicates. Similar Boolean terms and keywords were used across the identified database, and PICO framework guided the search strategy of the study. The search terms utilized across each databases are as mentioned in Appendix A Table A1, with search filters applied. Studies published between 2015 and 2025 were included in the screening for the final study sample.
2.3. Screening Questions
Two reviewers, S.S. and J.I., independently assessed and screened the studies, and any disagreements were resolved through discussion. In case of unresolved disagreement, a third reviewer, J.S., was consulted to reach a conclusive resolution. We used the following screening questions to determine whether to include or exclude studies. If a study met the following criteria during title, abstract, and body screening, it was included in the final sample.
- Is it about tobacco/smoking cessation interventions AND
- Is it conducted in the United States AND
- Is the study outcome tobacco cessation AND
- Are health/healthcare barriers and facilitators explored?
Barriers and facilitators to smoking cessation intervention implementation or outcomes were identified through inductive qualitative analysis of the Discussion and Results sections of each included study. Statements made by authors to explain their findings, i.e., challenges regarding recruitment, contextual factors, cultural relevance, acceptability, and resource availability, were extracted and coded. These were then categorized as barriers or facilitators, based on their influence on the intervention’s feasibility, effectiveness, or uptake.
2.4. Data Extraction, Analysis, and Quality Assessment
A standardized data extraction form was used to collect study details, including the author, study design, intervention type, population characteristics (i.e., smokers with mental illness, other conditions, or pregnancy), sample size, identified barriers, facilitators, and cessation outcomes. This review aimed to categorize the barriers and facilitators of both medical and non-medical tobacco cessation interventions at the population and healthcare system levels. A forest plot was created to pool the effect estimates of cessation interventions. Cochran’s Q test and the I-squared statistic were used to assess the heterogeneity of the included studies. A random-effects model was employed to account for variability across studies.
The quality assessment of the included studies was performed using a quality checklist for both RCT and non-RCT health intervention studies [22]. The quality assessment criteria included statistical conclusion validity, construct validity, i.e., power, reporting, and external validity, and internal validity, i.e., confounding and bias.
Barriers and facilitators were identified using inductive qualitative analysis. We extracted explanatory text from the Discussion, Limitations, and Conclusion sections of each study, in which authors interpreted significant or non-significant outcomes in light of contextual or structural factors. These factors were then coded and categorized based on whether they appeared to enhance (facilitators) or inhibit (barriers) the intervention’s impact or reach. This process enabled the synthesis of findings, even when the original study’s primary objective was not to evaluate barriers and facilitators directly. We extracted information on reported barriers and facilitators toward intervention implementation for the sub-population in the required studies. Barriers and facilitators were identified from both quantitative findings and qualitative content, including authors’ interpretations, contextual descriptions, and discussion of implementation issues, even when these factors were not directly assessed or statistically tested. This approach allowed us to capture relevant insights from all included studies, including those that did not demonstrate statistically significant differences in cessation outcomes.
A qualitative content analysis approach was used to synthesize these factors, examining how they were described and emphasized in each study. Common barriers and facilitators were identified based on both their recurrence across studies and the degree of emphasis placed on them by study authors, either regarding the results or the interpretation of the findings.
3. Results
A total of 35 studies met the inclusion criteria from an initial pool of 1555 identified records (Figure 1). Among these, 19 studies examined barriers and facilitators at the population level, with 10 assessing medical interventions and 9 evaluating non-medical interventions (Table 1A,B). Additionally, 16 studies focused on barriers and facilitators at the healthcare system level, with 6 investigating medical interventions and 10 exploring non-medical interventions (Table 2A,B).
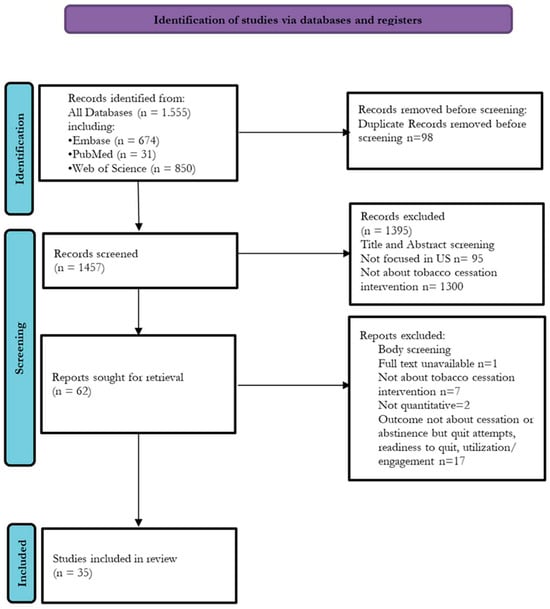
Figure 1.
PRISMA flowchart.

Table 1.
(A) Population level medical intervention study characteristics. (B) Population level non-medical intervention study characteristics.

Table 2.
(A) Health system level medical intervention study characteristics. (B) Health system level non-medical intervention study characteristics.
3.1. Population Level Barriers and Facilitators in Tobacco Cessation Interventions
3.1.1. Medical Interventions
The identified studies employed various medical interventions, including combined pharmacotherapy and behavioral counseling, financial incentives for pharmacotherapy, digital cessation interventions along with pharmacotherapy, or a combination of these with usual care (Table 1A). Key barriers included socioeconomic disadvantages such as homelessness, financial constraints, digital inequities, and mental or emotional issues like mental illness and low motivation. Facilitators that contributed to improved cessation outcomes encompassed financial incentives, peer motivation and social support, the incorporation of multilingual components in interventions for culturally diverse populations, patient-tailored therapies, nicotine replacement therapy (NRT), any combination of other therapies, integrated treatment services for tobacco cessation, or the implementation of digital intervention methods (Table A2).
The included studies assessed the effectiveness of various smoking cessation interventions among socioeconomically disadvantaged and high-risk populations. Effect sizes were reported as odds ratios (OR) or relative risks (RR), with corresponding 95% confidence intervals (CIs), reflecting the magnitude and statistical significance of each intervention’s impact. Interventions incorporating financial incentives demonstrated the strongest effects on smoking cessation. Baggett et al. reported an OR of 8.08 (95% CI: 3.35–19.5) for a program combining financial incentives, nicotine replacement therapy (NRT), and counseling among homeless smokers, indicating a highly significant effect. Similarly, Higgins et al. found that financial incentives combined with NRT increased cessation rates among socioeconomically disadvantaged mothers, with an OR of 5.88 (95% CI: 1.87–18.48). Kendzor et al. also reported a significant effect of financial incentives for low-income adults, with an OR of 3.18 (95% CI: 1.70–5.95). Community-based and behavioral interventions also demonstrated positive effects. Brooks et al. evaluated a community advocate-led cessation program for public housing residents, reporting an OR of 2.98 (95% CI: 1.56–5.68). Hooper et al. examined a culturally adapted video–text intervention combined with NRT among African American smokers, yielding an OR of 3.02 (95% CI: 0.53–7.37), although the wide CI suggests uncertainty in the estimate. Halpern et al. found that financial incentives combined with pharmacotherapy resulted in an OR of 2.27 (95% CI: 1.24–4.17), demonstrating statistical significance. Interventions targeting language barriers and mental health conditions showed varied effectiveness. Chen et al. assessed Asian-language behavioral counseling and NRT among Asian immigrants, reporting an OR of 0.40 (95% CI: 0.36–0.45), indicating reduced cessation rates compared to those for the controls. Meernik et al. examined a community-based cessation program for individuals with behavioral health conditions and found a modest effect (OR = 1.15, 95% CI: 1.08–1.21). Hickman et al. assessed a transtheoretical model-based intervention tailored for uninsured smokers with mental illness, reporting an OR of 1.80 (95% CI: 0.74–4.38), although the wide CI suggests limited precision. Finally, a mobile phone-delivered cessation intervention combined with NRT was evaluated by Vidrine et al. among socioeconomically disadvantaged smokers, yielding a relative risk of 2.11 (95% CI: 1.00–4.48), suggesting potential effectiveness, although with borderline statistical significance.
3.1.2. Non-Medical Interventions
Non-medical interventions at the population level included acceptance and commitment-based behavioral therapy implemented through a smartphone app, patient-tailored interactive and non-interactive text and web interventions, and cognitive behavioral counseling on its own (Table 1B). Common barriers included digital inequities, low motivation, digital literacy disparities, stress, stigma, household smoking exposure, being a racial minority, being a daily heavy smoker, and having less than a high school education. Effective facilitators consisted of behavioral support, self-paced engagement, personalized messaging, motivational support, cognitive behavioral therapy interventions, stricter indoor smoking restrictions, and the implementation of virtual interventions.
The efficacy of various smoking cessation interventions was evaluated across multiple randomized controlled trials (RCTs) and one cohort study, with effect sizes reported as the relative risk (RR) or odds ratio (OR), along with 95% confidence intervals (CIs). Bricker et al. (RR = 1.72, 95% CI: 1.45–2.05) demonstrated a significantly increased cessation rate among general adult smokers using an ACT-based smartphone application compared to the use of a standard app, indicating a robust effect. Similarly, Lee et al. (OR = 2.53, 95% CI: 1.21–5.28) found cognitive behavioral counseling (CBC) to be an effective intervention for pregnant and postpartum women, with a more than twofold increase in cessation likelihood. Mays et al. reported a significant effect of tailored mobile messaging for waterpipe smokers (OR = 1.9, 95% CI: 1.1–3.3), suggesting that personalized SMS interventions could be beneficial. Graham et al. (OR = 1.39, 95% CI: 1.15–1.68) and Villanti et al. (RR = 1.09, 95% CI: 1.04–1.15) reported significant, but comparatively modest, effects for mobile-based interventions targeting young adult e-cigarette smokers and socioeconomically disadvantaged young adults, respectively. Collins et al. (OR = 11.0, 95% CI: 6.3–19.2) reported the highest effect size, with behavioral counseling in mothers of young children associated with significantly increased smoking cessation, likely driven by household smoking restrictions. Conversely, Heffner et al. (OR = 0.91, 95% CI: 0.65–1.28) found no significant difference in cessation rates between sexual minority and nonminority smokers in web- and text-based interventions, suggesting that additional barriers such as minority stress and stigma may attenuate intervention effectiveness. Christiansen et al. also reported no significant difference in smoking cessation outcomes among low-socioeconomic status (SES) smokers receiving brief interventions in community settings. Similarly, Kamke et al.’s cohort study of text-based cessation interventions for pregnant women (OR = 1.01, 95% CI: 0.57–1.78) showed no significant impact, particularly when stratified by race and education level.
3.2. Healthcare System Level Barriers and Facilitators in Tobacco Cessation Interventions
3.2.1. Medical Interventions
Several studies identified medical interventions facilitated by healthcare system infrastructure, such as NRT, a combination of pharmacotherapy and proactive outreach, tobacco clinics in public housing, quitline services, and services involving community health workers for implementation (Table 2A). Healthcare system-level barriers included treatment access issues, rurality, cost of care, lack of provider engagement, and living in areas with limited health resources. Facilitators identified in the studies included free treatment availability and access, proactive encouragement, implementation of comprehensive treatments (a combination of pharmacotherapy and behavioral therapy), community-based clinic support, and social support provided by healthcare workers.
Among the randomized controlled trials (RCTs) reviewed, Dahne’s study assessing nicotine replacement therapy (NRT) sampling among general smokers reported an OR of 1.59 (95% CI: 0.97–2.59). Although suggestive of a positive effect, the confidence interval was 1.0, meaning the result is not statistically significant. In contrast, Fu et al. examined proactive outreach tobacco treatment among low-income smokers covered by Medicaid or MinnesotaCare, reporting an OR of 1.47 (95% CI: 1.12–1.93). The significant confidence interval suggests that proactive treatment, including free access to NRT and behavioral counseling, increases cessation rates in this population. Lee et al. investigated the impact of cessation advice receipt among U.S. adult smokers, finding an OR of 0.99 (95% CI: 0.95–1.00), indicating no meaningful association. Similarly, Kerkvliet et al. reported an OR of 0.78 (95% CI: 0.68–0.89) when examining quitline services combined with cessation treatment among smokers with and without mental health conditions. The significant negative effect suggests that additional psychological barriers may hinder the effectiveness of quitline interventions. Wewers et al. evaluated cessation treatment that involved community health workers and NRT among Appalachian smokers, finding an OR of 1.04 (95% CI: 1.01–1.06). While statistically significant, the effect size is modest, indicating only a slight improvement in cessation outcomes.
3.2.2. Non-Medical Interventions
System-level non-medical interventions included smartphone-based incentives, policy changes, i.e., Affordable Care Act (ACA) Medicaid expansion for tobacco treatment cessation coverage, and social media-based cessation intervention implementation (Table 2B). Common barriers included limited access to healthcare due to insurance coverage, observed racial discrimination within the health system, low awareness of pharmacotherapy, cultural obstacles, geographic isolation, digital access challenges, and various individual socioeconomic, mental, and emotional disadvantage factors. Facilitators included smartphone-based incentivized interventions; Medicaid expansion coverage for treatment; online peer and counselor support; automated prompts; specialized quitlines; live counseling engagement; greater acceptance of physical sensations, emotions, and thoughts; digital interventions; and coping response training.
Kurti et al. reported a substantial effect of a smartphone-based incentivized intervention for pregnant smokers, yielding an odds ratio (OR) of 9.33 (95% CI: 1.87–46.68), indicating a strong association between the intervention and smoking cessation. Similarly, Chen Lyu et al. found that social media-based cessation interventions for young adult smokers were associated with increased cessation likelihood (OR = 2.6, 95% CI: 1.8–4.2), despite identified digital access barriers. Bailey et al. investigated the impact of Medicaid expansion on smoking cessation among Medicaid-insured smokers and observed a modest but significant effect (OR = 1.35, 95% CI: 1.28–1.43), suggesting that healthcare access plays a critical role in cessation outcomes. Hooper, M.W. et al. examined cessation treatment incorporating cognitive-behavioral therapy (CBT) and generalized health education (GHE) among individuals facing racial barriers, with results showing an OR of 0.97 (95% CI: 0.94–0.99), reflecting a minimal but statistically significant effect. In studies evaluating digital interventions, Santiago-Torres et al. assessed the iCanQuit app for American Indian and Alaska Native smokers, yielding an OR of 1.97 (95% CI: 0.92–4.25), suggesting a potential but inconclusive effect. Similarly, an ACT-based smartphone cessation intervention for rural smokers resulted in an OR of 1.47 (95% CI: 0.96–2.27), indicating moderate efficacy. McCarthy et al. explored the effectiveness of an electronic health record (EHR)-enabled smoking treatment program among primary care patients, reporting a wide confidence interval (95% CI: 2.2–10.5), although the specific OR was not provided. Kreuter et al. found that specialized quitline services with navigation support for low-income smokers had a significant but negative effect on cessation (OR = 0.70, 95% CI: 0.50–0.95), implying that barriers such as low awareness of nicotine replacement therapy (NRT) and financial constraints may hinder cessation success. Thrul’s study on Facebook-based live counseling for young adult smokers demonstrated a small but positive impact (OR = 1.10, 95% CI: 1.02–1.20), emphasizing the role of social support in cessation. Webb Hooper et al. investigated CBT combined with an eight-week nicotine patch regimen in treatment-seeking smokers and reported an OR of 0.93 (95% CI: 0.89–0.98), indicating a small but statistically significant reduction in smoking likelihood associated with reductions in perceived distress and depressive symptoms.
4. Discussion
The study identifies key factors influencing the effectiveness of multifaceted tobacco cessation interventions at both the population and healthcare system levels. Common barriers include limited access to healthcare, digital literacy gaps, and psychological challenges, which hinder engagement with cessation programs. In contrast, facilitators that enhance tobacco cessation outcomes include tailored motivational support, behavioral therapy, expanded treatment coverage, improved healthcare access, and social support.
The findings suggest that integrating tailored interventions with expanded access to treatment can optimize cessation outcomes by addressing both individual and systemic barriers. Socioeconomic disadvantage, stress, low motivation, and mental illness significantly impact cessation success at the individual level. At the healthcare system level, structural and process-related components, i.e., healthcare infrastructure, provider engagement, and policy-driven interventions, play a critical role in ensuring equitable access to cessation treatments. A comprehensive approach that simultaneously tackles these barriers at multiple levels is essential for reducing disparities in treatment outcomes.
While financial incentives were identified as effective facilitators of smoking cessation in several studies, we acknowledge that their impact was often limited to short-term follow-ups. For example, studies such as those by Baggett and colleagues demonstrated significant cessation effects at early timepoints, but these effects tended to diminish over time [23,28]. Therefore, financial incentives should be interpreted as short-term facilitators that enhance initial cessation rates, particularly among highly disadvantaged populations, rather than as sustained solutions. Long-term effectiveness may require continued support or complementary behavioral interventions.
Multilingual services, materials, or personnel were noted as facilitators in several studies. These components were intentionally designed to address language barriers and improve cultural and linguistic alignment, thereby facilitating access to cessation services among racial and ethnic minoritized groups. We categorized such components as facilitators based on their intended function to promote engagement and inclusivity, rather than based solely on cessation outcomes. For instance, although a study [24] reported lower cessation rates among users of multilingual quitlines compared to those for English-speaking users, this comparison involved different cohorts across distinct time periods (2004–2008 vs. 2012–2019), with potential confounding factors. Despite the cessation outcome, the multilingual service model was introduced specifically to address known language and access disparities, aligning with our definition of facilitators in this review.
This study acknowledges certain limitations. The high heterogeneity observed across studies suggests variability in intervention effectiveness, likely influenced by differences in study design, population characteristics, and implementation contexts. While the random-effects model in the pooled analysis accounts for some degree of heterogeneity, future research should conduct stratified analyses to identify the most impactful intervention components for specific subpopulations. Additionally, publication bias and the reliance on self-reported cessation outcomes in some studies may limit the generalizability of the findings. Further studies employing objective cessation measures and longitudinal analyses are needed to strengthen the evidence base for effective tobacco cessation strategies. Our inclusion of the work of [12] reflects the broader scope of this review to capture structural and policy-level changes that function as population-level interventions. While the study is observational and subject to baseline differences between comparison groups, its difference-in-differences design supports a tentative association between Medicaid expansion and increased cessation, particularly among Hispanic populations. Nonetheless, we acknowledge the limitations in attributing causality due to potential confounding and lack of randomization.
It is essential to note that while our synthesis primarily includes studies focused on specific subpopulations, such as individuals of low socioeconomic status (SES), racial/ethnic minorities, or pregnant women, our objective was not to achieve national representativeness, but rather to integrate insights from diverse real-world implementation contexts. These contexts often overlap with priority populations disproportionately burdened by tobacco use, and the repetition of certain themes across multiple studies provides confidence in the generalizability of key barriers and facilitators. We acknowledge that this approach may not fully capture the experiences of higher SES or low-risk populations, as they are underrepresented in the literature. Nonetheless, by aggregating findings from a wide range of studies, our review offers a comprehensive overview of the most persistent challenges and enablers across healthcare and behavioral domains.
This review contributes to the field by addressing limitations in previous work that either aggregated diverse populations or focused exclusively on intervention efficacy. By synthesizing implementation barriers and facilitators across studies involving racially/ethnically minoritized and SGM populations, our review uniquely centers equity-related contextual and structural considerations. Unlike prior reviews, this study also includes both clinical and policy-level interventions, highlighting how systems-level changes like Medicaid expansion which may influence cessation in underrepresented groups. The originality of this review lies in its focus on implementation contexts, its explicit attention to subgroup-specific dynamics, and the integration of findings across heterogeneous intervention types, offering practical insights for equity-focused program design and future research.
5. Recommendations for Public Health and Healthcare Systems
This review established the significance of a multi-tiered approach to tobacco cessation interventions that considers both individual and systemic determinants. Improving health care access, workforce engagement, and digital solutions can help achieve the effectiveness of cessation in a real-world setting. Prioritizing digital health, telemedicine, mobile health, text messaging or relevant virtual modes of implementation can help improve access, particularly for populations facing geographic and healthcare access barriers. Incorporating culturally and linguistically tailored interventions can help improve treatment reach and effectiveness among culturally diverse and some hard-to-reach target populations. Finally, improving healthcare workforce training in inculcating cessation interventions during routine practices can help strengthen cessation treatment utilization, engagement, and outcomes.
Author Contributions
Conceptualization, methodology, software, validation—S.S.; formal analysis, investigation, resources, data curation—J.I. and S.J.; writing—original draft preparation—S.S.; writing—review and editing, visualization, supervision, project administration—J.I. and S.J.; funding acquisition—N/A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors have reviewed and edited the output and take full responsibility for the content of this publication.
Conflicts of Interest
The authors declare no conflicts of interest.
Appendix A

Table A1.
Search strategy.
Table A1.
Search strategy.
| Search Details | Results | Database |
|---|---|---|
| ((“Tobacco Use Cessation”[MeSH Terms] OR “Smoking Cessation”[MeSH Terms] OR “Smoking Reduction”[MeSH Terms] OR (“Tobacco Control”[MeSH Terms] OR “Smoking Reduction”[MeSH Terms]) OR “Nicotine Replacement Therapy”[MeSH Terms]) AND (“outcome assessment, health care”[MeSH Terms] OR “quality assurance, health care”[MeSH Terms] OR “Treatment Outcome”[MeSH Terms] OR “Program Evaluation”[MeSH Terms] OR “Health Services Accessibility”[MeSH Terms]) AND (“Healthcare Disparities”[MeSH Terms] OR “Health Status Disparities”[MeSH Terms] OR “Health Inequities”[MeSH Terms] OR “Socioeconomic Disparities in Health”[MeSH Terms] OR (“Social Determinants of Health”[MeSH Terms] OR “Health Information Management”[MeSH Terms] OR “Health Impact Assessment”[MeSH Terms]) OR “Health Equity”[MeSH Terms] OR “Vulnerable Populations”[MeSH Terms] OR (“Minority Health”[MeSH Terms] OR “Race Factors”[MeSH Terms]))) AND (y_10[Filter]) | 50 | PubMed |
| ((‘tobacco use cessation’/exp OR ‘tobacco use cessation’ OR ‘smoking cessation’/exp OR ‘smoking cessation’ OR ‘tobacco control’/exp OR ‘tobacco control’ OR ‘smoking reduction’/exp OR ‘smoking reduction’ OR ‘nicotine replacement therapy’/exp OR ‘nicotine replacement therapy’) AND [2015,2016,2017,2018,2019,2020,2021,2022,2023,2024,2025]/py) AND ((‘outcome assessment, health care’/exp OR ‘outcome assessment, health care’ OR ‘quality assurance, health care’/exp OR ‘quality assurance, health care’ OR ‘treatment outcome’/exp OR ‘treatment outcome’ OR ‘program evaluation’/exp OR ‘program evaluation’ OR ‘health services accessibility’/exp OR ‘health services accessibility’) AND [2015,2016,2017,2018,2019,2020,2021,2022,2023,2024,2025]/py) AND ((‘healthcare disparities’/exp OR ‘healthcare disparities’ OR ‘health status disparities’/exp OR ‘health status disparities’ OR ‘health inequities’/exp OR ‘health inequities’ OR ‘socioeconomic disparities in health’/exp OR ‘socioeconomic disparities in health’ OR ‘social determinants of health’/exp OR ‘social determinants of health’ OR ‘health information management’/exp OR ‘health information management’ OR ‘health impact assessment’/exp OR ‘health impact assessment’ OR ‘health equity’/exp OR ‘health equity’ OR ‘vulnerable populations’/exp OR ‘vulnerable populations’ OR ‘minority health’/exp OR ‘minority health’ OR ‘race factors’/exp OR ‘race factors’) AND [2015,2016,2017,2018,2019,2020,2021,2022,2023,2024,2025]/py) | 936 | Embase |
| ((((TS = (smoking cessation)) OR TS = (Smoking Reduction)) OR TS = (Tobacco Control)) OR TS = (Tobacco Use Cessation)) OR TS = (Nicotine Replacement Therapy) and Preprint Citation Index (Exclude–Database) AND ((((TS = (Health Care Outcome Assessment)) OR TS = (Health Care Quality Assurance)) OR TS = (Treatment Outcome)) OR TS = (Program Evaluation)) OR TS = (Health Services Accessibility) and Preprint Citation Index (Exclude–Database) AND ((((((((((TS = (Healthcare Disparities)) OR TS = (Health Status Disparities)) OR TS = (Health Inequities)) OR TS = (Socioeconomic Disparities in Health)) OR TS = (Social Determinants of Health)) OR TS = (Health Information Management)) OR TS = (Health Impact Assessment)) OR TS = (Health Equity)) OR TS = (Vulnerable Populations)) OR TS = (Minority Health)) OR TS = (Race Factors) Preprint Citation Index (Exclude–Database) and 2015 or 2016 or 2017 or 2018 or 2019 or 2020 or 2021 or 2022 or 2023 or 2024 or 2025 (Publication Years) and Article or Review Article (Document Types) | 1426 | Web of Science |

Table A2.
PRISMA Checklist.
Table A2.
PRISMA Checklist.
| Section and Topic | Item # | Checklist Item | Location Where Item Is Reported |
|---|---|---|---|
| TITLE | |||
| Title | 1 | Identify the report as a systematic review. | Line 2–3, Page 1 |
| ABSTRACT | |||
| Abstract | 2 | See the PRISMA 2020 for Abstracts checklist. | The abstract section was prepared accordingly. |
| INTRODUCTION | |||
| Rationale | 3 | Describe the rationale for the review in the context of existing knowledge. | Lines 63–91 |
| Objectives | 4 | Provide an explicit statement of the objective(s) or question(s) the review addresses. | Lines 91–98 |
| METHODS | |||
| Eligibility criteria | 5 | Specify the inclusion and exclusion criteria for the review and how studies were grouped for the syntheses. | Lines 101–126 |
| Information sources | 6 | Specify all databases, registers, websites, organisations, reference lists and other sources searched or consulted to identify studies. Specify the date when each source was last searched or consulted. | Lines 109–115 |
| Search strategy | 7 | Present the full search strategies for all databases, registers and websites, including any filters and limits used. | Lines 109–115 and Appendix A Table A1 |
| Selection process | 8 | Specify the methods used to decide whether a study met the inclusion criteria of the review, including how many reviewers screened each record and each report retrieved, whether they worked independently, and if applicable, details of automation tools used in the process. | Lines 109–126 and Figure 1 |
| Data collection process | 9 | Specify the methods used to collect data from reports, including how many reviewers collected data from each report, whether they worked independently, any processes for obtaining or confirming data from study investigators, and if applicable, details of automation tools used in the process. | Lines 109–126 and Figure 1 |
| Data items | 10a | List and define all outcomes for which data were sought. Specify whether all results that were compatible with each outcome domain in each study were sought (e.g., for all measures, time points, analyses), and if not, the methods used to decide which results to collect. | Table 1A and Table 2B |
| 10b | List and define all other variables for which data were sought (e.g., participant and intervention characteristics, funding sources). Describe any assumptions made about any missing or unclear information. | Table 1A and Table 2B | |
| Study risk of bias assessment | 11 | Specify the methods used to assess risk of bias in the included studies, including details of the tool(s) used, how many reviewers assessed each study and whether they worked independently, and if applicable, details of automation tools used in the process. | Lines 127–130 |
| Effect measures | 12 | Specify for each outcome the effect measure(s) (e.g., risk ratio, mean difference) used in the synthesis or presentation of results. | Table 1A and Table 2B |
| Synthesis methods | 13a | Describe the processes used to decide which studies were eligible for each synthesis (e.g., tabulating the study intervention characteristics and comparing against the planned groups for each synthesis (item #5)). | Lines 117–126 and Figure 1 |
| 13b | Describe any methods required to prepare the data for presentation or synthesis, such as handling of missing summary statistics, or data conversions. | NA | |
| 13c | Describe any methods used to tabulate or visually display results of individual studies and syntheses. | Table 1A and Table 2B | |
| 13d | Describe any methods used to synthesize results and provide a rationale for the choice(s). If meta-analysis was performed, describe the model(s), method(s) to identify the presence and extent of statistical heterogeneity, and software package(s) used. | Lines 133–136 | |
| 13e | Describe any methods used to explore possible causes of heterogeneity among study results (e.g., subgroup analysis, meta-regression). | NA | |
| 13f | Describe any sensitivity analyses conducted to assess robustness of the synthesized results. | NA | |
| Reporting bias assessment | 14 | Describe any methods used to assess risk of bias due to missing results in a synthesis (arising from reporting biases). | Lines 133–136 |
| Certainty assessment | 15 | Describe any methods used to assess certainty (or confidence) in the body of evidence for an outcome. | NA |
| RESULTS | |||
| Study selection | 16a | Describe the results of the search and selection process, from the number of records identified in the search to the number of studies included in the review, ideally using a flow diagram. | Figure 1 |
| 16b | Cite studies that might appear to meet the inclusion criteria, but which were excluded, and explain why they were excluded. | Lines 143–147 | |
| Study characteristics | 17 | Cite each included study and present its characteristics. | Table 1A and Table 2B |
| Risk of bias in studies | 18 | Present assessments of risk of bias for each included study. | Table 1A and Table 2B |
| Results of individual studies | 19 | For all outcomes, present, for each study: (a) summary statistics for each group (where appropriate) and (b) an effect estimate and its precision (e.g., confidence/credible interval), ideally using structured tables or plots. | Table 1A and Table 2B; Figure 1 |
| Results of syntheses | 20a | For each synthesis, briefly summarise the characteristics and risk of bias among contributing studies. | Table 1A and Table 2B |
| 20b | Present results of all statistical syntheses conducted. If meta-analysis was done, present for each the summary estimate and its precision (e.g., confidence/credible interval) and measures of statistical heterogeneity. If comparing groups, describe the direction of the effect. | NA | |
| 20c | Present results of all investigations of possible causes of heterogeneity among study results. | Lines 320–344 | |
| 20d | Present results of all sensitivity analyses conducted to assess the robustness of the synthesized results. | NA | |
| Reporting biases | 21 | Present assessments of risk of bias due to missing results (arising from reporting biases) for each synthesis assessed. | NA |
| Certainty of evidence | 22 | Present assessments of certainty (or confidence) in the body of evidence for each outcome assessed. | NA |
| DISCUSSION | |||
| Discussion | 23a | Provide a general interpretation of the results in the context of other evidence. | Lines 377–390 |
| 23b | Discuss any limitations of the evidence included in the review. | Lines 391–399 | |
| 23c | Discuss any limitations of the review processes used. | Lines 391–399 | |
| 23d | Discuss implications of the results for practice, policy, and future research. | Lines 401–411 | |
| OTHER INFORMATION | |||
| Registration and protocol | 24a | Provide registration information for the review, including register name and registration number, or state that the review was not registered. | Not registered |
| 24b | Indicate where the review protocol can be accessed, or state that a protocol was not prepared. | Not prepared | |
| 24c | Describe and explain any amendments to information provided at registration or in the protocol. | Not registered/not prepared | |
| Support | 25 | Describe sources of financial or non-financial support for the review, and the role of the funders or sponsors in the review. | NA |
| Competing interests | 26 | Declare any competing interests of review authors. | NA |
| Availability of data, code and other materials | 27 | Report which of the following are publicly available and where they can be found: template data collection forms; data extracted from included studies; data used for all analyses; analytic code; any other materials used in the review. | NA |
References
- Tobacco. World Health Organization. 2025. Available online: https://www.who.int/health-topics/tobacco#tab=tab_2 (accessed on 10 February 2025).
- Burden of Cigarette Use in the U.S. Office on Smoking and Health, National Center for Chronic Disease Prevention and Health Promotion, Centers for Disease Control and Prevention. 2024. Available online: https://www.cdc.gov/tobacco/campaign/tips/resources/data/cigarette-smoking-in-united-states.html (accessed on 10 February 2025).
- Improving Tobacco-Related Health Disparities. National Center for Chronic Disease Prevention and Health Promotion; Office on Smoking and Health. Available online: https://www.cdc.gov/tobacco/tobacco-features/health-equity.html (accessed on 10 February 2025).
- Simmons, V.N.; Piñeiro, B.; Hooper, M.W.; Gray, J.E.; Brandon, T.H. Tobacco-Related Health Disparities Across the Cancer Care Continuum. Cancer Control 2016, 23, 434. Available online: https://pmc.ncbi.nlm.nih.gov/articles/PMC5972388/ (accessed on 10 February 2025). [CrossRef] [PubMed]
- Marbin, J.; Balk, S.J.; Gribben, V.; Groner, J. Health disparities in tobacco use and exposure: A structural competency approach. Pediatrics 2021, 147, e2020040253. Available online: https://publications.aap.org/pediatrics/article/147/1/e2020040253/33415/Health-Disparities-in-Tobacco-Use-and-Exposure-A (accessed on 10 February 2025). [CrossRef] [PubMed]
- United States Public Health Service Office of the Surgeon General; National Center for Chronic Disease Prevention and Health Promotion (US) Office on Smoking and Health, Washington (DC). Interventions for Smoking Cessation and Treatments for Nicotine Dependence—Smoking Cessation—NCBI Bookshelf. In Smoking Cessation: A Report of the Surgeon General; US Department of Health and Human Services: Washington, DC, USA, 2020. Available online: https://www.ncbi.nlm.nih.gov/books/NBK555596/ (accessed on 10 February 2025).
- Benefits of Quitting Smoking. National Center for Chronic Disease Prevention and Health Promotion; Office on Smoking and Health. Available online: https://www.cdc.gov/tobacco/about/benefits-of-quitting.html (accessed on 10 February 2025).
- Brooks, D.R.; Burtner, J.L.; Borrelli, B.; Heeren, T.C.; Evans, T.; Davine, J.A.; Greenbaum, J.; Scarpaci, M.; Kane, J.; Rees, V.W.; et al. Twelve-month outcomes of a group-randomized community health advocate-led smoking cessation intervention in public housing. Nicotine Tob. Res. 2018, 20, 1434–1441. [Google Scholar] [CrossRef] [PubMed]
- Chen Lyu, J.; Meacham, M.C.; Nguyen, N.; Ramo, D.; Ling, P.M. Factors Associated with Abstinence Among Young Adult Smokers Enrolled in a Real-world Social Media Smoking Cessation Program. Nicotine Tob. Res. 2024, 26, S27–S35. [Google Scholar]
- Hickman, N.J.; Delucchi, K.L.; Prochaska, J.J. Treating tobacco dependence at the intersection of diversity, poverty, and mental illness: A randomized feasibility and replication trial. Nicotine Tob. Res. 2015, 17, 1012–1021. [Google Scholar] [CrossRef]
- Kurti, A.N.; Tang, K.; Bolivar, H.A.; Evemy, C.; Medina, N.; Skelly, J.; Nighbor, T.; Higgins, S.T. Smartphone-based financial incentives to promote smoking cessation during pregnancy: A pilot study. Prev. Med. 2020, 140, 106201. [Google Scholar] [CrossRef]
- Bailey, S.R.; Marino, M.; Ezekiel-Herrera, D.; Schmidt, T.; Angier, H.; Hoopes, M.J.; DeVoe, J.E.; Heintzman, J.; Huguet, N. Tobacco Cessation in Affordable Care Act Medicaid Expansion States Versus Non-expansion States. Nicotine Tob. Res. 2020, 22, 1016–1022. Available online: https://academic.oup.com/ntr/article/22/6/1016/5498071 (accessed on 10 February 2025). [CrossRef] [PubMed]
- Thrul, J.; Meacham, M.C.; Tice, C.; Kelly, O.; Ramo, D.E. Live counselor contact in a facebook intervention predicts smoking cessation outcomes. Psychol. Addict. Behav. 2020, 34, 360–369. [Google Scholar] [CrossRef]
- Kreuter, M.W.; Garg, R.; Fu, Q.; Caburnay, C.; Thompson, T.; Roberts, C.; Sandheinricha, D.; Javeda, I.; Wolffa, J.M.; Butler, T.; et al. Helping low-income smokers quit: Findings from a randomized controlled trial comparing specialized quitline services with and without social needs navigation. Lancet Reg. Health–Am. 2023, 23, 100529. Available online: https://www.embase.com/search/results?subaction=viewrecord&id=L2025307733&from=export (accessed on 10 February 2025). [CrossRef]
- Pacek, L.R.; Joseph McClernon, F.; Bosworth, H.B. Adherence to pharmacological smoking cessation interventions: A literature review and synthesis of correlates and barriers. Nicotine Tob. Res. 2018, 20, 1163–1172. Available online: https://www.embase.com/search/results?subaction=viewrecord&id=L624465157&from=export (accessed on 10 February 2025). [CrossRef]
- Register, S.J.; Harrington, K.F.; Agne, A.A.; Cherrington, A.L. Effectiveness of Non-Primary Care-Based Smoking Cessation Interventions for Adults with Diabetes: A Systematic Literature Review. Curr. Diab. Rep. 2016, 16, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Kock, L.; Brown, J.; Hiscock, R.; Tattan-Birch, H.; Smith, C.; Shahab, L. Individual-level behavioural smoking cessation interventions tailored for disadvantaged socioeconomic position: A systematic review and meta-regression. Lancet Public Health 2019, 4, e628–e644. Available online: https://www.embase.com/search/results?subaction=viewrecord&id=L2004927263&from=export (accessed on 10 February 2025). [CrossRef] [PubMed]
- Patel, N.; Karimi, S.M.; Little, B.; Egger, M.; Antimisiaris, D. Applying Evidence Synthesis for Constructing Directed Acyclic Graphs to Identify Causal Pathways Affecting U.S. Early-Stage Non-Small Cell Lung Cancer Treatment Receipt and Overall Survival. Therapeutics 2024, 1, 64–94. [Google Scholar] [CrossRef]
- Dijk, S.W.; Caulley, L.M.; Hunink, M.; Labrecque, J. From complexity to clarity: How directed acyclic graphs enhance the study design of systematic reviews and meta-analyses. Eur. J. Epidemiol. 2024, 39, 27–33. Available online: https://link.springer.com/article/10.1007/s10654-023-01042-z. (accessed on 10 February 2025). [CrossRef]
- Eriksen, M.B.; Frandsen, T.F. The impact of patient, intervention, comparison, outcome (PICO) as a search strategy tool on literature search quality: A systematic review. J. Med. Libr. Assoc. 2018, 106, 420. Available online: https://pmc.ncbi.nlm.nih.gov/articles/PMC6148624/ (accessed on 10 February 2025). [CrossRef]
- Connected Papers. Available online: https://www.connectedpapers.com/ (accessed on 12 February 2025).
- Downs, S.H.; Black, N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J. Epidemiol. Community Health 1998, 52, 377. Available online: https://pmc.ncbi.nlm.nih.gov/articles/PMC1756728/ (accessed on 10 February 2025). [CrossRef]
- Baggett, T.P.; Chang, Y.; Yaqubi, A.; McGlave, C.; Higgins, S.T.; Rigotti, N.A. Financial incentives for smoking abstinence in homeless smokers: A pilot randomized controlled trial. Nicotine Tob. Res. 2018, 20, 1442–1450. [Google Scholar] [CrossRef]
- Chen, C.; Anderson, C.M.; Babb, S.D.; Frank, R.; Wong, S.; Kuiper, N.M.; Zhu, S.H. Evaluation of the Asian Smokers’ Quitline: A Centralized Service for a Dispersed Population. Am. J. Prev. Med. 2021, 60, S154–S162. [Google Scholar] [CrossRef]
- Higgins, S.T.; Plucinski, S.; Orr, E.; Nighbor, T.D.; Coleman, S.R.M.; Skelly, J.; DeSarno, M.; Bunn, J. Randomized clinical trial examining financial incentives for smoking cessation among mothers of young children and possible impacts on child secondhand smoke exposure. Prev. Med. 2023, 176, 107651. [Google Scholar] [CrossRef]
- Hooper, M.W.; Miller, D.B.; Saldivar, E.; Mitchell, C.; Johnson, L.; Burns, M.; Huang, M.C. Randomized Controlled Trial Testing a Video-Text Tobacco Cessation Intervention Among Economically Disadvantaged African American Adults. Psychol. Addict. Behav. 2021, 35, 769–777. [Google Scholar] [CrossRef]
- Halpern, S.D.; French, B.; Small, D.S.; Saulsgiver, K.; Harhay, M.O.; Audrain-McGovern, J.; Loewenstein, G.; Brennan, T.A.; Asch, D.A.; Volpp, K.G.; et al. Randomized Trial of Four Financial-Incentive Programs for Smoking Cessation. N. Engl. J. Med. 2015, 372, 2108–2117. Available online: https://www.embase.com/search/results?subaction=viewrecord&id=L604555159&from=export (accessed on 10 February 2025). [CrossRef] [PubMed]
- Kendzor, D.E.; Businelle, M.S.; Frank-Pearce, S.G.; Waring, J.J.C.; Chen, S.; Hébert, E.T.; Swartz, M.D.; Alexander, A.C.; Sifat, M.S.; Boozary, L.K.; et al. Financial Incentives for Smoking Cessation among Socioeconomically Disadvantaged Adults: A Randomized Clinical Trial. JAMA Netw. Open. 2024, 7, e2418821. [Google Scholar] [CrossRef] [PubMed]
- Meernik, C.; McCullough, A.; Ranney, L.; Walsh, B.; Goldstein, A.O. Evaluation of Community-Based Cessation Programs: How Do Smokers with Behavioral Health Conditions Fare? Community Ment. Health J. 2018, 54, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Vidrine, D.J.; Frank-Pearce, S.G.; Vidrine, J.I.; Tahay, P.D.; Marani, S.K.; Chen, S.; Yuan, Y.; Cantor, S.B.; Prokhorov, A.V. Efficacy of Mobile Phone-Delivered Smoking Cessation Interventions for Socioeconomically Disadvantaged Individuals: A Randomized Clinical Trial. JAMA Intern. Med. 2019, 179, 167–174. [Google Scholar] [CrossRef]
- Bricker, J.B.; Santiago-Torres, M.; Mull, K.E.; Sullivan, B.M.; David, S.P.; Schmitz, J.; Stotts, A.; Rigotti, N.A. Do medications increase the efficacy of digital interventions for smoking cessation? Secondary results from the iCanQuit randomized trial. Addiction 2024, 119, 664–676. [Google Scholar] [CrossRef]
- Villanti, A.C.; Peasley-Miklus, C.; Cha, S.; Schulz, J.; Klemperer, E.M.; LePine, S.E.; West, J.C.; Mays, D.; Mermelstein, R.; Higgins, S.T.; et al. Tailored text message and web intervention for smoking cessation in U.S. socioeconomically-disadvantaged young adults: A randomized controlled trial. Prev. Med. 2022, 165, 107209. Available online: https://www.embase.com/search/results?subaction=viewrecord&id=L2019908359&from=export (accessed on 10 February 2025). [CrossRef]
- Heffner, J.L.; Mull, K.E.; Watson, N.L.; McClure, J.B.; Bricker, J.B. Long-Term smoking cessation outcomes for sexual minority versus nonminority smokers in a large randomized controlled trial of two web-based interventions. Nicotine Tob. Res. 2020, 22, 1596–1604. [Google Scholar] [CrossRef]
- Mays, D.; Johnson, A.C.; Phan, L.; Sanders, C.; Shoben, A.; Tercyak, K.P.; Wagener, T.L.; Brinkman, M.C.; Lipkus, I.M. Tailored Mobile Messaging Intervention for Waterpipe Tobacco Cessation in Young Adults: A Randomized Trial. Am. J. Public Health 2021, 111, 1685–1695. Available online: https://www.embase.com/search/results?subaction=viewrecord&id=L636002421&from=export (accessed on 10 February 2025). [CrossRef]
- Lee, M.; Miller, S.M.; Wen, K.Y.; Hui, S.K.A.; Roussi, P.; Hernandez, E. Cognitive-behavioral intervention to promote smoking cessation for pregnant and postpartum inner city women. J. Behav. Med. 2015, 38, 932–943. [Google Scholar] [CrossRef]
- Christiansen, B.A.; Reeder, K.M.; Terbeek, E.G.; Fiore, M.C.; Baker, T.B. Motivating low socioeconomic status smokers to accept evidence-based smoking cessation treatment: A brief intervention for the community agency setting. Nicotine Tob. Res. 2015, 17, 1002–1011. [Google Scholar] [CrossRef]
- Collins, B.N.; Nair, U.S.; Davis, S.M.; Rodriguez, D. Increasing home smoking restrictions boosts underserved MOMs’ bioverified quit success. Am. J. Health Behav. 2019, 43, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Graham, A.L.; Amato, M.S.; Cha, S.; Jacobs, M.A.; Bottcher, M.M.; Papandonatos, G.D. Effectiveness of a Vaping Cessation Text Message Program among Young Adult e-Cigarette Users: A Randomized Clinical Trial. JAMA Intern. Med. 2021, 181, 923–930. [Google Scholar] [CrossRef]
- Kamke, K.; Grenen, E.; Robinson, C.; El-Toukhy, S. Dropout and abstinence outcomes in a national text messaging smoking cessation intervention for pregnant women, smokefreemom: Observational study. JMIR mHealth uHealth 2019, 7, e14699. Available online: https://www.embase.com/search/results?subaction=viewrecord&id=L629551580&from=export (accessed on 10 February 2025). [CrossRef]
- Dahne, J.; Wahlquist, A.E.; Smith, T.T.; Carpenter, M.J. The differential impact of nicotine replacement therapy sampling on cessation outcomes across established tobacco disparities groups. Prev. Med. 2020, 136, 106096. Available online: https://linkinghub.elsevier.com/retrieve/pii/S0091743520301201 (accessed on 10 February 2025). [CrossRef] [PubMed]
- Fu, S.S.; Van Ryn, M.; Nelson, D.; Burgess, D.J.; Thomas, J.L.; Saul, J.; Clothier, B.; Nyman, J.A.; Hammett, P.; Joseph, A.M. Proactive tobacco treatment offering free nicotine replacement therapy and telephone counselling for socioeconomically disadvantaged smokers: A randomised clinical trial. Thorax 2016, 71, 446–453. [Google Scholar] [CrossRef] [PubMed]
- Galiatsatos, P.; Soybel, A.; Jassal, M.; Cruz, S.A.P.; Spartin, C.; Shaw, K.; Cunningham, J.; Kanarek, N.F. Tobacco treatment clinics in urban public housing: Feasibility and outcomes of a hands-on tobacco dependence service in the community. BMC Public Health. 2021, 21, 1514. [Google Scholar] [CrossRef]
- Lee, J.; Contrera Avila, J.; Ahluwalia, J.S. Differences in cessation attempts and cessation methods by race/ethnicity among US adult smokers, 2016–2018. Addict. Behav. 2023, 137, 107523. Available online: https://www.embase.com/search/results?subaction=viewrecord&id=L2020808761&from=export (accessed on 10 February 2025). [CrossRef]
- Kerkvliet, J.L.; Wey, H.; Fahrenwald, N.L. Cessation among state quitline participants with a mental health condition. Nicotine Tob. Res. 2015, 17, 735–741. [Google Scholar] [CrossRef]
- Wewers, M.E.; Shoben, A.; Conroy, S.; Curry, E.; Ferketich, A.K.; Murray, D.M.; Nemeth, J.; Wermert, A. Effectiveness of Two Community Health Worker Models of Tobacco Dependence Treatment Among Community Residents of Ohio Appalachia. Nicotine Tob. Res. 2017, 19, 1499–1507. [Google Scholar] [CrossRef]
- Hooper, M.W.; Calixte-Civil, P.; Verzijl, C.; Brandon, K.O.; Asfar, T.; Koru-Sengul, T.; Antoni, M.H.; Lee, D.J.; Simmons, V.N.; Brandon, T.H. Associations between perceived racial discrimination and tobacco cessation among diverse treatment seekers. Ethn. Dis. 2020, 30, 411–420. [Google Scholar] [CrossRef]
- McCarthy, D.E.; Baker, T.B.; Zehner, M.E.; Adsit, R.T.; Kim, N.; Zwaga, D.; Coates, K.; Wallenkamp, H.; Nolan, M.; Steiner, M.; et al. A comprehensive electronic health record-enabled smoking treatment program: Evaluating reach and effectiveness in primary care in a multiple baseline design. Prev. Med. 2022, 165, 107101. Available online: https://www.embase.com/search/results?subaction=viewrecord&id=L2018636055&from=export (accessed on 10 February 2025). [CrossRef] [PubMed]
- Santiago-Torres, M.; Mull, K.E.; Sullivan, B.M.; Ferketich, A.K.; Bricker, J.B. Efficacy of an acceptance and commitment therapy-based smartphone application for helping rural populations quit smoking: Results from the iCanQuit randomized trial. Prev. Med. 2022, 157, 107008. [Google Scholar] [CrossRef] [PubMed]
- Santiago-Torres, M.; Mull, K.E.; Sullivan, B.M.; Kwon, D.M.; Nez Henderson, P.; Nelson, L.A.; Patten, C.A.; Bricker, J.B. Efficacy and Utilization of Smartphone Applications for Smoking Cessation among American Indians and Alaska Natives: Results from the iCanQuit Trial. Nicotine Tob. Res. 2022, 24, 544–554. [Google Scholar] [CrossRef] [PubMed]
- Webb Hooper, M.; Kolar, S.K. Distress, race/ethnicity and smoking cessation in treatment-seekers: Implications for disparity elimination. Addiction 2015, 110, 1495–1504. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).