Impact of Climate Change on Schistosomiasis Transmission and Distribution—Scoping Review
Abstract
1. Introduction
2. Methodology
2.1. Research Questions
2.2. Eligibility Criteria
2.3. Identification of Relevant Studies
2.4. Study Selection Process
2.5. Data Extraction, Synthesis and Analysis of Results
3. Results
3.1. Study Characteristics
3.2. Environmental Changes and Schistosomiasis Transmission
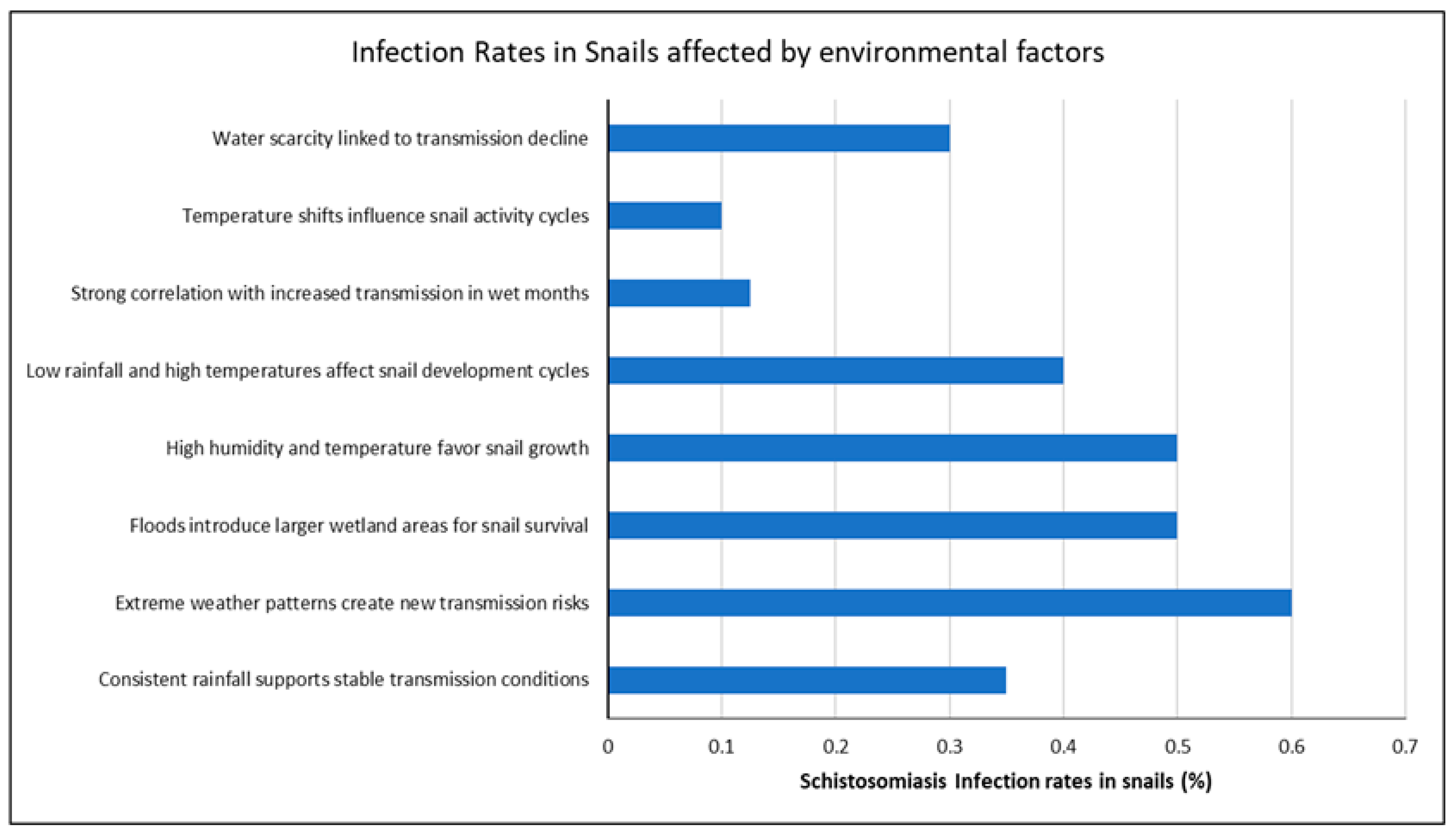
3.3. How Environmental Factors Influence Schistosoma Infection in Freshwater Snails
3.4. Countries with High Schistosoma Infection Rates in Snails
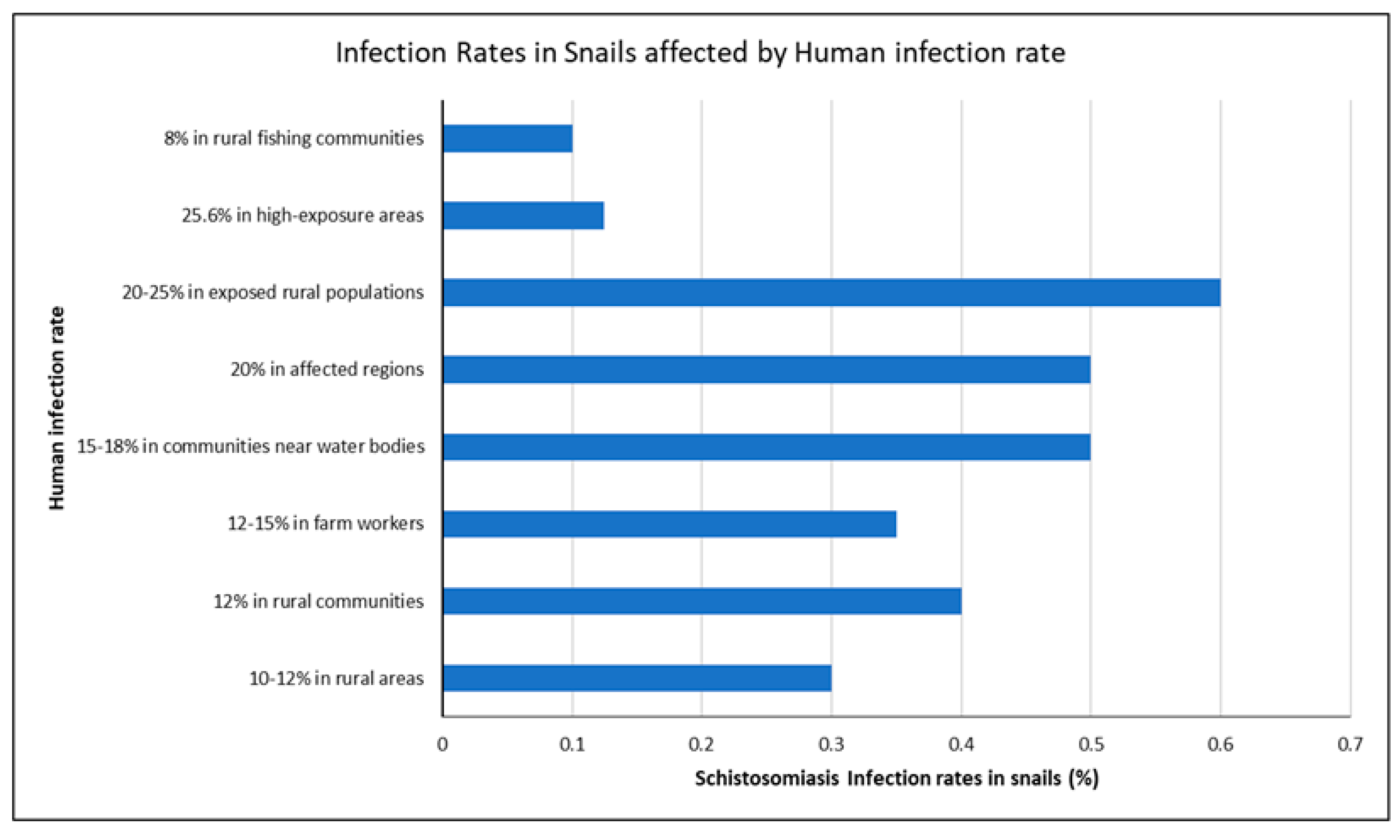
3.5. Link Between Snail Infection Rates and Human Schistosomiasis
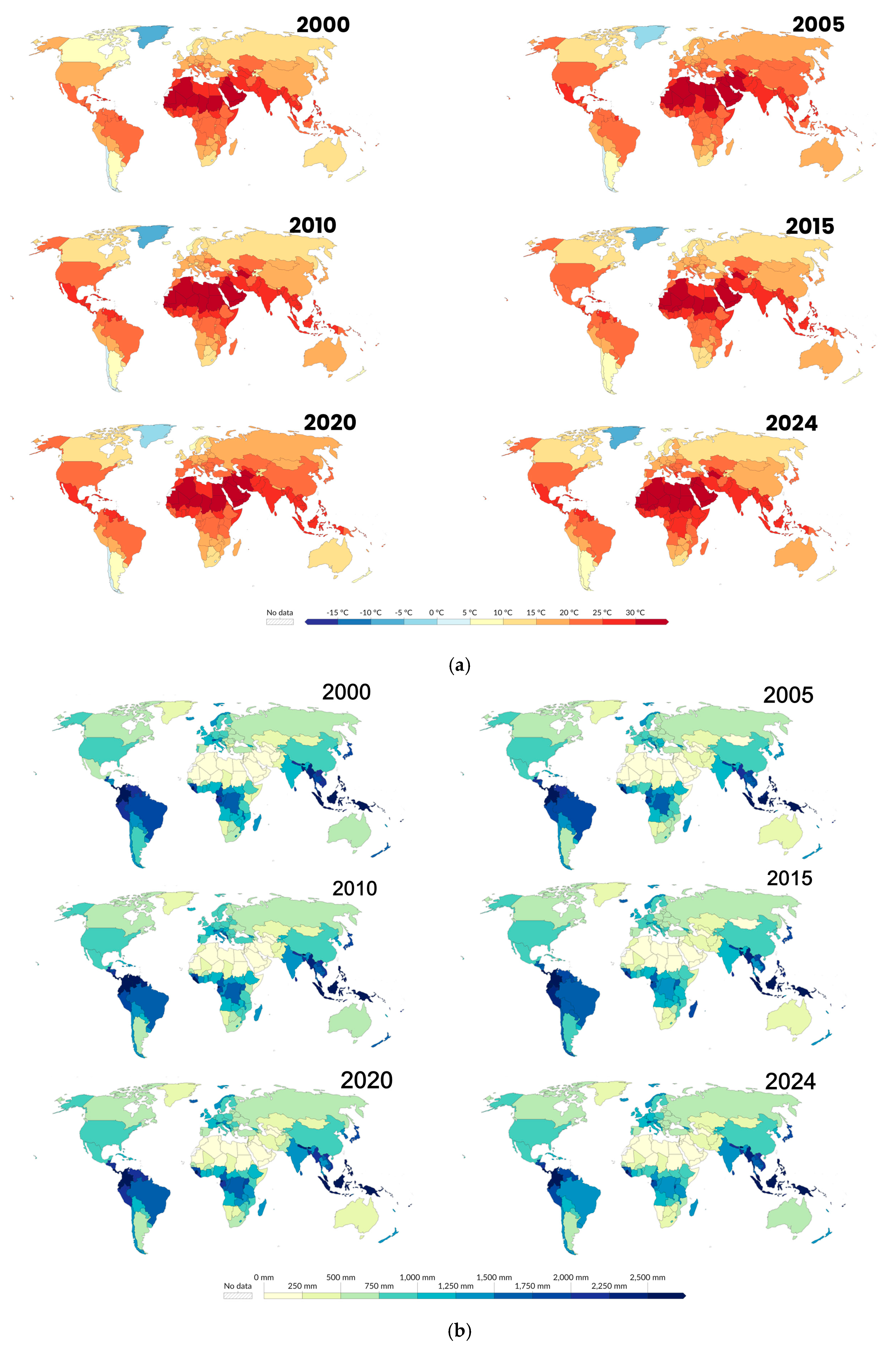
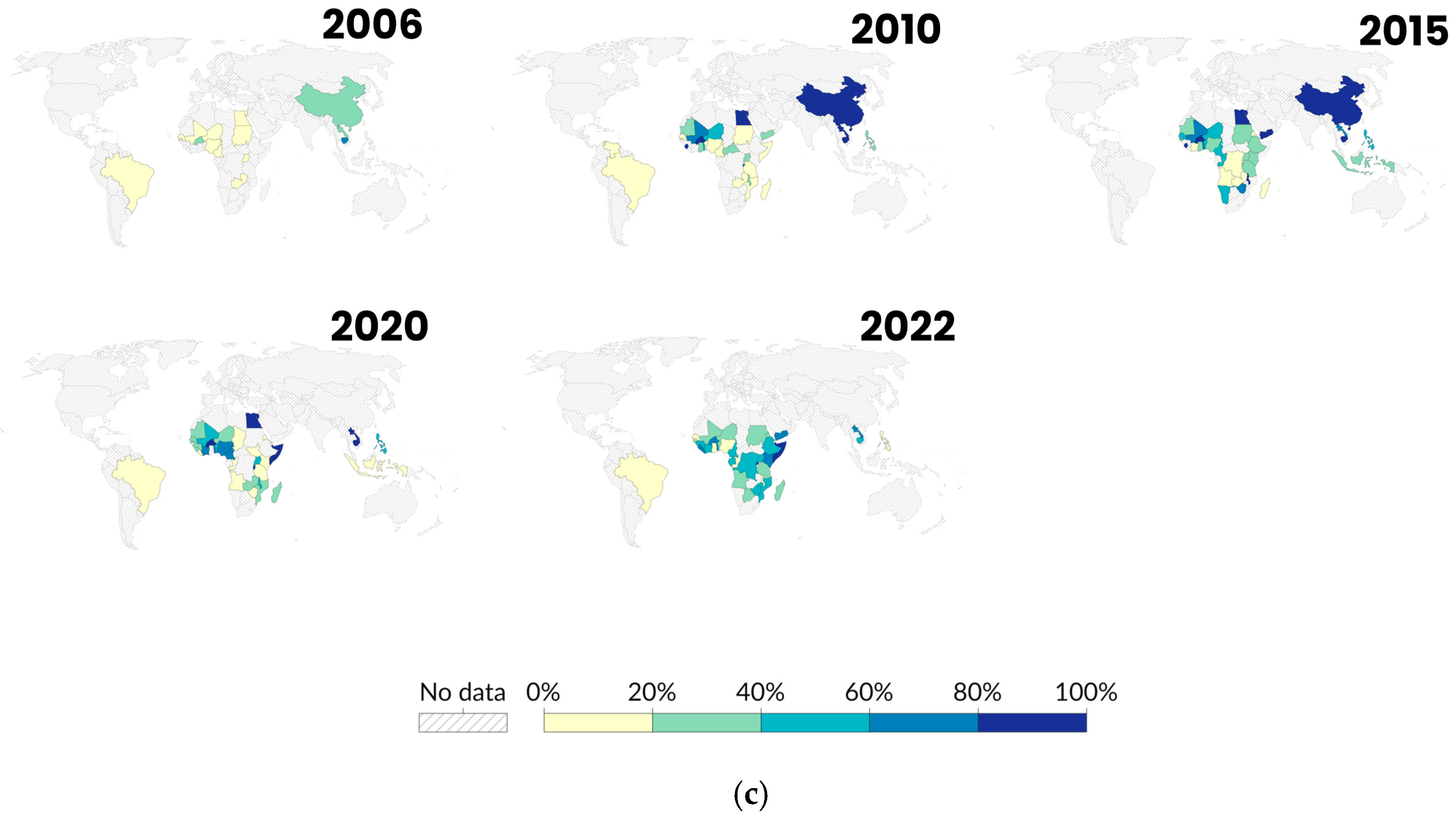
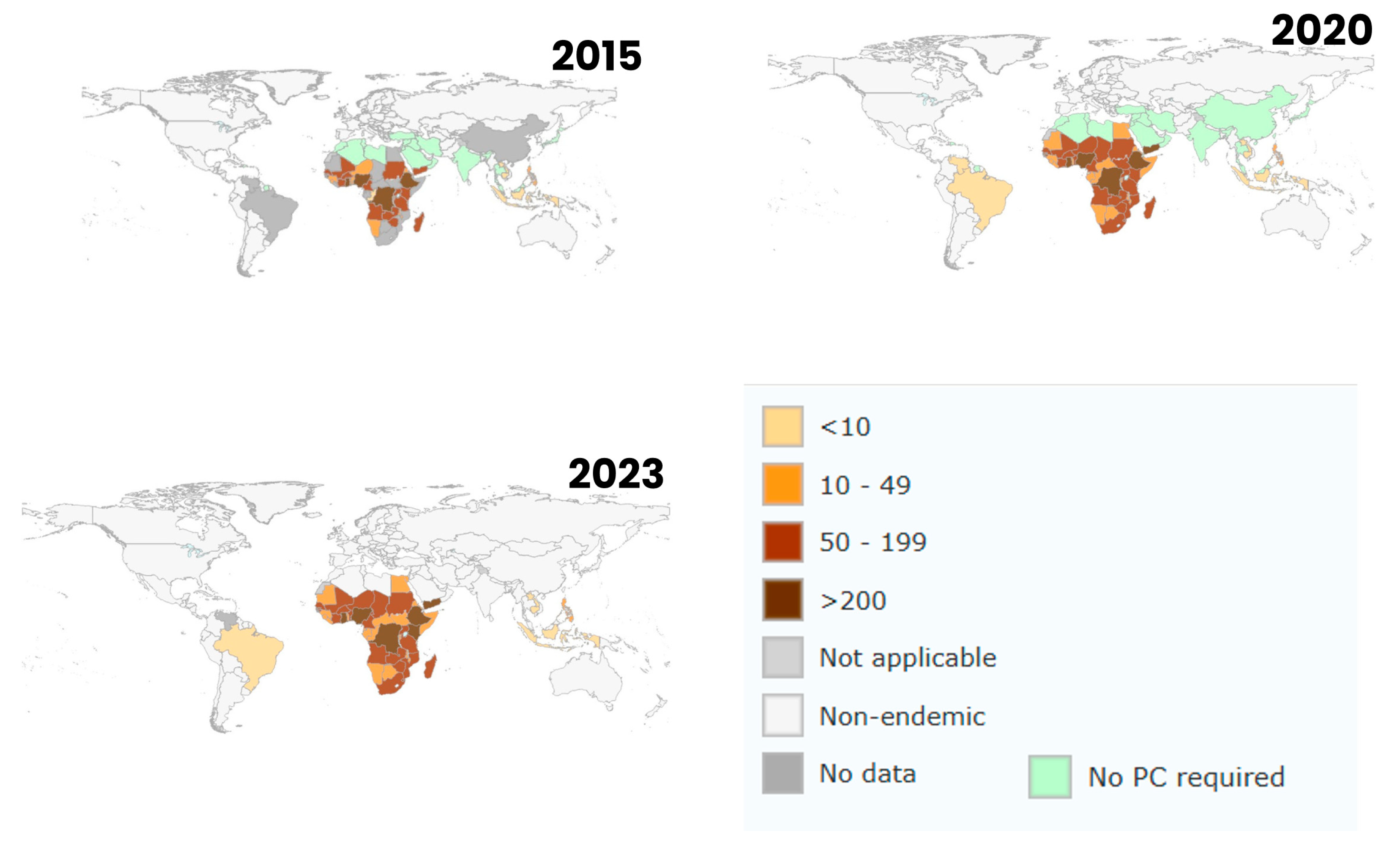
3.6. Climate Change and Emerging Schistosomiasis Hotspots
3.7. Climate Adaptation Policies and Schistosomiasis Control
4. Discussion
5. Limitations of the Study
6. Future Research
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Aula, O.P.; McManus, D.P.; Jones, M.K.; Gordon, C.A. Schistosomiasis with a focus on Africa. Trop. Med. Infect. Dis. 2021, 6, 109. [Google Scholar] [CrossRef] [PubMed]
- Adenowo, A.F.; Oyinloye, B.E.; Ogunyinka, B.I.; Kappo, A.P. Impact of human schistosomiasis in sub-Saharan Africa. Braz. J. Infect. Dis. 2015, 19, 196–205. [Google Scholar] [CrossRef]
- Loker, E.S.; DeJong, R.J.; Brant, S.V. Scratching the itch: Updated perspectives on the schistosomes responsible for swimmer’s itch around the world. Pathogens 2022, 11, 587. [Google Scholar] [CrossRef]
- Mawa, P.A.; Kincaid-Smith, J.; Tukahebwa, E.M.; Webster, J.P.; Wilson, S. Schistosomiasis morbidity hotspots: Roles of the human host, the parasite and their interface in the development of severe morbidity. Front. Immunol. 2021, 12, 635869. [Google Scholar] [CrossRef] [PubMed]
- Verjee, M.A. Schistosomiasis: Still a cause of significant morbidity and mortality. Res. Rep. Trop. Med. 2019, 10, 153–163. [Google Scholar] [CrossRef]
- O’Ferrall, A.M.; Musaya, J.; Stothard, J.R.; Roberts, A.P. Aligning antimicrobial resistance surveillance with schistosomiasis research: An interlinked One Health approach. Trans. R. Soc. Trop. Med. Hyg. 2024, 118, 498–504. [Google Scholar] [CrossRef] [PubMed]
- Yadav, N.; Upadhyay, R.K. Global effect of climate change on seasonal cycles, vector population and rising challenges of communicable diseases: A review. J. Atmos. Sci. Res. 2023, 6, 21–59. [Google Scholar] [CrossRef]
- Robinson, W.A. Climate change and extreme weather: A review focusing on the continental United States. J. Air Waste Manag. Assoc. 2021, 71, 1186–1209. [Google Scholar] [CrossRef]
- Sivaramanan, S. Global Warming and Climate change, causes, impacts and mitigation. Cent. Environ. Auth. 2015. [Google Scholar] [CrossRef]
- Saaristo, M.; Brodin, T.; Balshine, S.; Bertram, M.G.; Brooks, B.W.; Ehlman, S.M.; McCallum, E.S.; Sih, A.; Sundin, J.; Wong, B.B.; et al. Direct and indirect effects of chemical contaminants on the behaviour, ecology and evolution of wildlife. Proc. R. Soc. B 2018, 285, 20181297. [Google Scholar] [CrossRef]
- Rubaba, O.; Chimbari, M.J.; Mukaratirwa, S. The role of snail aestivation in transmission of schistosomiasis in changing climatic conditions. Afr. J. Aquat. Sci. 2016, 41, 143–150. [Google Scholar] [CrossRef]
- Kalinda, C.; Chimbari, M.; Mukaratirwa, S. Implications of changing temperatures on the growth, fecundity and survival of intermediate host snails of schistosomiasis: A systematic review. Int. J. Environ. Res. Public Health 2017, 14, 80. [Google Scholar] [CrossRef]
- Ozretich, R.W.; Wood, C.L.; Allan, F.; Koumi, A.R.; Norman, R.; Brierley, A.S.; De Leo, G.A.; Little, D.C. The potential for aquaculture to reduce poverty and control schistosomiasis in Côte d’Ivoire (Ivory Coast) during an era of climate change: A systematic review. Rev. Fish. Sci. Aquac. 2022, 30, 467–497. [Google Scholar] [CrossRef]
- Jones, I.; Lund, A.; Riveau, G.; Jouanard, N.; Ndione, R.A.; Sokolow, S.H.; De Leo, G.A. Ecological control of schistosomiasis in Sub-Saharan Africa: Restoration of predator-prey dynamics to reduce transmission. In Ecology and Evolution of Infectious Disease: Pathogen Control and Public Health Management in Low-Income Countries; Oxford University Press: Oxford, UK, 2018; pp. 236–251. [Google Scholar]
- Ayob, N.; Burger, R.P.; Belelie, M.D.; Nkosi, N.C.; Havenga, H.; de Necker, L.; Cilliers, D.P. Modelling the historical distribution of schistosomiasis-transmitting snails in South Africa using ecological niche models. PLoS ONE 2023, 18, e0295149. [Google Scholar] [CrossRef]
- Tabo, Z.; Kalinda, C.; Breuer, L.; Albrecht, C. Exploring the interplay between climate change and schistosomiasis transmission dynamics. Infect. Dis. Model. 2024, 9, 158–176. [Google Scholar] [CrossRef]
- Stensgaard, A.S.; Vounatsou, P.; Sengupta, M.E.; Utzinger, J. Schistosomes, snails and climate change: Current trends and future expectations. Acta Trop. 2019, 190, 257–268. [Google Scholar] [CrossRef]
- Mas-Coma, S.; Valero, M.A.; Bargues, M.D. Climate change effects on trematodiases, with emphasis on zoonotic fascioliasis and schistosomiasis. Vet. Parasitol. 2009, 163, 264–280. [Google Scholar] [CrossRef] [PubMed]
- McCreesh, N.; Booth, M. Challenges in predicting the effects of climate change on Schistosoma mansoni and Schistosoma haematobium transmission potential. Trends Parasitol. 2013, 29, 548–555. [Google Scholar] [CrossRef] [PubMed]
- McCreesh, N.; Nikulin, G.; Booth, M. Predicting the effects of climate change on Schistosoma mansoni transmission in eastern Africa. Parasites Vectors 2015, 8, 4. [Google Scholar] [CrossRef]
- Blum, A.J.; Hotez, P.J. Global “worming”: Climate change and its projected general impact on human helminth infections. PLoS Neglected Trop. Dis. 2018, 12, e0006370. [Google Scholar] [CrossRef]
- Short, E.E.; Caminade, C.; Thomas, B.N. Climate change contribution to the emergence or re-emergence of parasitic diseases. Infect. Dis. Res. Treat. 2017, 10, 1178633617732296. [Google Scholar] [CrossRef]
- Perez Saez, F.J. A Field-Based Modelling Framework of the Ecohydrology of Schistosomiasis. 2018. Available online: https://infoscience.epfl.ch/entities/publication/22cb92c7-f956-47da-9ee6-da13b4106bff (accessed on 27 December 2024).
- Letlaila, R.F. The Historical and Seasonal Distribution of Schistosomiasis Transmitting Vectors in the Mpumalanga Province, South Africa. Ph.D. Dissertation, North-West University, Potchefstroom, South Africa, 2023. [Google Scholar]
- Lund, A.J.; Lopez-Carr, D.; Sokolow, S.H.; Rohr, J.R.; De Leo, G.A. Agricultural innovations to reduce the health impacts of dams. Sustainability 2021, 13, 1869. [Google Scholar] [CrossRef]
- Wood, C.L.; Sokolow, S.H.; Jones, I.J.; Chamberlin, A.J.; Lafferty, K.D.; Kuris, A.M.; Jocque, M.; Hopkins, S.; Adams, G.; Buck, J.C.; et al. Precision mapping of snail habitat provides a powerful indicator of human schistosomiasis transmission. Proc. Natl. Acad. Sci. USA 2019, 116, 23182–23191. [Google Scholar] [CrossRef] [PubMed]
- Arksey, H.; O’Malley, L. Scoping studies: Towards a methodological framework. Int. J. Soc. Res. Methodol. 2005, 8, 19–32. [Google Scholar] [CrossRef]
- Levac, D.; Rivard, L.; Missiuna, C. Defining the active ingredients of interactive computer play interventions for children with neuromotor impairments: A scoping review. Res. Dev. Disabil. 2012, 33, 214–223. [Google Scholar] [CrossRef] [PubMed]
- McGowan, J.; Straus, S.; Moher, D.; Langlois, E.V.; O’Brien, K.K.; Horsley, T.; Aldcroft, A.; Zarin, W.; Garitty, C.M.; Hempel, S.; et al. Reporting scoping reviews—PRISMA ScR extension. J. Clin. Epidemiol. 2020, 123, 177–179. [Google Scholar] [CrossRef] [PubMed]
- Andrus, P.S.; Stothard, J.R.; Wade, C.M. Seasonal patterns of Schistosoma mansoni infection within Biomphalaria snails at the Ugandan shorelines of Lake Albert and Lake Victoria. PLoS Neglected Trop. Dis. 2023, 17, e0011506. [Google Scholar] [CrossRef]
- Omuok, D.A. A Malacology Survey to Map Out Schistosomiasis Transmission Sites on Mageta Island, Siaya County, Western Kenya. Ph.D. Dissertation, University of Nairobi, Nairobi, Kenya, 2022. [Google Scholar]
- Asante-Kwatia, E.; Gyimah, L.; Forkuo, A.D.; Anyan, W.K.; Gbemu, M.A.; Armah, F.A.; Mensah, A.Y. Ethnobotanical Survey and Cercaricidal Activity Screening of Medicinal Plants Used for Schistosomiasis Treatment in Atwima-Nwabiagya District, Ashanti Region, Ghana. J. Parasitol. Res. 2023, 2023, 6707157. [Google Scholar] [CrossRef]
- Adekiya, T.A.; Aruleba, R.T.; Oyinloye, B.E.; Okosun, K.O.; Kappo, A.P. The effect of climate change and the snail-schistosome cycle in transmission and bio-control of schistosomiasis in Sub-Saharan Africa. Int. J. Environ. Res. Public Health 2020, 17, 181. [Google Scholar] [CrossRef]
- Nzalawahe, J. Trematode Infections in Freshwater Snails and Seasonal Variations in Iringa and Arumeru Districts, Tanzania. Tanzan. Vet. J. 2021, 36, 23–33. [Google Scholar] [CrossRef]
- Phillips, A.E.; Gazzinelli-Guimarães, P.H.; Aurelio, H.O.; Dhanani, N.; Ferro, J.; Nala, R.; Deol, A.; Fenwick, A. Urogenital schistosomiasis in Cabo Delgado, northern Mozambique: Baseline findings from the SCORE study. Parasites Vectors 2018, 11, 30. [Google Scholar] [CrossRef]
- Muhammed, H. Floods: An Increasing Threat to Schistosomiasis Control in Nigeria. Afro-Egypt. J. Infect. Endem. Dis. 2023, 13, 280–286. [Google Scholar] [CrossRef]
- El-Khayat, H.M.; Mossalem, H.S.; El-Hommossany, K.; Sayed, S.S.; Mohammed, W.A.; Zayed, K.M.; Saied, M.; Habib, M.R. Assessment of schistosomiasis transmission in the River Nile at Greater Cairo using malacological surveys and cercariometry. J. Parasit. Dis. 2022, 46, 1090–1102. [Google Scholar] [CrossRef]
- Starkloff, N.C.; Angelo, T.; Mahalila, M.P.; Charles, J.; Kinung’hi, S.; Civitello, D.J. Spatio-temporal variability in transmission risk of human schistosomes and animal trematodes in a seasonally desiccating East African landscape. Proc. R. Soc. B 2024, 291, 20231766. [Google Scholar] [CrossRef] [PubMed]
- Stensgaard, A.S.; Booth, M.; Nikulin, G.; McCreesh, N. Combining process-based and correlative models improves predictions of climate change effects on Schistosoma mansoni transmission in eastern Africa. Geospat. Health 2016, 11, 94–101. [Google Scholar] [CrossRef]
- Appleton, C.C.; Madsen, H. Human schistosomiasis in wetlands in southern Africa. Wetl. Ecol. Manag. 2012, 20, 253–269. [Google Scholar] [CrossRef]
- Codjoe, S.N.; Larbi, R.T. Climate change/variability and schistosomiasis transmission in Ga district, Ghana. Clim. Dev. 2016, 8, 58–71. [Google Scholar] [CrossRef]
- van der Deure, T.; Maes, T.; Huyse, T.; Stensgaard, A.S. Climate change could fuel urinary schistosomiasis transmission in Africa and Europe. Glob. Change Biol. 2024, 30, e17434. [Google Scholar] [CrossRef]
- Gordon, C.A.; Kurscheid, J.; Williams, G.M.; Clements, A.C.; Li, Y.; Zhou, X.N.; Utzinger, J.; McManus, D.P.; Gray, D.J. Asian schistosomiasis: Current status and prospects for control leading to elimination. Trop. Med. Infect. Dis. 2019, 4, 40. [Google Scholar] [CrossRef]
- Recopuerto-Medina, L.M.; Gutierrez, F.C.; San Diego, J.A.; Alviar, N.A.; Santos, J.R.; Dagamac, N.H. MaxEnt modeling of the potential risk of schistosomiasis in the Philippines using bioclimatic factors. Parasitol. Int. 2024, 98, 102827. [Google Scholar] [CrossRef]
- Petney, T.N.; Sithithaworn, P.; Andrews, R.H. Parasite diversity, dynamics, and climate change. In Biodiversity of Southeast Asian Parasites and Vectors Causing Human Disease; Springer International Publishing: Cham, Switzerland, 2021; pp. 183–204. [Google Scholar]
- Sato, M.O.; Adsakwattana, P.; Fontanilla, I.K.; Kobayashi, J.; Sato, M.; Pongvongsa, T.; Fornillos, R.J.; Waikagul, J. Odds, challenges and new approaches in the control of helminthiasis, an Asian study. Parasite Epidemiol. Control 2019, 4, e00083. [Google Scholar] [CrossRef] [PubMed]
- Glidden, C.K.; Singleton, A.L.; Chamberlin, A.; Tuan, R.; Palasio, R.G.; Caldeira, R.L.; Monteiro, A.M.; Lwiza, K.M.; Liu, P.; Silva, V.; et al. Climate and urbanization drive changes in the habitat suitability of Schistosoma mansoni competent snails in Brazil. Nat. Commun. 2024, 15, 4838. [Google Scholar] [CrossRef]
- Qiu, J.; Han, D.; Li, R.; Xiao, Y.; Zhu, H.; Xia, J.; Jiang, J.; Han, Y.; Shao, Q.; Yan, Y.; et al. Satellite Imagery-Based Identification of High-Risk Areas of Schistosome Intermediate Snail Hosts Spread after Flood. Remote Sens. 2022, 14, 3707. [Google Scholar] [CrossRef]
- Kalinda, C.; Chimbari, M.J.; Grant, W.E.; Wang, H.H.; Odhiambo, J.N.; Mukaratirwa, S. Simulation of population dynamics of Bulinus globosus: Effects of environmental temperature on production of Schistosoma haematobium cercariae. PloS Neglected Trop. Dis. 2018, 12, e0006651. [Google Scholar] [CrossRef]
- Malone, J.B.; Yilma, J.M.; McCarroll, J.C.; Erko, B.; Mukaratirwa, S.; Zhou, X. Satellite climatology and the environmental risk of Schistosoma mansoni in Ethiopia and east Africa. Acta Trop. 2001, 79, 59–72. [Google Scholar] [CrossRef]
- Kim, C.S.; Echaubard, P.; Suwannatrai, A.; Kaewkes, S.; Wilcox, B.A.; Sripa, B. Seasonal and spatial environmental influence on Opisthorchis viverrini intermediate hosts, abundance, and distribution: Insights on transmission dynamics and sustainable control. PLoS Neglected Trop. Dis. 2016, 10, e0005121. [Google Scholar] [CrossRef]
- Liu, L.; Mondal, M.M.; Idris, M.A.; Lokman, H.S.; Rajapakse, P.J.; Satrija, F.; Diaz, J.L.; Upatham, E.S.; Attwood, S.W. The phylogeography of Indoplanorbis exustus (Gastropoda: Planorbidae) in Asia. Parasites Vectors 2010, 3, 57. [Google Scholar] [CrossRef] [PubMed]
- Afshan, K.; Fortes-Lima, C.A.; Artigas, P.; Valero, M.A.; Qayyum, M.; Mas-Coma, S. Impact of climate change and man-made irrigation systems on the transmission risk, long-term trend and seasonality of human and animal fascioliasis in Pakistan. Geospat. Health 2014, 8, 317–334. [Google Scholar] [CrossRef]
- Mccreesh, N. Modelling the Effects of Temperature Changes on Schistosoma mansoni Transmission. Ph.D. Dissertation, Durham University, Durham, UK, 2015. [Google Scholar]
- Urbani, C.; Sinoun, M.; Socheat, D.; Pholsena, K.; Strandgaard, H.; Odermatt, P.; Hatz, C. Epidemiology and control of mekongi schistosomiasis. Acta Trop. 2002, 82, 157–168. [Google Scholar] [CrossRef]
- Spear, R.C.; Seto, E.Y.; Carlton, E.J.; Liang, S.; Remais, J.V.; Zhong, B.; Qiu, D. The challenge of effective surveillance in moving from low transmission to elimination of schistosomiasis in China. Int. J. Parasitol. 2011, 41, 1243–1247. [Google Scholar] [CrossRef]
- Rabone, M.; Wiethase, J.H.; Allan, F.; Gouvras, A.N.; Pennance, T.; Hamidou, A.A.; Webster, B.L.; Labbo, R.; Emery, A.M.; Garba, A.D.; et al. Freshwater snails of biomedical importance in the Niger River Valley: Evidence of temporal and spatial patterns in abundance, distribution and infection with Schistosoma spp. Parasites Vectors 2019, 12, 498. [Google Scholar] [CrossRef] [PubMed]
- Monde, C.; Syampungani, S.; van den Brink, P.J. Natural and human induced factors influencing the abundance of Schistosoma host snails in Zambia. Environ. Monit. Assess. 2016, 188, 370. [Google Scholar] [CrossRef] [PubMed]
- Perez-Saez, J.; Mande, T.; Rinaldo, A. Space and time predictions of schistosomiasis snail host population dynamics across hydrologic regimes in Burkina Faso. Geospat. Health 2019, 14. [Google Scholar] [CrossRef]
- Al-Delaimy, A.K. The prospective effects of climate change on neglected tropical diseases in the Eastern Mediterranean region: A review. Curr. Environ. Health Rep. 2022, 9, 315–323. [Google Scholar] [CrossRef]
- Oleaga, A.; Rey, O.; Polack, B.; Grech-Angelini, S.; Quilichini, Y.; Pérez-Sánchez, R.; Boireau, P.; Mulero, S.; Brunet, A.; Rognon, A.; et al. Epidemiological surveillance of schistosomiasis outbreak in Corsica (France): Are animal reservoir hosts implicated in local transmission? PLoS Neglected Trop. Dis. 2019, 13, e0007543. [Google Scholar] [CrossRef] [PubMed]
- Neira, M.; Erguler, K.; Ahmady-Birgani, H.; Al-Hmoud, N.D.; Fears, R.; Gogos, C.; Hobbhahn, N.; Koliou, M.; Kostrikis, L.G.; Lelieveld, J.; et al. Climate change and human health in the Eastern Mediterranean and Middle East: Literature review, research priorities and policy suggestions. Environ. Res. 2023, 216, 114537. [Google Scholar] [CrossRef]
- EL-hassouni, S.; Mansouri, D.; Jaghror, H.; Kaioua, S.; Slim, M.; Dahmani, J. Schistosomiasis and Geographical Distribution of Aquatic Mollusks and Disease Vectors in the Gharb Plain (Morocco). Egypt. J. Aquat. Biol. Fish. 2024, 28, 755–767. [Google Scholar]
- Yang, G.J.; Bergquist, R. Potential impact of climate change on schistosomiasis: A global assessment attempt. Trop. Med. Infect. Dis. 2018, 3, 117. [Google Scholar] [CrossRef]
- Clennon, J.A.; King, C.H.; Muchiri, E.M.; Kitron, U. Hydrological modelling of snail dispersal patterns in Msambweni, Kenya and potential resurgence of Schistosoma haematobium transmission. Parasitology 2006, 134, 683–693. [Google Scholar] [CrossRef]
- Dalila, M.N. The Distribution and Diversity of Land Snails in Shimba Hills National Reserve, Kenya. Ph.D. Dissertation, University of Nairobi, Nairobi, Kenya, 2009. [Google Scholar]
- Lydig, A. Factors Conditioning the Distribution of Fresh Water Pulmonates, Biomphalaria spp., Bulinus spp., and Lymnea spp., in Babati District, Tanzania. Bachelor’s Dissertation, Södertörn University, Huddinge, Sweden, 2009. [Google Scholar]
- Akpinar-Elci, M.; Sealy, H. Climate change and public health in small island states and Caribbean countries. In Global Climate Change and Public Health; Humana: New York, NY, USA, 2014; pp. 279–292. [Google Scholar]
- da Paz, W.S.; Duthie, M.S.; de Jesus, A.R.; de Araújo, K.C.; Dos Santos, A.D.; Bezerra-Santos, M. Population-based, spatiotemporal modeling of social risk factors and mortality from schistosomiasis in Brazil between 1999 and 2018. Acta Trop. 2021, 218, 105897. [Google Scholar] [CrossRef]
- Malek, E.A. Snail Transmitted Parasitic Diseases: Volume II; CRC Press: Boca Raton, FL, USA, 2018. [Google Scholar]
- Bargues, M.D.; Angles, R.; Coello, J.; Artigas, P.; Funatsu, I.R.; Cuervo, P.F.; Buchon, P.; Mas-Coma, S. One Health initiative in the Bolivian Altiplano human fascioliasis hyperendemic area: Lymnaeid biology, population dynamics, microecology and climatic factor influences. Rev. Bras. Parasitol. Veterinária 2021, 30, e025620. [Google Scholar] [CrossRef]
- Leonardo, L.; Varona, G.; Fornillos, R.J.; Manalo, D.; Tabios, I.K.; Moendeg, K.; de Cadiz, A.; Kikuchi, M.; Chigusa, Y.; Mistica, M.; et al. Oncomelania hupensis quadrasi: Snail intermediate host of Schistosoma japonicum in the Philippines. Acta Trop. 2020, 210, 105547. [Google Scholar] [CrossRef] [PubMed]
- Diakité, N.R.; Koffi, P.B.; Konan, C.K.; Bassa, F.K.; Chamberlin, A.J.; Ouattara, M.; De Leo, G.A.; N’Goran, E.K. Variability of biological traits of Bulinus truncatus and Biomphalaria pfeifferi, the intermediate host snails of schistosomiasis, from three climatic zones of Côte d’Ivoire. Front. Environ. Sci. 2023, 11, 1193239. [Google Scholar] [CrossRef]
- Anorue, C.O.; Onyali, I.O.; Anyanwu, I.N.; Nweke, C.J.; Okesanya, O.J. Impacts of Water Physicochemical Parameters on Schistosomiasis Vector Snail Distributional-Abundance and Infectivity rate in South-Eastern Nigeria. Niger. J. Parasitol. 2024, 45, 152–163. [Google Scholar] [CrossRef]
- Kiatsopit, N.; Sithithaworn, P.; Kopolrat, K.; Andrews, R.H.; Petney, T.N. Seasonal cercarial emergence patterns of Opisthorchis viverrini infecting Bithynia siamensis goniomphalos from Vientiane Province, Lao PDR. Parasites Vectors 2014, 7, 551. [Google Scholar] [CrossRef]
- Tañan, V.B.; Sumaya, N.H. Bio-inventory of terrestrial gastropod species in Northern Mindanao, Philippines. Biodiversitas J. Biol. Divers. 2024, 25, 3391–3402. [Google Scholar] [CrossRef]
- Perez, K.M.; Sabino, L.L.; Rebancos, C.M.; Gonzalez, J.C.; DEChavez, E.R.; Cuevas, V.C. Local ecological knowledge on land snail diversity in mount Banahaw, Philippines. Int. J. Conserv. Sci. 2024, 15, 1533–1552. [Google Scholar] [CrossRef]
- Arifin, A.; Biba, M.A.; Syafiuddin, S. Determinants of Production and Income Risks of Rainfed Lowland Farming: A Case Study in Maros Regency, Indonesia. Caraka Tani J. Sustain. Agric. 2021, 36, 319–328. [Google Scholar] [CrossRef]
- Basheer, R.M. Prevalence of Bo vine Fasciolosis in Hilla Kuku, Khartoum State, Sudan. Ph.D. Dissertation, Sudan University of Science & Technology, Khartoum, Sudan, 2022. [Google Scholar]
- Ahmed, M.K. Transmission of Schistsomiasis in The Blue Nile State, Sudan, (An investigated study in May 2009–April 2010). Gezira J. Health Sci. 2018, 14, 508–526. [Google Scholar]
- Asefa, D.I. A Cross-Sectional Study on Prevalence of Bovine Trematodiasis and Associated Risk Factors in Damot Sore District, Wolaita Zone, Southern Ethiopia. SSRN Electron. J. 2022, 3, 1–72. [Google Scholar] [CrossRef]
- Gaye, P.M.; Doucouré, S.; Sow, D.; Sokhna, C.; Ranque, S. Freshwater snail-borne parasitic diseases in Africa. Trop. Med. Health 2024, 52, 61. [Google Scholar] [CrossRef] [PubMed]
- Siama, A.; Saotoing, P.; Nloga, A.M. Malacological survey and dynamic of Lymnaea natalensis population intermediate host of Fasciola gigantica in the Douvar dam freshwater of Farth Nord region Cameroon. J. Entomol. Zool. Stud. 2020, 8, 1213–1221. [Google Scholar]
- Namsanor, J.; Sithithaworn, P.; Kopolrat, K.; Kiatsopit, N.; Pitaksakulrat, O.; Tesana, S.; Andrews, R.H.; Petney, T.N. Seasonal transmission of Opisthorchis viverrini sensu lato and a lecithodendriid trematode species in Bithynia siamensis goniomphalos snails in northeast Thailand. Am. J. Trop. Med. Hyg. 2015, 93, 87. [Google Scholar] [CrossRef]
- Lanza, G.R.; Upatham, S.; Chen, A. A Place-Based Conceptual Model (PBCM) of Neotricula aperta/Schistosoma mekongi habitat before and after dam construction in the Lower Mekong River. PLOS Neglected Trop. Dis. 2023, 17, e0011122. [Google Scholar] [CrossRef] [PubMed]
- Ruth, A. Impact of Climate Variability on the Socioeconomic Livelihoods in Arapai Division, Soroti City, Uganda. Ph.D. Dissertation, Kampala International University, Kampala, Uganda, 2023. [Google Scholar]
- Daly, S.W. Using Monte Carlo Simulations and Molecular Biology Laboratory Methods to Assess Human Exposure to Fecal Contamination and Enteric Pathogens Through the Environment. Ph.D. Dissertation, North Carolina State University, Raleigh, NC, USA, 2023. [Google Scholar]
- Han, K.T.; Wai, K.T.; Aye, K.H.; Kyaw, K.W.; Maung, W.P.; Oo, T. Emerging neglected helminthiasis and determinants of multiple helminth infections in flood-prone township in Myanmar. Trop. Med. Health 2019, 47, 1. [Google Scholar] [CrossRef]
- Sangwalee, W.; Rattanapitoon, N.; Thanchomnang, T. Intestinal parasitic infections and risk factors among Myanmar migrant workers in northeast Thailand. Asian Pac. J. Trop. Med. 2021, 14, 17–26. [Google Scholar] [CrossRef]
- Khieu, V.; Sayasone, S.; Muth, S.; Kirinoki, M.; Laymanivong, S.; Ohmae, H.; Huy, R.; Chanthapaseuth, T.; Yajima, A.; Phetsouvanh, R.; et al. Elimination of schistosomiasis mekongi from endemic areas in Cambodia and the Lao People’s Democratic Republic: Current status and plans. Trop. Med. Infect. Dis. 2019, 4, 30. [Google Scholar] [CrossRef]
- Wrable, M. Exploring the Association Between Remotely Sensed Environmental Parameters and Surveillance Disease Data: An Application to the Spatiotemporal Modelling of Schistosomiasis in Ghana. Master’s Dissertation, Tufts University, Medford, MA, USA, 2017. [Google Scholar]
- Ikpeze, O.O.; Obikwelu, M.E. Factors affecting seasonal abundance of gastropods of public health importance found at Agulu Lake shorelines in Nigeria. Int. J. Pure App. Biosci 2016, 4, 91–102. [Google Scholar] [CrossRef]
- Aksu, S.; Başkurt, S.; Emiroğlu, Ö.; Tarkan, A. Establishment and range expansion of non-native fish species facilitated by hot springs: The case study from the Upper Sakarya Basin (NW, Turkey). Int. J. Oceanogr. Hydrobiol. 2021, 50, 247–258. [Google Scholar] [CrossRef]
- El-Sayed, A.; Kamel, M. Climatic changes and their role in emergence and re-emergence of diseases. Environ. Sci. Pollut. Res. 2020, 27, 22336–22352. [Google Scholar] [CrossRef]
- Lenik, J.M.; Achuo-Egbe, Y.; Harley, J.M. S2264 GI Meets ID: Two Unusual Findings on Endoscopy Leading to the Life-Saving Diagnosis and Treatments. Off. J. Am. Coll. Gastroenterol. 2021, 116, S965–S966. [Google Scholar] [CrossRef]
- Blood-Siegfried, J.; Zeantoe, G.C.; Evans, L.J.; Bondo, J.; Forstner, J.R.; Wood, K. The impact of nurses on neglected tropical disease management. Public Health Nurs. 2015, 32, 680–701. [Google Scholar] [CrossRef] [PubMed]
- Hayman, S.K.; Mihelcic, J.R. Evaluation of Appropriate Groundwater Supply Technologies That Increase Access to Sufficient and Improved Water in Rural Coastal Communities in Panama. In World Environmental and Water Resources Congress 2016; American Society of Civil Engineers: Reston, VA, USA, 2016; pp. 319–328. [Google Scholar]
- Harada, Y.; Iwashita, H.; Moriyasu, T.; Nagi, S.; Saito, N.; Sugawara-Mikami, M.; Yoshioka, K.; Yotsu, R.; Japan NTD Study Group. The current status of neglected tropical diseases in Japan: A scoping review. PLOS Neglected Trop. Dis. 2024, 18, e0011854. [Google Scholar] [CrossRef] [PubMed]
- Inoue, M.; Sawada, N.; Matsuda, T.; Iwasaki, M.; Sasazuki, S.; Shimazu, T.; Shibuya, K.; Tsugane, S. Attributable causes of cancer in Japan in 2005—Systematic assessment to estimate current burden of cancer attributable to known preventable risk factors in Japan. Ann. Oncol. 2012, 23, 1362–1369. [Google Scholar] [CrossRef]
- Choi, M.H.; Yu, J.R.; Hong, S.T. Who neglects neglected tropical diseases?-Korean perspective. J. Korean Med. Sci. 2015, 30 (Suppl. S2), S122–S130. [Google Scholar] [CrossRef]
- Kali, A. Schistosome infections: An Indian perspective. J. Clin. Diagn. Res. JCDR 2015, 9, DE01. [Google Scholar] [CrossRef]
- Kulinkina, A.V.; Sarkar, R.; Mohan, V.R.; Walz, Y.; Kaliappan, S.P.; Ajjampur, S.S.; Ward, H.; Naumova, E.N.; Kang, G. Prediction of hookworm prevalence in southern India using environmental parameters derived from Landsat 8 remotely sensed data. Int. J. Parasitol. 2020, 50, 47–54. [Google Scholar] [CrossRef]
- Labony, S.S.; Hossain, M.S.; Hatta, T.; Dey, A.R.; Mohanta, U.K.; Islam, A.; Shahiduzzaman, M.; Hasan, M.M.; Alim, M.A.; Tsuji, N.; et al. Mammalian and avian larval schistosomatids in Bangladesh: Molecular characterization, epidemiology, molluscan vectors, and occurrence of human cercarial dermatitis. Pathogens 2022, 11, 1213. [Google Scholar] [CrossRef] [PubMed]
- Bennett, N.R.; Few, R.; Geere, J.L.; Omasete, J. Climate Change and Drowning Risk in Bangladesh and Tanzania and the Implications for RNLI Programmes. 2023. Available online: https://devresearch.uea.ac.uk/wp-content/uploads/2023/01/DEV-RPP-24-V4.pdf (accessed on 17 January 2025).
- Mwai, J.; Omogi, J.O.; Abdi, M.H. Environmental factors influencing prevention and control of schistosomiasis infection in Mwea, Kirinyaga County Kenya: A cross sectional study. East Afr. Health Res. J. 2021, 5, 99. [Google Scholar] [CrossRef]
- Namanya, D.B.; Berrang-Ford, L.; Harper, S.L.; Ford, J.; Bikaitwoha, E.M.; Lwasa, S.; Wright, C.J.; Kazaana, C. Geography, Climate Change and Health Adaptation Planning in Uganda. In Practicing Health Geography: The African Context; Springer International Publishing: Cham, Switzerland, 2021; pp. 175–190. [Google Scholar]
- Sindato, C.; Mboera, L.E. Climate Change Impacts, Adaptation and Mitigation Strategies in Tanzania. In Climate Change and Human Health Scenarios: International Case Studies; Springer Nature: Cham, Switzerland, 2024; pp. 317–331. [Google Scholar]
- Reed, A.L.; Al-Harbi, M.H.; Makaula, P.; Condemine, C.; Hesketh, J.; Archer, J.; Jones, S.; Kayuni, S.A.; Musaya, J.; Stanton, M.C.; et al. A geospatial analysis of local intermediate snail host distributions provides insight into schistosomiasis risk within under-sampled areas of southern Lake Malawi. Parasites Vectors 2024, 17, 272. [Google Scholar] [CrossRef]
- Awuni, S.; Adarkwah, F.; Ofori, B.D.; Purwestri, R.C.; Bernal, D.C.; Hajek, M. Managing the challenges of climate change mitigation and adaptation strategies in Ghana. Heliyon 2023, 9, e15491. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, K. Don’t Forget to Wash! Water, Sanitation, and Hygiene Among Zambian Students. 2022. Available online: https://digitalcommons.unl.edu/nutritionglobalresearch/11/ (accessed on 17 January 2025).
- Phillips, A.E.; Gazzinelli-Guimaraes, P.H.; Aurelio, H.O.; Ferro, J.; Nala, R.; Clements, M.; King, C.H.; Fenwick, A.; Fleming, F.M.; Dhanani, N. Assessing the benefits of five years of different approaches to treatment of urogenital schistosomiasis: A SCORE project in Northern Mozambique. PLoS Neglected Trop. Dis. 2017, 11, e0006061. [Google Scholar] [CrossRef] [PubMed]
- Perez-Saez, J.; Mari, L.; Bertuzzo, E.; Casagrandi, R.; Sokolow, S.H.; De Leo, G.A.; Mande, T.; Ceperley, N.; Froehlich, J.M.; Sou, M.; et al. A theoretical analysis of the geography of schistosomiasis in Burkina Faso highlights the roles of human mobility and water resources development in disease transmission. PLoS Neglected Trop. Dis. 2015, 9, e0004127. [Google Scholar] [CrossRef]
- Gal, L.B.; Bruck, M.; Tal, R.; Baum, S.; Ali, J.M.; Weldegabriel, L.L.; Sabar, G.; Golan, R.; Bentwich, Z. Sustainable Elimination of Schistosomiasis in Ethiopia—A Five-Year Follow-Up Study. Trop. Med. Infect. Dis. 2022, 7, 218. [Google Scholar] [CrossRef]
- Kaawa-Mafigiri, D.; Kato, F.; Megan, S.S. Tackling Deadly Diseases in Africa: Key Considerations for Epidemic Response and Preparedness in Uganda. 2021. Available online: https://www.socialscienceinaction.org/wp-content/uploads/2021/09/TDDAP-Key-Considerations-Epidemic-Response-Preparedness-UGANDA.pdf (accessed on 17 January 2025).
- Kara, S.L.; Jusabani, A. CHOICE Tanzania Annual Report for 2023. Available online: https://www.aku.edu/ighd/research-programmes/Documents/CHOICE-Country-Report-Tanzania-2023.pdf (accessed on 17 January 2025).
- Essiet, A.G.; Gordon, A.A.; Inyang, I.B.; Mkpan, S.B.; Kohol, B.I. Epidemiology of Waterborne Diseases in South-South Nigeria: An Investigation of Risk Factors and Control Strategies. Fac. Nat. Appl. Sci. J. Health Sports Sci. Recreat. 2024, 2, 155–165. [Google Scholar]
- Bah, Y.M.; Paye, J.; Bah, M.S.; Conteh, A.; Saffa, S.; Tia, A.; Sonnie, M.; Veinoglou, A.; Hodges, M.H.; Zhang, Y. Schistosomiasis in school age children in Sierra Leone after 6 years of mass drug administration with praziquantel. Front. Public Health 2019, 7, 1. [Google Scholar] [CrossRef]
- Gerard, J.; Kibaara, T.; Blom, I.M.; Falconer, J.; Mohammed, S.; Kadri-Alabi, Z.; Taylor, R.; Abdullahi, L.; Hughes, R.C.; Onyango, B.; et al. Climate Sensitive Health Outcomes in Kenya: A Scoping Review of Environmental Exposures and Health Outcomes Research, 2000 to 2023. medRxiv 2024. [Google Scholar] [CrossRef]
- Velleman, Y.; Blair, L.; Fleming, F.; Fenwick, A. Water-, Sanitation-, and Hygiene-Related Diseases. In Infectious Diseases; Springer: New York, NY, USA, 2023; pp. 189–219. [Google Scholar]
- Kpoto, L.M. Integrated Approach to the Control of Lymphatic Filariasis, Schistosomiasis, and Soil-transmitted Helminthiasis in Liberia, West Africa. Ph.D. Dissertation, The University of Liverpool, Liverpool, UK, 2020. [Google Scholar]
- Taïbi, A.N.; Kane, A.; Bourlet, M.; Lorin, M.; Ballouche, A. The Senegal River, a disturbed lifeline in the Sahel. In River Culture: Life as a Dance to the Rhythm of the Waters; UNESCO: Paris, France, 2023; pp. 79–113. [Google Scholar]
- Godfrey, S.; Van der Velden, M.; Muianga, A.; Vigh, M.; Gunning, J.W.; Elbers, C. Impact study of the One Million Initiative rural water and sanitation programme in Mozambique. Waterlines 2014, 33, 35–44. [Google Scholar] [CrossRef]
- Fuhrimann, S.; Winkler, M.S.; Kabatereine, N.B.; Tukahebwa, E.M.; Halage, A.A.; Rutebemberwa, E.; Medlicott, K.; Schindler, C.; Utzinger, J.; Cissé, G. Risk of intestinal parasitic infections in people with different exposures to wastewater and fecal sludge in Kampala, Uganda: A cross-sectional study. PLoS Neglected Trop. Dis. 2016, 10, e0004469. [Google Scholar] [CrossRef]
- Wairimu, M.J. Community Participation in Schistosomiasis and Soil-Transmitted Helminths Control and Related Research in Kwale County, Coastal Kenya. Ph.D. Dissertation, Jomo Kenyatta University of Agriculture and Technology, Juja, Kenya, 2015. [Google Scholar]
- Luhunga, P.M.; Songoro, A.E. Analysis of climate change and extreme climatic events in the Lake Victoria Region of Tanzania. Front. Clim. 2020, 2, 559584. [Google Scholar] [CrossRef]
- Nyandwi, E.; Veldkamp, A.; Amer, S.; Karema, C.; Umulisa, I. Schistosomiasis mansoni incidence data in Rwanda can improve prevalence assessments, by providing high-resolution hotspot and risk factors identification. BMC Public Health 2017, 17, 845. [Google Scholar] [CrossRef] [PubMed]
- Elliott, A.M.; Roestenberg, M.; Wajja, A.; Opio, C.; Angumya, F.; Adriko, M.; Egesa, M.; Gitome, S.; Mfutso-Bengo, J.; Bejon, P.; et al. Ethical and scientific considerations on the establishment of a controlled human infection model for schistosomiasis in Uganda: Report of a stakeholders’ meeting held in Entebbe, Uganda. AAS Open Res. 2018, 1, 2. [Google Scholar] [CrossRef] [PubMed]
- Sidze, S.M. Empowering Community Resilience to Climate Change in Cameroon Using Technology-Enhanced Learning. 2017. Available online: https://core.ac.uk/download/pdf/236374643.pdf (accessed on 17 January 2025).
- Kulinkina, A.V. Community Based Methods for Schistosomiasis Prediction and Sustainable Control in Ghana. Ph.D. Dissertation, Tufts University, Medford, MA, USA, 2017. [Google Scholar]
- Joshi, Y.P.; Harmon, W.M. Assessment of Climate Vulnerabilities and Risks in the Health Sector of Liberia. 2021. Available online: https://www.atachcommunity.com/fileadmin/uploads/atach/Documents/Country_documents/Liberia_VA_2021.pdf (accessed on 17 January 2025).
- Alex, S. The Impact of Climate Change on Rural Agro-Pastoralist Communities in Aweil East County, South Sudan. Ph.D. Dissertation, Nkumba University, Entebbe, Uganda, 2018. [Google Scholar]
- Phalira, W.; Kossam, F.; Maliwichi-Nyirenda, C.; Mphepo, G.; Pullanikkatil, D.; Chiotha, S.; Kamlongera, C. Scientific Validation of Traditional Early Warning Signals for Floods and Drought in Nsanje and Chikwawa Districts, Malawi. In Socio-Ecological Systems and Decoloniality: Convergence of Indigenous and Western Knowledge; Springer International Publishing: Cham, Switzerland, 2023; pp. 129–155. [Google Scholar]
- Mensah, H.; Amponsah, O.; Opoku, P.; Ahadzie, D.K.; Takyi, S.A. Resilience to climate change in Ghanaian cities and its implications for urban policy and planning. SN Soc. Sci. 2021, 1, 118. [Google Scholar] [CrossRef]
- Gutierrez, E.S.; Murtagh, V.; Crété, E. Detailed Shelter Response Profile Ethiopia: Local Building Cultures for Sustainable and Resilient Habitats. 2018. Available online: https://hal.science/hal-02888183v1/file/Ethiopia.pdf (accessed on 17 January 2025).
- Godwin-Akpan, T.G.; Chowdhury, S.; Rogers, E.J.; Kollie, K.K.; Zaizay, F.Z.; Wickenden, A.; Zawolo, G.V.; Parker, C.B.; Dean, L. Recommendations for an Optimal Model of integrated case detection, referral, and confirmation of Neglected Tropical Diseases: A case study in Bong County, Liberia. medRxiv 2022. [Google Scholar] [CrossRef]
- Russell, D.J.; Thuesen, P.A.; Thomson, F.E. A review of the biology, ecology, distribution and control of Mozambique tilapia, Oreochromis mossambicus (Peters 1852)(Pisces: Cichlidae) with particular emphasis on invasive Australian populations. Rev. Fish Biol. Fish. 2012, 22, 533–554. [Google Scholar]
- Libanda, B.; Rand, E.; Gyang, G.N.; Sindano, C.T.; Simwanza, L.; Chongo, M. Recent and future exposure of water, sanitation, and hygiene systems to climate-related hazards in Zambia. J. Water Clim. Change 2024, 15, 958–977. [Google Scholar] [CrossRef]
- Arostegui, M.C.; Wood, C.L.; Jones, I.J.; Chamberlin, A.J.; Jouanard, N.; Faye, D.S.; Kuris, A.M.; Riveau, G.; De Leo, G.A.; Sokolow, S.H. Potential biological control of schistosomiasis by fishes in the lower Senegal river basin. Am. J. Trop. Med. Hyg. 2018, 100, 117. [Google Scholar] [CrossRef]
- Bompangue, D.; Moore, S.; Taty, N.; Impouma, B.; Sudre, B.; Manda, R.; Balde, T.; Mboussou, F.; Vandevelde, T. Description of the targeted water supply and hygiene response strategy implemented during the cholera outbreak of 2017–2018 in Kinshasa, DRC. BMC Infect. Dis. 2020, 20, 226. [Google Scholar] [CrossRef]
- Tupps, C.; Kargbo-Labour, I.; Paye, J.; Dhakal, S.; Hodges, M.H.; Jones, A.H.; Davlin, S.; Sonnie, M.; Manah, S.; Imtiaz, R.; et al. Community-wide prevalence and intensity of soil-transmitted helminthiasis and Schistosoma mansoni in two districts of Sierra Leone. PLOS Neglected Trop. Dis. 2022, 16, e0010410. [Google Scholar] [CrossRef]
- Labbe, J.; Ford, J.D.; Berrang-Ford, L.; Donnelly, B.; Lwasa, S.; Namanya, D.B.; Twesigomwe, S.; IHACCResearch Team Harper, S.L. Vulnerability to the health effects of climate variability in rural southwestern Uganda. Mitig. Adapt. Strateg. Glob. Change 2016, 21, 931–953. [Google Scholar] [CrossRef]
- Codjoe, S.N.; Gough, K.V.; Wilby, R.L.; Kasei, R.; Yankson, P.W.; Amankwaa, E.F.; Abarike, M.A.; Atiglo, D.Y.; Kayaga, S.; Mensah, P.; et al. Impact of extreme weather conditions on healthcare provision in urban Ghana. Soc. Sci. Med. 2020, 258, 113072. [Google Scholar] [CrossRef] [PubMed]
- Mwanga, J.R.; Kinung’hi, S.M.; Mosha, J.; Angelo, T.; Maganga, J.; Campbell Jr, C.H. Village response to mass drug administration for schistosomiasis in Mwanza region, northwestern Tanzania: Are we missing socioeconomic, cultural, and political dimensions? Am. J. Trop. Med. Hyg. 2020, 103, 1969. [Google Scholar] [CrossRef]
- Liu, M.M.; Feng, Y.; Yang, K. Impact of micro-environmental factors on survival, reproduction and distribution of Oncomelania hupensis snails. Infect. Dis. Poverty 2021, 10, 47. [Google Scholar] [CrossRef] [PubMed]
- Stensgaard, A.S.; Utzinger, J.; Vounatsou, P.; Hürlimann, E.; Schur, N.; Saarnak, C.F.; Simoonga, C.; Mubita, P.; Kabatereine, N.B.; Tchuenté, L.A.; et al. Large-scale determinants of intestinal schistosomiasis and intermediate host snail distribution across Africa: Does climate matter? Acta Trop. 2013, 128, 378–390. [Google Scholar] [CrossRef]
- Pedersen, U.B.; Stendel, M.; Midzi, N.; Mduluza, T.; Soko, W.; Stensgaard, A.S.; Vennervald, B.J.; Mukaratirwa, S.; Kristensen, T.K. Modelling climate change impact on the spatial distribution of fresh water snails hosting trematodes in Zimbabwe. Parasites Vectors 2014, 7, 536. [Google Scholar] [CrossRef]
- Paull, S.H.; Johnson, P.T. High temperature enhances host pathology in a snail–trematode system: Possible consequences of climate change for the emergence of disease. Freshw. Biol. 2011, 56, 767–778. [Google Scholar] [CrossRef]
- Bănăduc, D.; Simić, V.; Cianfaglione, K.; Barinova, S.; Afanasyev, S.; Öktener, A.; McCall, G.; Simić, S.; Curtean-Bănăduc, A. Freshwater as a sustainable resource and generator of secondary resources in the 21st century: Stressors, threats, risks, management and protection strategies, and conservation approaches. Int. J. Environ. Res. Public Health 2022, 19, 16570. [Google Scholar] [CrossRef]
- Rohr, J.R.; Barrett, C.B.; Civitello, D.J.; Craft, M.E.; Delius, B.; DeLeo, G.A.; Hudson, P.J.; Jouanard, N.; Nguyen, K.H.; Ostfeld, R.S.; et al. Emerging human infectious diseases and the links to global food production. Nat. Sustain. 2019, 2, 445–456. [Google Scholar] [CrossRef]
- Djeddour, D.; Pratt, C.; Makale, F.; Rwomushana, I.; Day, R. The apple snail, Pomacea canaliculata: An evidence note on invasiveness and potential economic impacts for East Africa. CABI Work. Pap. 2021, 21, 77. [Google Scholar]
- Watts, S.; El Katsha, S. Irrigation, farming and schistosomiasis: A case study in the Nile delta. Int. J. Environ. Health Res. 1997, 7, 101–113. [Google Scholar] [CrossRef]
- Wright, C.Y.; Kapwata, T.; Naidoo, N.; Asante, K.P.; Arku, R.E.; Cissé, G.; Simane, B.; Atuyambe, L.; Berhane, K. Climate Change and Human Health in Africa in Relation to Opportunities to Strengthen Mitigating Potential and Adaptive Capacity: Strategies to Inform an African “Brains Trust”. Ann. Glob. Health 2024, 90, 7. [Google Scholar] [CrossRef] [PubMed]
- Rocklöv, J.; Ahlm, C.; Scott, M.E.; Humphries, D.L. Climate change pathways and potential future risks to nutrition and infection. In Nutrition and Infectious Diseases: Shifting the Clinical Paradigm; Humana: Cham, Switzerland, 2021; pp. 429–458. [Google Scholar]
- Conlon, K.C.; Austin, C.M. Climate change and public health interventions. In Climate Change and Global Public Health; Humana: Cham, Switzerland, 2021; pp. 549–564. [Google Scholar]
- Koehn, P. Climate policy and action ‘underneath’Kyoto and Copenhagen: China and the United States. Wiley Interdiscip. Rev. Clim. Change 2010, 1, 405–417. [Google Scholar] [CrossRef]
- Levia, D.F.; Bergquist, R.; Meydani, A.; Hu, Y.; Hannah, D.M. Hydrological extremes heighten vulnerability to schistosomiasis. Earths Future 2024, 12, e2024EF004659. [Google Scholar] [CrossRef]
- Verma, A.; Nagarajan, H.D.; Jaiswal, V.; Tamdamba, H.; Sah, S.; Mehta, R.; Bushi, G.; Balaraman, A.K.; Pandey, S.; Brar, M.; et al. Schistosomiasis infection in Europe: A climate-driven public health challenge. Clin. Infect. Pract. 2025, 25, 100402. [Google Scholar] [CrossRef]
- Afful, P.; Abotsi, G.K.; Adu-Gyamfi, C.O.; Benyem, G.; Katawa, G.; Kyei, S.; Arndts, K.; Ritter, M.; Asare, K.K. Schistosomiasis–Microbiota Interactions: A Systematic Review and Meta-Analysis. Pathogens 2024, 13, 906. [Google Scholar] [CrossRef]
- Asare, K.K.; Afful, P.; Abotsi, G.K.; Adu-Gyamfi, C.O.; Benyem, G.; Katawa, G.; Arndts, K.; Ritter, M. Schistosomiasis endemicity and its role in sexually transmitted infections—A systematic review and meta-analysis. Front. Parasitol. 2024, 3, 1451149. [Google Scholar] [CrossRef]


| Region | Current Transmission Suitability | Predicted Impact of Climate Change | Future Hotspot | Key Factors | Potential Effects | Infection Rates (Snails) | Human Infection Rates | Key Findings | References |
|---|---|---|---|---|---|---|---|---|---|
| Sub-Saharan Africa | High | Increased transmission risk | Eastern Africa | Temperature rise, seasonal rainfall | Higher transmission during wet seasons | High | Increased in wet seasons | Climate change leads to more favorable conditions for snail reproduction in wetter areas | [11,16,38,39] |
| Southern Africa | Moderate | Increased transmission risk | Southern Africa | Increased temperatures, higher rainfall | Longer transmission seasons | Moderate | Moderate | Increased rainfall and temperature increase snail density, extending transmission seasons | [15,24,40] |
| West Africa | Moderate | Decreased transmission risk | West Africa | Temperature rise, water body changes | Decreased transmission risk due to temperature rise | Low | Low | Higher temperatures limit snail survival, reducing transmission risk | [12,33,41,42] |
| Southeast Asia | Low | Increased transmission risk | Southeast Asia | Monsoon, temperature increase | Increased transmission risk due to wet season | Low | Low | Monsoon shifts could introduce new areas for snail host survival | [43,44,45,46] |
| South America | Low | Increased transmission risk | Northern South America | Rainfall, temperature rise | Increased risk due to seasonal flooding | Low | Low | Changes in rainfall patterns increase areas of suitable habitat for snails | [33,47,48] |
| Central Africa | High | Increased transmission risk | Central Africa | Higher temperatures, extended rainy season | Snail population densities increase in warmer months | High | Increased in wet season | Climate-driven increases in water bodies lead to higher snail populations and infection rates | [12,16,22,49] |
| East Africa | Moderate | Increased transmission risk | Eastern Africa | Changing rainfall patterns, rising temperatures | Prolonged wet season increases transmission risk | Moderate | Increased | Increased rainfall leads to an extended breeding period for snails | [11,19,38,50] |
| South East Asia | Low | Increased transmission risk | Southeast Asia | Higher monsoon intensity, rising temperatures | Increased snail populations in monsoon season | Low | Low | Rising temperatures and monsoon lengthening transmission windows | [51,52,53] |
| Central Asia | Low | No significant change | Central Asia | Mild temperature fluctuations | Stable snail populations in mild conditions | Low | Low | Little to no impact from climate change in stable, low-transmission regions | [43,54,55,56] |
| Western Africa | Moderate | Increased transmission risk | Western Africa | Rainfall variation, temperature increases | Snail population peak during rainy season | Moderate | Moderate | Variable rainfall patterns increase risks for schistosomiasis transmission | [13,57,58,59] |
| Mediterranean Region | Low | Increased transmission risk | Mediterranean countries | Temperature rise, water body alterations | Longer transmission seasons due to higher temperatures | Low | Low | Shifting water bodies due to temperature changes could introduce new transmission foci | [60,61,62,63] |
| Southern Asia | Moderate | Increased transmission risk | South Asia | Increased monsoons, rising temperatures | Increased snail populations during monsoon periods | Moderate | Moderate | Extended monsoon season likely increases snail populations, raising transmission risk | [43,64] |
| East Africa | High | Increased transmission risk | Kenya, Tanzania | Higher temperatures, reduced water bodies | Decreased transmission in drier periods | High | Increased in wet seasons | Drying water bodies may reduce snail habitats in some areas, but wet season populations may spike | [38,65,66,67] |
| Caribbean Islands | Low | No significant change | Caribbean islands | Stable temperature, rainfall fluctuations | Stable transmission rates | Low | Low | Stable environmental conditions limit changes to transmission risk | [68,69] |
| Central America | Moderate | Increased transmission risk | Central America | Rising temperatures, changing rainfall patterns | Longer wet season extends transmission risks | Moderate | Increased | Lengthened wet season extends periods of snail-host availability | [70,71] |
| Pacific Islands | Low | Increased transmission risk | Pacific Islands | Rising sea levels, increased rainfall | Increased transmission in flooded areas | Low | Low | Flooded areas may support new snail populations, raising infection risks | [44,72] |
| West Africa | High | Increased transmission risk | West Africa | Longer rainy season, rising temperatures | Increased transmission during wet periods | High | Increased in wet seasons | Longer rainy season increases snail-host survival, leading to increased infection rates | [73,74] |
| South East Asia | Moderate | Decreased transmission risk | Vietnam, Laos | Temperature fluctuations, rainfall variability | Shorter wet season reduces transmission risk | Moderate | Low | Shorter wet season may limit available breeding conditions for snails | [75] |
| Tropical Asia | Moderate | Increased transmission risk | Indonesia, Philippines | Rising temperature, longer wet season | Extended breeding period for snails | Moderate | High | Extended rainy season and warmer temperatures increase risks for both snail population and human rates | [76,77,78] |
| Middle East | Low | No significant change | Middle East | Stable temperatures, periodic rainfall | Stable transmission levels | Low | Low | Dry conditions and stable water levels result in minimal change to transmission | [62] |
| East Africa | High | Increased transmission risk | Ethiopia, Sudan | Temperature rise, flooding | Snail population densities increase during floods | High | Increased in wet seasons | Flooding due to higher rainfall extends snail-host habitats, raising transmission risk | [79,80,81] |
| Central Africa | Moderate | Decreased transmission risk | Cameroon, Central African Republic | Temperature increase, dry conditions | Lower transmission due to lack of water bodies | Moderate | Low | Higher temperatures reduce available snail habitats, decreasing infection rates | [82,83] |
| South East Asia | High | Increased transmission risk | Thailand, Cambodia | Rising temperatures, extended wet season | Increase in snail populations and infection rates | High | Increased in wet season | Temperature rise extends breeding season for snails, increasing human transmission risk | [84,85] |
| East Africa | Moderate | Increased transmission risk | Uganda, Rwanda | Rainfall changes, temperature fluctuations | Transmission peak in rainy season | Moderate | High | Climate-induced rainfall shifts may extend wet season transmission period for schistosomiasis | [86,87] |
| Southeast Asia | Low | Increased transmission risk | Myanmar, Cambodia | Longer rainy season, rising temperature | Increased snail populations in new wetland areas | Low | Low | Seasonal flooding creates new areas for snail-host survival, raising transmission risk | [88,89,90] |
| West Africa | Moderate | Increased transmission risk | Nigeria, Ghana | Temperature rise, seasonal rainfall changes | Increased risk due to favorable breeding conditions | Moderate | Increased | Warmer wet season leads to increased snail density and infection risk | [41,91,92] |
| Eastern Mediterranean | Low | Decreased transmission risk | Türkiye, Greece | Rising temperatures, changing rainfall patterns | Reduced snail populations due to less favorable conditions | Low | Low | Rising temperatures reduce optimal snail habitats in the region | [93,94] |
| Central America | High | Increased transmission risk | Honduras, Panama | Increased rainfall, rising temperature | Higher transmission rates during wet season | High | Increased in wet season | Longer rainy periods contribute to increased snail population densities | [95,96,97] |
| East Asia | Low | No significant change | Japan, South Korea | Stable temperatures, minimal rainfall changes | Stable transmission levels | Low | Low | Stable environmental conditions lead to minimal impact on transmission risk | [98,99,100] |
| South Asia | Moderate | Decreased transmission risk | India, Bangladesh | Increased temperatures, seasonal rainfall | Shorter rainy seasons reduce transmission risk | Moderate | Low | Shorter rainy seasons may limit periods of transmission for schistosomiasis | [101,102,103,104] |
| Year | Location | Climate Adaptation Policy | Impact on Schistosomiasis Control | Policy Integration with Public Health | Key Findings | Implications for Schistosomiasis Transmission | References |
|---|---|---|---|---|---|---|---|
| 2012–2014 | Mozambique | Sustainable Irrigation, Water Supply, and Sanitation for Climate Challenges | Climate-smart irrigation lowers snail populations; improved rural water systems reduce contamination | Incorporated into irrigation policy frameworks and community health initiatives. | Improved irrigation management decreases snail habitats and schistosomiasis prevalence. | Enhanced sanitation reduces disease risk and prevents agricultural zone outbreaks. | [122,136] |
| 2015–2017 | Burkina Faso, Kenya; Uganda; Mozambique; Rwanda; Cameroon; Ghana; Mozambique | Integrated approaches including river basin planning, climate-resilient infrastructure, flood risk management, sustainable agriculture, urban resilience, and coordinated water and sanitation strategies. | Controlling water accumulation through river management, improved storage, drainage, and floodplain management reduces snail breeding and strengthens climate and health resilience. | Climate and health resilience measures are integrated across national river management, flood risk, water distribution, agricultural, urban planning, and health emergency systems. | Significant reductions in snail populations (up to 25%) and schistosomiasis transmission, especially in flood-prone areas, through improved sanitation and management. | Adapted health infrastructure, sustainable farming, and water management strategies reduce seasonal transmission spikes, prevent outbreaks, and control disease spread in urban and rural areas. | [111,112,123,124,126,128,129,141] |
| 2018–2020 | Uganda; South Sudan; Ethiopia; Senegal; Sierra Leone; Liberia; Tanzania; Democratic Republic of Congo | Comprehensive strategies for climate resilience, including water purification, flood management, disaster risk reduction, and climate-adapted infrastructure | Community-based water purification, flood protection, and climate-adapted systems reduce contamination risks and prevent snail population growth, ensuring better public health outcomes. | Integrated strategies linking local health, disaster management, food security, and community-based risk programs to enhance resilience and health outcomes | Snail population reduced by up to 40%, leading to decreased transmission and infection rates, especially in rural, flood-prone, and coastal areas, with improved healthcare access during climate extremes. | Water purification, flood management, and resilient infrastructure reduce transmission risks and prevent schistosomiasis outbreaks, particularly in vulnerable regions. | [117,120,125,127,131,134,138,139] |
| 2021–2023 | Kenya; Uganda; Liberia; Ghana; Zambia; Ethiopia; Sierra Leone; Nigeria; Tanzania; Democratic Republic of Congo; Senegal; Malawi | Integrated strategies for climate resilience, including water resource management, flood prevention, climate-smart agriculture, sanitation, and early warning systems. | Climate-resilient infrastructure, early flood warnings, improved water quality, and sanitation reduce snail habitats, prevent contamination, and lower schistosomiasis transmission. | Integrated strategies linking water, sanitation, agriculture, public health, and disaster response to enhance community health resilience and reduce disease risks. | Targeted interventions reduce schistosomiasis cases and transmission by up to 40%, with significant reductions in vulnerable regions, improved access to treatment, and lower disease burden. | Policies and strategies, including flood management, early warnings, water quality improvement, and sanitation, significantly reduce schistosomiasis transmission, especially in rural, urban, and high-risk communities. | [36,105,106,109,110,113,114,115,119,121,130,132,133,135,140,142,143] |
| 2024 | Tanzania; Malawi; Nigeria; Kenya; Zambia | Integrated strategies for climate resilience, including coastal adaptation, early warning systems, water management, and sanitation improvement. | Coastal defenses, water stagnation reduction, and improved sanitation reduce schistosomiasis transmission, with increased awareness and urban resilience policies enhancing disease control. | Integrated strategies linking national health, water management, disaster preparedness, and sanitation programs to enhance disease control and resilience. | Targeted interventions reduced schistosomiasis transmission by 30%, lowered snail populations, and decreased waterborne disease risk in agricultural, coastal, and urban areas. | Coastal and urban resilience policies, along with prevention measures and improved sanitation, play key roles in reducing schistosomiasis transmission. | [107,108,116,118,137] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Asare, K.K.; Mohammed, M.-D.W.; Aboagye, Y.O.; Arndts, K.; Ritter, M. Impact of Climate Change on Schistosomiasis Transmission and Distribution—Scoping Review. Int. J. Environ. Res. Public Health 2025, 22, 812. https://doi.org/10.3390/ijerph22050812
Asare KK, Mohammed M-DW, Aboagye YO, Arndts K, Ritter M. Impact of Climate Change on Schistosomiasis Transmission and Distribution—Scoping Review. International Journal of Environmental Research and Public Health. 2025; 22(5):812. https://doi.org/10.3390/ijerph22050812
Chicago/Turabian StyleAsare, Kwame Kumi, Muhi-Deen Wonwana Mohammed, Yussif Owusu Aboagye, Kathrin Arndts, and Manuel Ritter. 2025. "Impact of Climate Change on Schistosomiasis Transmission and Distribution—Scoping Review" International Journal of Environmental Research and Public Health 22, no. 5: 812. https://doi.org/10.3390/ijerph22050812
APA StyleAsare, K. K., Mohammed, M.-D. W., Aboagye, Y. O., Arndts, K., & Ritter, M. (2025). Impact of Climate Change on Schistosomiasis Transmission and Distribution—Scoping Review. International Journal of Environmental Research and Public Health, 22(5), 812. https://doi.org/10.3390/ijerph22050812






