Cardiovascular, Hemodynamic, and Anthropometric Adaptations Induced by Walking Training at FATmax in Obese Males and Females over 45 Years Old
Abstract
1. Introduction
2. Methods
2.1. Study Design and Participants
2.2. Assessment and Measurements
2.3. Graded Exercise Test (GXT)
2.4. Calculations
2.5. Training Protocol
2.6. Statistical Analysis
3. Results
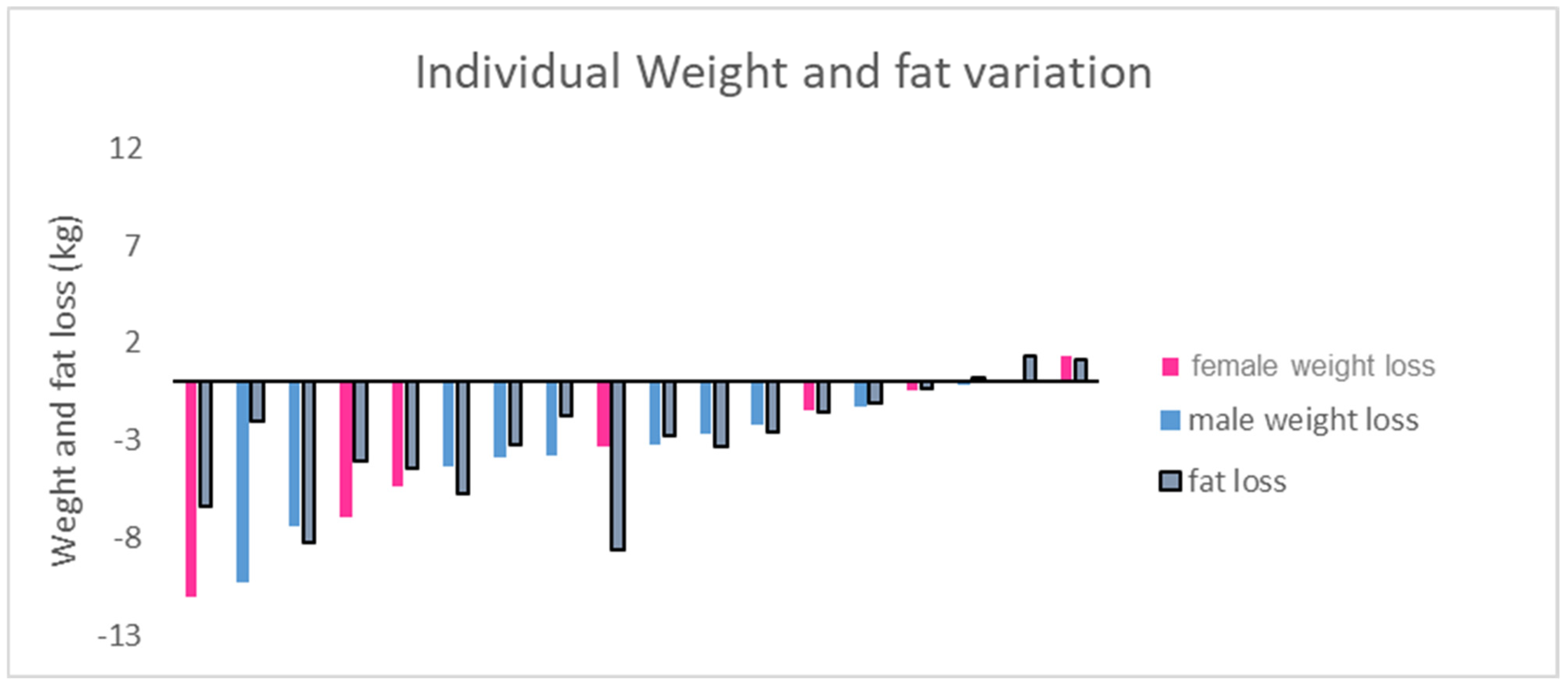
3.1. Body Composition
3.2. Exercise Performance
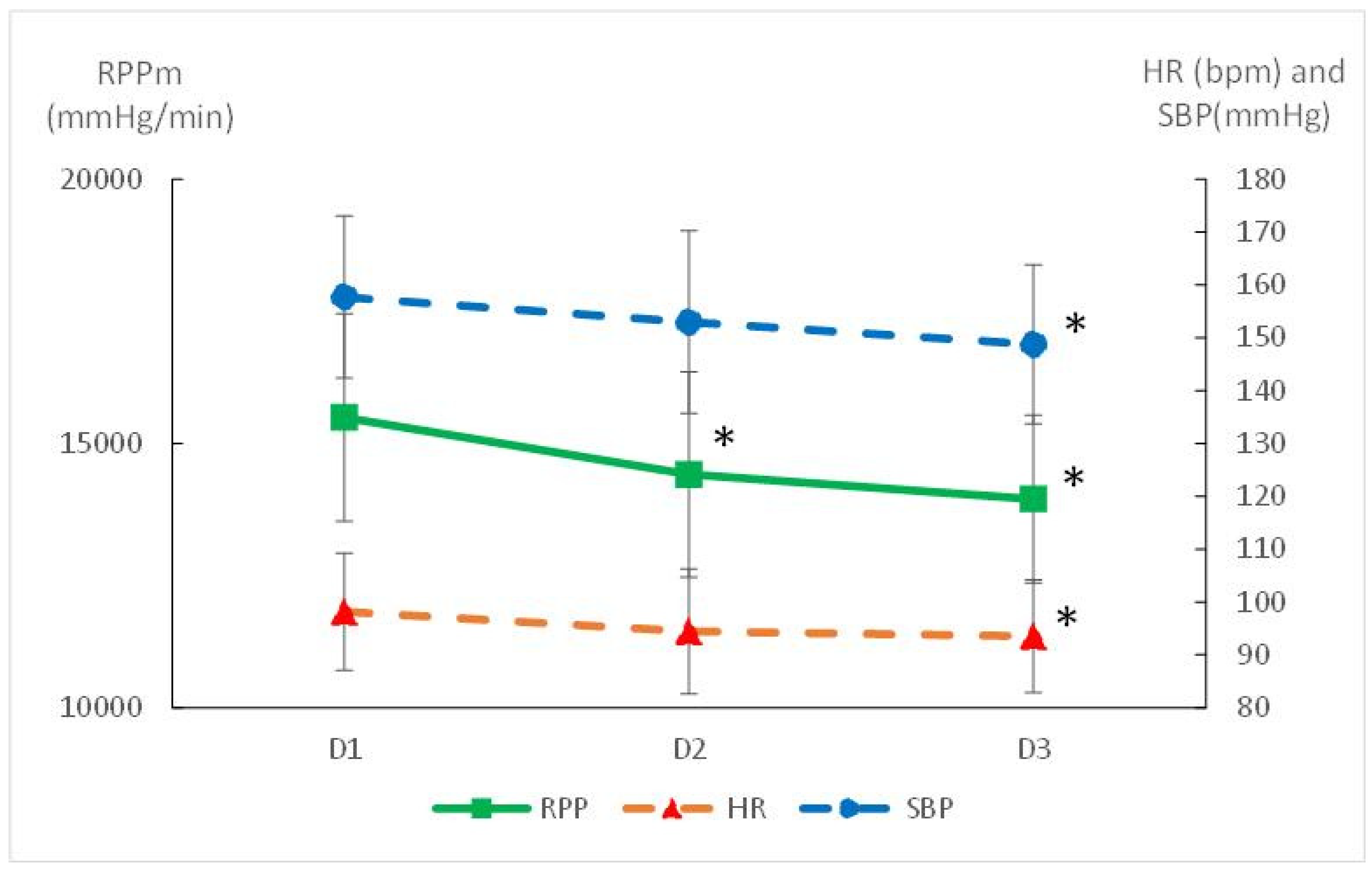
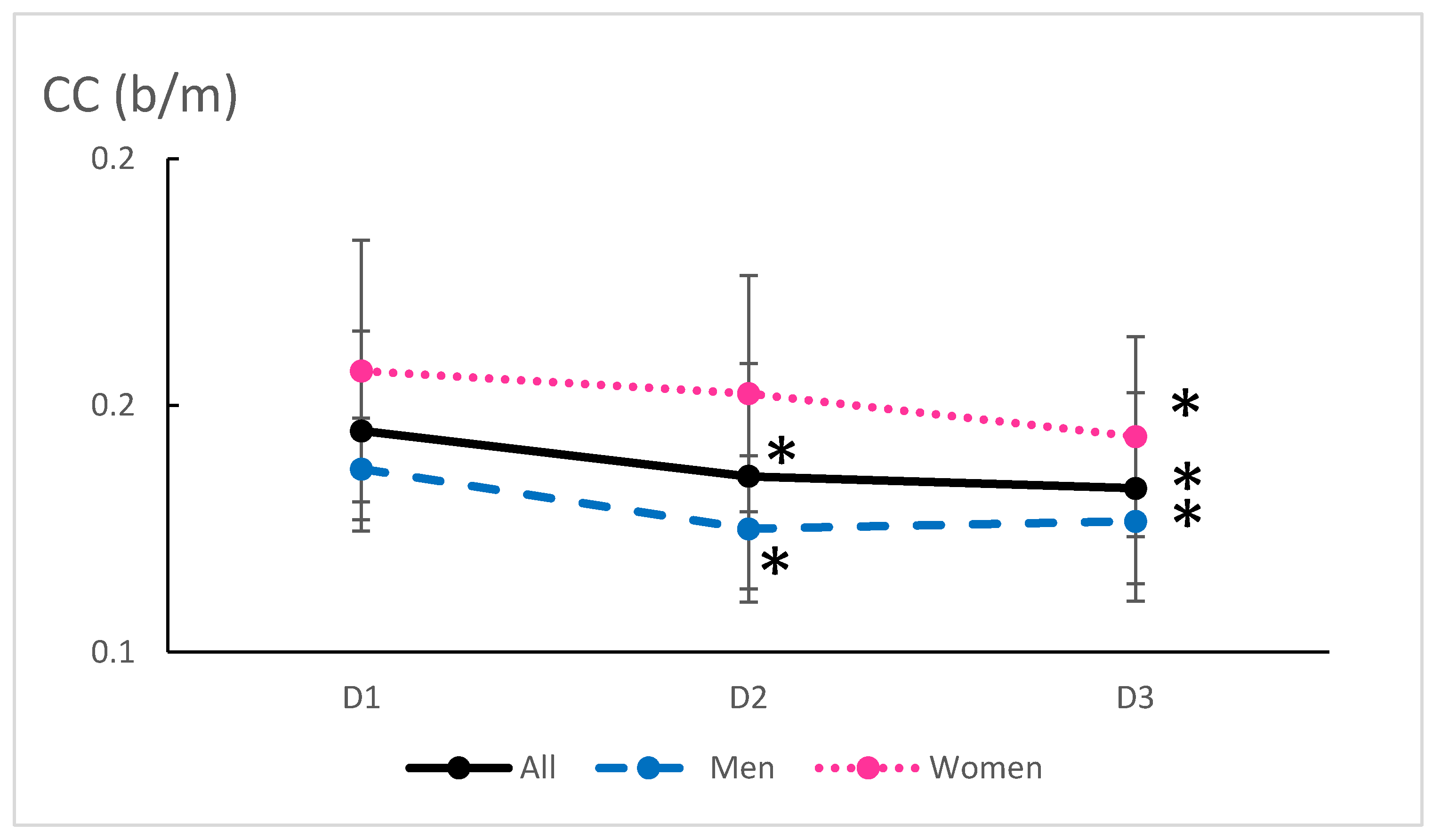
3.3. Cardiovascular Adaptations
4. Discussion
4.1. Benefits of FATmax Training and Body Composition
4.2. Cardiovascular Adaptations Through FATmax Training
4.3. The Role of Autonomy in FATmax Training
4.4. Implications for Long-Term Health and Disease Prevention
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Phelps, N.H.; Singleton, R.K.; Zhou, B.; Heap, R.A.; Mishra, A.; Bennett, J.E.; Paciorek, C.J.; Lhoste, V.P.; Carrillo-Larco, R.M.; Stevens, G.A.; et al. Worldwide trends in underweight and obesity from 1990 to 2022: A pooled analysis of 3663 population-representative studies with 222 million children, adolescents, and adults. Lancet 2024, 403, 1027–1050. [Google Scholar] [CrossRef] [PubMed]
- Prevalence of Overweight and Obesity in France: The 2020 Obepi-Roche Study by the “Ligue Contre l’Obésité”—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/36769573/ (accessed on 20 February 2025).
- Zhao, M.; Zhang, N.; Wang, M.; Yao, S.; Wang, C.; Yun, C.; Zhang, S.; Sun, Y.; Hou, Z.; Chen, S.; et al. Transitions in Metabolic Health and Onset Age of Cardiovascular Diseases. Am. J. Prev. Med. 2023, 65, 1059–1068. [Google Scholar] [CrossRef] [PubMed]
- Kouvari, M.; Panagiotakos, D.B.; Yannakoulia, M.; Georgousopoulou, E.; Critselis, E.; Chrysohoou, C.; Tousoulis, D.; Pitsavos, C. Transition from metabolically benign to metabolically unhealthy obesity and 10-year cardiovascular disease incidence: The ATTICA cohort study. Metab.-Clin. Exp. 2019, 93, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Tazzeo, C.; Zucchelli, A.; Vetrano, D.L.; Demurtas, J.; Smith, L.; Schoene, D.; Sanchez-Rodriguez, D.; Onder, G.; Balci, C.; Bonetti, S.; et al. Risk factors for multimorbidity in adulthood: A systematic review. Ageing Res. Rev. 2023, 91, 102039. [Google Scholar] [CrossRef]
- Bundy, J.D.; Li, C.; Stuchlik, P.; Bu, X.; Kelly, T.N.; Mills, K.T.; He, H.; Chen, J.; Whelton, P.K.; He, J. Systolic Blood Pressure Reduction and Risk of Cardiovascular Disease and Mortality: A Systematic Review and Network Meta-analysis. JAMA Cardiol. 2017, 2, 775–781. [Google Scholar] [CrossRef]
- Yano, Y.; Kim, H.C.; Lee, H.; Azahar, N.; Ahmed, S.; Kitaoka, K.; Kaneko, H.; Kawai, F.; Mizuno, A.; Viera, A.J. Isolated Diastolic Hypertension and Risk of Cardiovascular Disease: Controversies in Hypertension—Pro Side of the Argument. Hypertension 2022, 79, 1563–1570. [Google Scholar] [CrossRef]
- Triantafyllidi, H.; Birmpa, D.; Benas, D.; Fabri, A.; Schoinas, A.; Thymis, I.; Kostelli, G.; Ikonomidis, I. Isolated Diastolic Hypertension Is Not a Benign Situation Regarding Hypertension Mediated Damage. Available online: https://journals.lww.com/jhypertension/abstract/2022/06001/isolated_diastolic_hypertension_is_not_a_benign.308.aspx (accessed on 19 April 2025).
- Sabaka, P.; Dukat, A.; Gajdosik, J.; Bendzala, M.; Caprnda, M.; Simko, F. The effects of body weight loss and gain on arterial hypertension control: An observational prospective study. Eur. J. Med. Res. 2017, 22, 43. [Google Scholar] [CrossRef]
- Fazliana, M.; Liyana, A.Z.; Omar, A.; Ambak, R.; Mohamad Nor, N.S.; Shamsudin, U.K.; Salleh, N.A.; Aris, T. Effects of weight loss intervention on body composition and blood pressure among overweight and obese women: Findings from the MyBFF@home study. BMC Womens Health 2018, 18, 93. [Google Scholar] [CrossRef]
- Lin, C.H.; Kurup, S.; Herrero, P.; Schechtman, K.B.; Eagon, J.C.; Klein, S.; Dávila-Román, V.G.; Stein, R.I.; Dorn-II, G.W.; Gropler, R.J.; et al. Myocardial Oxygen Consumption Change Predicts Left Ventricular Relaxation Improvement in Obese Humans After Weight Loss. Obesity 2011, 19, 1804–1812. [Google Scholar] [CrossRef]
- Pescatello, L.S.; Buchner, D.M.; Jakicic, J.M.; Powell, K.E.; Kraus, W.E.; Bloodgood, B.; Campbell, W.W.; Dietz, S.; Dipietro, L.; George, S.M.; et al. Physical Activity to Prevent and Treat Hypertension: A Systematic Review. Med. Sci. Sports Exerc. 2019, 51, 1314. [Google Scholar] [CrossRef]
- Filippou, C.D.; Tsioufis, C.P.; Thomopoulos, C.G.; Mihas, C.C.; Dimitriadis, K.S.; Sotiropoulou, L.I.; Chrysochoou, C.A.; Nihoyannopoulos, P.I.; Tousoulis, D.M. Dietary Approaches to Stop Hypertension (DASH) Diet and Blood Pressure Reduction in Adults with and without Hypertension: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Adv. Nutr. 2020, 11, 1150–1160. [Google Scholar] [CrossRef] [PubMed]
- Madhusudhan, U. Effect of high and low intensities of aerobic training on rate pressure product. Natl. J. Physiol. Pharm. Pharmacol. 2018, 8, 550. [Google Scholar] [CrossRef]
- Ha, J.-W.; Juracan, E.M.; Mahoney, D.W.; Oh, J.K.; Shub, C.; Seward, J.B.; Pellikka, P.A. Hypertensive response to exercise: A potential cause for new wall motion abnormality in the absence of coronary artery disease. J. Am. Coll. Cardiol. 2002, 39, 323–327. [Google Scholar] [CrossRef] [PubMed]
- Brun, J.-F.; Myzia, J.; Varlet-Marie, E.; Raynaud De Mauverger, E.; Mercier, J. Beyond the Calorie Paradigm: Taking into Account in Practice the Balance of Fat and Carbohydrate Oxidation during Exercise? Nutrients 2022, 14, 1605. [Google Scholar] [CrossRef]
- Pescatello, L.S.; Franklin, B.A.; Fagard, R.; Farquhar, W.B.; Kelley, G.A.; Ray, C.A.; By, T. pronouncement was written for the A.C. of S.M. Exercise and Hypertension. Med. Sci. Sports Exerc. 2004, 36, 533. [Google Scholar] [CrossRef]
- Romain, A.J.; Carayol, M.; Desplan, M.; Fedou, C.; Ninot, G.; Mercier, J.; Avignon, A.; Brun, J.F. Physical Activity Targeted at Maximal Lipid Oxidation: A Meta-Analysis. J. Nutr. Metab. 2012, 2012, 285395. [Google Scholar] [CrossRef]
- Tan, S.; Wang, J.; Cao, L.; Guo, Z.; Wang, Y. Positive effect of exercise training at maximal fat oxidation intensity on body composition and lipid metabolism in overweight middle-aged women. Clin. Physiol. Funct. Imaging 2016, 36, 225–230. [Google Scholar] [CrossRef]
- Chávez-Guevara, I.A.; Urquidez-Romero, R.; Pérez-León, J.A.; González-Rodríguez, E.; Moreno-Brito, V.; Ramos-Jiménez, A. Chronic Effect of Fatmax Training on Body Weight, Fat Mass, and Cardiorespiratory Fitness in Obese Subjects: A Meta-Analysis of Randomized Clinical Trials. Int. J. Environ. Res. Public Health 2020, 17, 7888. [Google Scholar] [CrossRef]
- Cao, L.; Jiang, Y.; Li, Q.; Wang, J.; Tan, S. Exercise Training at Maximal Fat Oxidation Intensity for Overweight or Obese Older Women: A Randomized Study. J. Sports Sci. Med. 2019, 18, 413–418. [Google Scholar]
- Wang, J.; Tan, S.; Cao, L. Exercise training at the maximal fat oxidation intensity improved health-related physical fitness in overweight middle-aged women. J. Exerc. Sci. Fit. 2015, 13, 111–116. [Google Scholar] [CrossRef]
- Cebula, A.; Tyka, A.K.; Tyka, A.; Pałka, T.; Pilch, W.; Luty, L.; Mucha, D. Physiological response and cardiorespiratory adaptation after a 6-week Nordic Walking training targeted at lipid oxidation in a group of post-menopausal women. PLoS ONE 2020, 15, e0230917. [Google Scholar] [CrossRef] [PubMed]
- Besnier, F.; Lenclume, V.; Gérardin, P.; Fianu, A.; Martinez, J.; Naty, N.; Porcherat, S.; Boussaid, K.; Schneebeli, S.; Jarlet, E.; et al. Individualized Exercise Training at Maximal Fat Oxidation Combined with Fruit and Vegetable-Rich Diet in Overweight or Obese Women: The LIPOXmax-Réunion Randomized Controlled Trial. PLoS ONE 2015, 10, e0139246. [Google Scholar] [CrossRef] [PubMed]
- Jackson, A.; Pollock, M. Generalized equations for predicting body density of men. Br. J. Nutr. 1978, 40, 497–504. [Google Scholar] [CrossRef] [PubMed]
- Péronnet, F.; Massicotte, D. Table of nonprotein respiratory quotient: An update. Can. J. Sport Sci. 1991, 16, 23–29. Available online: https://www.semanticscholar.org/paper/Table-of-nonprotein-respiratory-quotient%3A-an-P%C3%A9ronnet-Massicotte/21c9d33f5b8cf8ab46c5b7550343601e80e76a42 (accessed on 14 March 2025).
- Cohen, J. A power primer. Psychol. Bull. 1992, 112, 155–159. [Google Scholar] [CrossRef]
- Bogdanis, G.C.; Vangelakoudi, A.; Maridaki, M. Peak Fat Oxidation Rate During Walking in Sedentary Overweight Men and Women. J. Sports Sci. Med. 2008, 7, 525–531. [Google Scholar]
- Haufe, S.; Engeli, S.; Budziarek, P.; Utz, W.; Schulz-Menger, J.; Hermsdorf, M.; Wiesner, S.; Otto, C.; Fuhrmann, J.C.; Luft, F.C.; et al. Determinants of Exercise-induced Fat Oxidation in Obese Women and Men. Horm. Metab. Res. 2009, 42, 215–221. [Google Scholar] [CrossRef]
- Pérez-Martin, A.; Dumortier, M.; Raynaud, E.; Brun, J.F.; Fédou, C.; Bringer, J.; Mercier, J. Balance of substrate oxidation during submaximal exercise in lean and obese people. Diabetes Metab. 2001, 27, 466–474. [Google Scholar]
- Preda, A.; Carbone, F.; Tirandi, A.; Montecucco, F.; Liberale, L. Obesity phenotypes and cardiovascular risk: From pathophysiology to clinical management. Rev. Endocr. Metab. Disord. 2023, 24, 901–919. [Google Scholar] [CrossRef]
- Hawkins, S.A.; Wiswell, R.A. Rate and Mechanism of Maximal Oxygen Consumption Decline with Aging. Sports Med. 2003, 33, 877–888. [Google Scholar] [CrossRef]
- Antonopoulos, A.S.; Goliopoulou, A.; Vogiatzi, G.; Tousoulis, D. Myocardial Oxygen Consumption. In Coronary Artery Disease; Tousoulis, D., Ed.; Academic Press: Cambridge, MA, USA, 2018; pp. 127–136. ISBN 978-0-12-811908-2. [Google Scholar]
- Phoemsapthawee, J. Combined exercise training improves blood pressure at rest and during exercise in young obese prehypertensive men. J. Sports Med. Phys. Fit. 2020, 61, 468–479. [Google Scholar] [CrossRef] [PubMed]
- Kozakova, M.; Palombo, C. Vascular Ageing and Aerobic Exercise. Int. J. Environ. Res. Public Health 2021, 18, 10666. [Google Scholar] [CrossRef] [PubMed]
- Battista, F.; Ermolao, A.; van Baak, M.A.; Beaulieu, K.; Blundell, J.E.; Busetto, L.; Carraça, E.V.; Encantado, J.; Dicker, D.; Farpour-Lambert, N.; et al. Effect of exercise on cardiometabolic health of adults with overweight or obesity: Focus on blood pressure, insulin resistance, and intrahepatic fat—A systematic review and meta-analysis. Obes. Rev. 2021, 22, e13269. [Google Scholar] [CrossRef] [PubMed]
- Cornelissen, V.A.; Fagard, R.H. Effects of Endurance Training on Blood Pressure, Blood Pressure–Regulating Mechanisms, and Cardiovascular Risk Factors. Hypertension 2005, 46, 667–675. [Google Scholar] [CrossRef]
- Cortes, M.B.; da Silva, R.S.N.; de Oliveira, P.C.; da Silva, D.S.; Irigoyen, M.C.C.; Waclawovsky, G.; Schaun, M.I. Effect of aerobic and resistance exercise training on endothelial function in individuals with overweight and obesity: A systematic review with meta-analysis of randomized clinical trials. Sci. Rep. 2023, 13, 11826. [Google Scholar] [CrossRef]
- Rivera-Torres, S.; Fahey, T.D.; Rivera, M.A. Adherence to Exercise Programs in Older Adults: Informative Report. Gerontol. Geriatr. Med. 2019, 5, 2333721418823604. [Google Scholar] [CrossRef]
- Hanssen, H.; Boardman, H.; Deiseroth, A.; Moholdt, T.; Simonenko, M.; Kränkel, N.; Niebauer, J.; Tiberi, M.; Abreu, A.; Solberg, E.E.; et al. Personalized exercise prescription in the prevention and treatment of arterial hypertension: A Consensus Document from the European Association of Preventive Cardiology (EAPC) and the ESC Council on Hypertension. Eur. J. Prev. Cardiol. 2022, 29, 205–215. [Google Scholar] [CrossRef]
- Guiraudou, M.; Fédou, C.; Romain, A.-J.; Sferlazza, A.; Calas, E.; Brun, J.-F. Effects over one year of low-intensity endurance exercise targeted at the level of maximal lipid oxidation. Sci. Sports 2015, 30, e127–e131. [Google Scholar] [CrossRef]
| D1 | D2 | D3 | p (D1 vs. D3) | Effect Size | |
|---|---|---|---|---|---|
| Body weight (kg) | 95.3 (14.9) | 91.4 (15.4) | 91.6 (14.3) | 0.032 | 0.25 |
| female | 85.9 (13.1) | 83.1 (11.3) | 82.0 (9.7) | 0.089 | 0.34 |
| male | 101.3 (13.3) | 96.7 (15.8) | 97.7 (13.6) | 0.004 | 0.27 |
| BMI (kg/m2) | 31.8 (3.8) | 30.4 (4.1) | 30.5 (3.5) | 0.0002 | 0.36 |
| female | 31.5 (4.8) | 30.3 (4.3) | 29.9 (11.2) | 0.019 | 0.40 |
| male | 32.0 (3.2) | 30.5 (4.2) | 30.8 (3.4) | 0.03 | 0.30 |
| Waist circumference (cm) | 109.2 (9.0) | 103.6 (9.9) | 103.6 (7.4) | 0.0003 | 0.64 |
| female | 108.7 (12.4) | 104.4 (10.4) | 101.9 (7.9) | 0.013 | 0.65 |
| male | 109.4 (6.8) | 103.0 (9.9) | 104.8 (7.3) | 0.050 | 0.64 |
| % Fat | 33.6 (7.1) | 31.4 (8.3) | 31.6 (7.2) | 0.022 | 0.27 |
| female | 41.7 (3.3) | 40.4 (2.8) | 39.6 (1.2) | 0.390 | 0.80 |
| male | 28.5 (2.0) | 25.5 (4.6) | 26.6 (3.7) | 0.034 | 0.59 |
| Lean body mass (kg) | 63.6 (13.5) | 62.7 (13.2) | 62.8 (12.9) | 0.512 | 0.06 |
| female | 49.9 (6.8) | 49.3 (4.9) | 49.5 (5.4) | 0.259 | 0.07 |
| male | 72.3 (8.1) | 71.3 (8.8) | 71.4 (7.7) | 0.096 | 0.01 |
| At Rest | During Exercise | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| D1 | D2 | D3 | p (D1 vs. D3) | Cohen’s D | D1 | D2 | D3 | p (D1 vs. D3) | Cohen’s D | |
| HR (BPM) | 77 (13) | 72 (14) | 73 (11) | 0.097 | 0.36 | 98 (11) | 94 (12) | 94 (11) | 0.022 | 0.42 |
| Female | 79 (15) | 78 (17) | 73 (13) | 0.007 | 0.46 | 102 (14) | 99 (13) | 95 (12) | 0.069 | 0.57 |
| Male | 76 (12) | 68 (12) | 73 (10) | 0.059 | 0.27 | 96 (8) | 91 (10) | 93 (10) | 0.327 | 0.30 |
| SV (mL) | 63.8 (12.1) | 68.9 (18.3) | 60.5 (17.4) | 0.334 | 0.22 | 93.3 (17.6) | 96.5 (19.3) | 88.1 (18.9) | 0.248 | 0.29 |
| Female | 60.0 (9.4) | 62.5 (13.5) | 55.0 (18.2) | 0.432 | 0.35 | 82.0 (14.2) | 84.3 (16.6) | 79.8 (20.8) | 0.368 | 0.13 |
| Male | 66.2 (13.4) | 72.7 (20.3) | 64.0 (16.8) | 0.222 | 0.15 | 100.5 (16.1) | 103.2 (17.9) | 93.4 (16.5) | 0.385 | 0.44 |
| Qc (L·min−1) | 4.9 (1.0) | 4.8 (1.0) | 4.3 (0.9) | 0.245 | 0.60 | 9.1 (1.6) | 9.0 (1.9) | 8.3 (1.6) | 0.353 | 0.49 |
| Female | 4.8 (1.2) | 4.6 (0.8) | 3.9 (1.0) | 0.201 | 0.77 | 8.1 (1.7) | 8.1 (1.1) | 7.7 (1.9) | 0.224 | 0.23 |
| Male | 5.0 (0.9) | 4.9 (1.1) | 4.6 (0.8) | 0.205 | 0.47 | 9.7 (1.3) | 9.4 (2.1) | 8.7 (1.3) | 0.436 | 0.78 |
| SBP (mmHg) | 131 (14) | 130 (15) | 123 (11) | 0.012 | 0.59 | 158 (15) | 153 (17) | 149 (15) | 0.001 | 0.57 |
| Female | 124 (11) | 121 (12) | 116 (11) | 0.834 | 0.64 | 150 (13) | 142 (10) | 143 (16) | 0.074 | 0.50 |
| Male | 135 (15) | 135 (14) | 127 (9) | 0.168 | 0.65 | 162 (15) | 160 (17) | 152 (14) | 0.407 | 0.66 |
| DBP (mmHg) | 85 (9) | 86 (9) | 81 (5) | 0.014 | 0.54 | 96 (9) | 95 (9) | 90 (6) | <0.001 | 0.79 |
| Female | 80 (5) | 81 (5) | 78 (4) | 0.175 | 0.48 | 91 (5) | 89 (6) | 86 (4) | 0.092 | 0.99 |
| Male | 89 (10) | 89 (9) | 83 (5) | 0.432 | 0.67 | 99 (10) | 99 (9) | 92 (6) | 0.012 | 0.84 |
| RPP (mmHg·min−1) | 10,107 (1967) | 9333 (1940) | 8984 (1595) | 0.004 | 0.28 | 15,493 (2412) | 14,414 (2220) | 13,949 (2348) | <0.001 | 0.62 |
| Female | 9844 (2360) | 9479 (2470) | 8486 (1843) | 0.105 | 1.82 | 15,430 (3218) | 14,075 (2242) | 13,607 (2701) | 0.02 | 0.61 |
| Male | 10,274 (1777) | 9239 (1646) | 9302 (1395) | 0.183 | 0.59 | 15,533 (1917) | 14,630 (2286) | 14,166 (2204) | 0.03 | 0.64 |
| CC (beat·m−1) | 1.45 (0.20) | 1.36 (0.23) | 1.33 (0.19) | <0.001 | 0.57 | |||||
| Female | 1.57 (0.26) | 1.52 (0.24) | 1.44 (0.20) | 0.020 | 0.56 | |||||
| Male | 1.37 (0.10) | 1.25 (0.15) | 1.27 (0.16) | 0.019 | 0.74 | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mille-Hamard, L.; Momken, I.; Koralsztein, J.-P.; Billat, V.L. Cardiovascular, Hemodynamic, and Anthropometric Adaptations Induced by Walking Training at FATmax in Obese Males and Females over 45 Years Old. Int. J. Environ. Res. Public Health 2025, 22, 701. https://doi.org/10.3390/ijerph22050701
Mille-Hamard L, Momken I, Koralsztein J-P, Billat VL. Cardiovascular, Hemodynamic, and Anthropometric Adaptations Induced by Walking Training at FATmax in Obese Males and Females over 45 Years Old. International Journal of Environmental Research and Public Health. 2025; 22(5):701. https://doi.org/10.3390/ijerph22050701
Chicago/Turabian StyleMille-Hamard, Laurence, Iman Momken, Jean-Pierre Koralsztein, and Véronique Louise Billat. 2025. "Cardiovascular, Hemodynamic, and Anthropometric Adaptations Induced by Walking Training at FATmax in Obese Males and Females over 45 Years Old" International Journal of Environmental Research and Public Health 22, no. 5: 701. https://doi.org/10.3390/ijerph22050701
APA StyleMille-Hamard, L., Momken, I., Koralsztein, J.-P., & Billat, V. L. (2025). Cardiovascular, Hemodynamic, and Anthropometric Adaptations Induced by Walking Training at FATmax in Obese Males and Females over 45 Years Old. International Journal of Environmental Research and Public Health, 22(5), 701. https://doi.org/10.3390/ijerph22050701







