Impact of Praziquantel Mass Drug Administration on Schistosomiasis: A Comparison of Prevalence and Risk Factors Between Treated School Aged Children and Untreated Adults in Abuja, Nigeria
Abstract
1. Introduction
2. Problem Statement
3. Materials and Methods
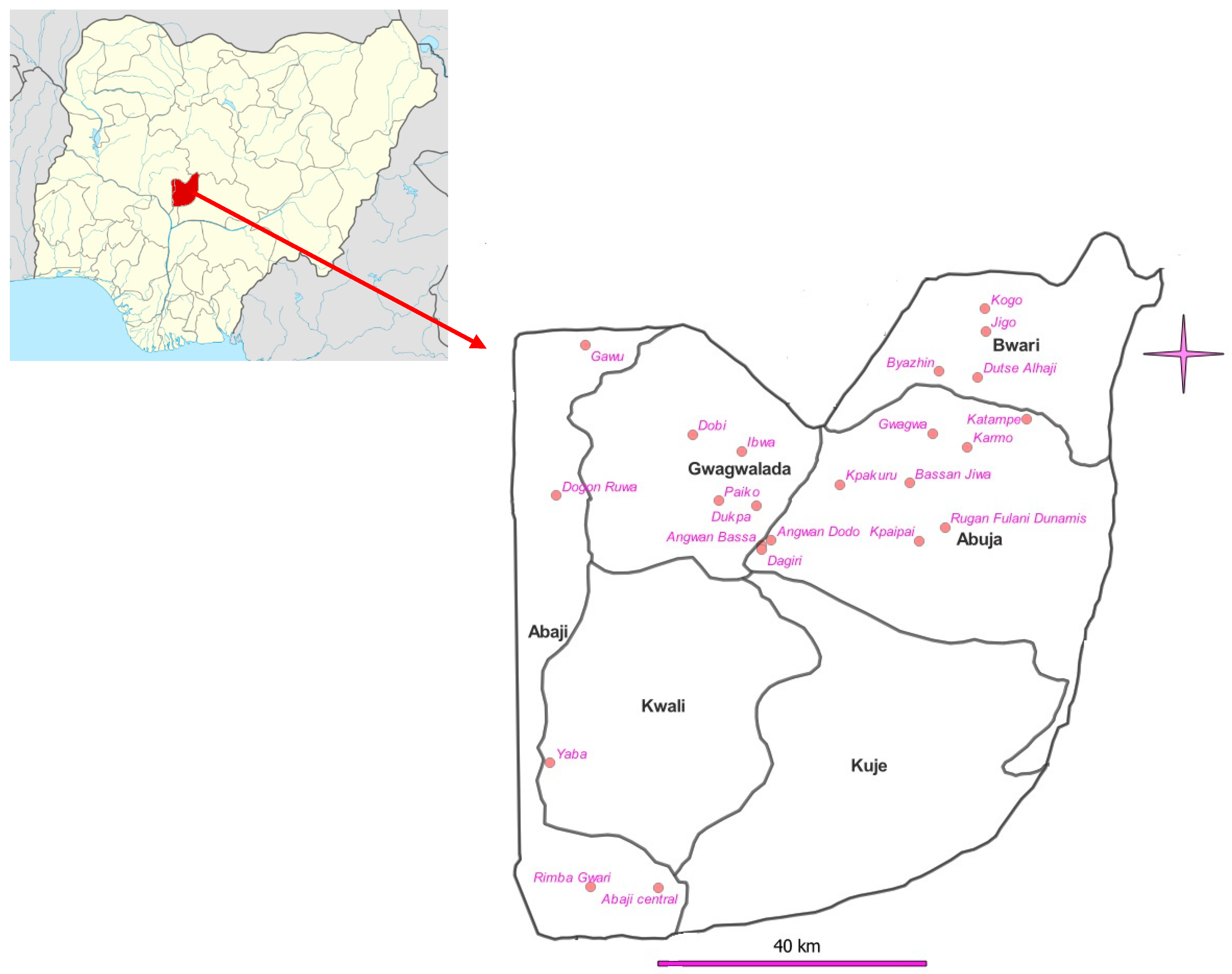
3.1. Study Area
3.2. Study Design
3.3. Specimen Collection
3.4. Urine Filtration Technique
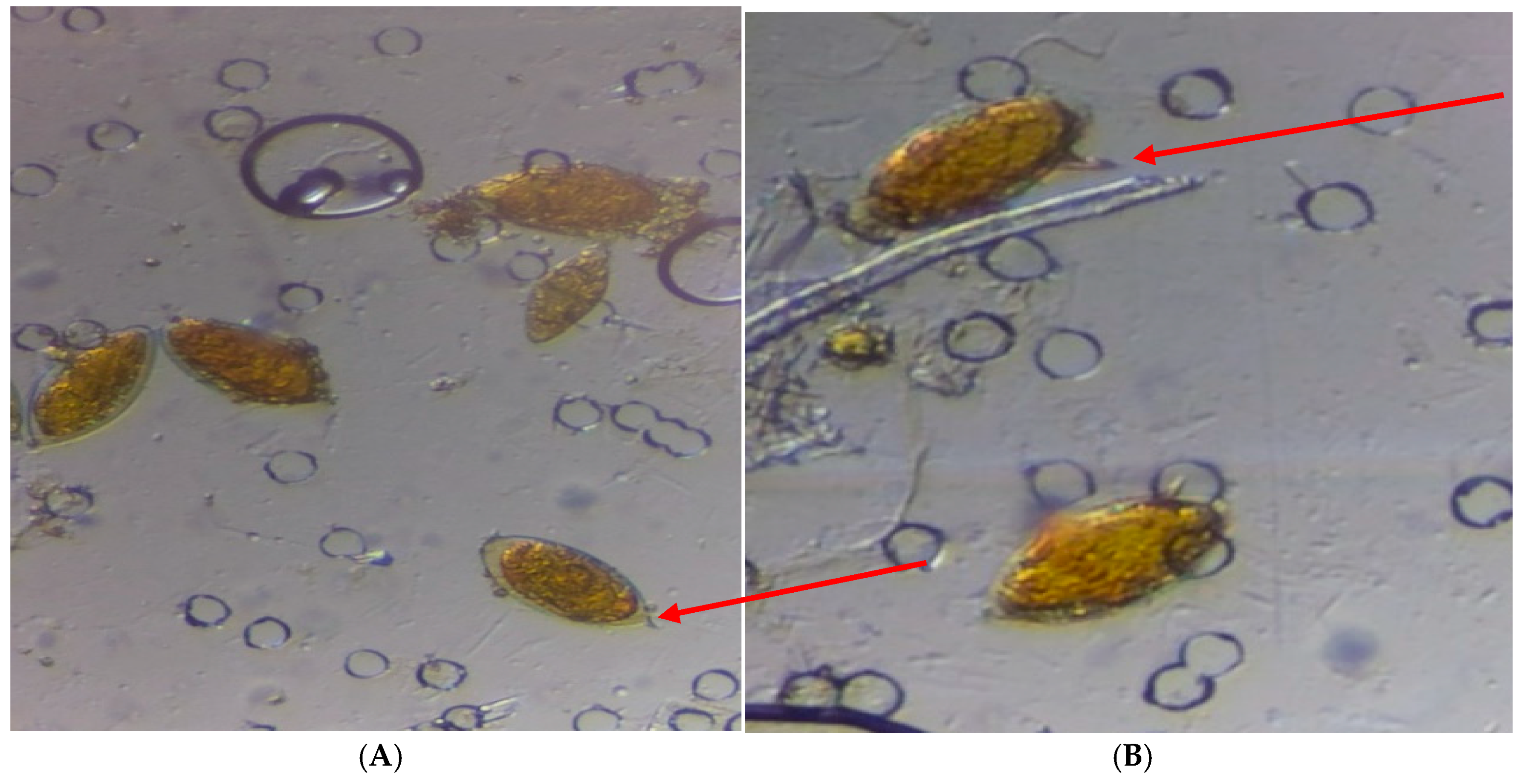
3.5. Kato-Katz Technique for Stool Examination
3.6. Statistical Analysis
4. Results
4.1. Demographic Characteristics of the Studied Population
4.2. Prevalence of Schistosomiasis by Local Area Councils
4.3. Prevalence and Intensity by Schistosoma Species
4.4. Comparison of the Impact Prevalence with Baseline Prevalence
4.5. Relationship Between Gender and Schistosoma Infection
4.6. Relationship Between Schistosomiasis Infection and Age Category (SAC and Adult)
4.7. Association Between Sources/Activities in the River and Prevalence of Schistosomiasis
5. Discussion
6. Limitations of the Study
7. Conclusions
8. Recommendation
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McManus, D.P.; Dunne, D.W.; Sacko, M.; Utzinger, J.; Vennervald, B.J.; Zhou, X.-N. Schistosomiasis. Nat. Rev. Dis. Primers 2018, 4, 13. [Google Scholar] [CrossRef]
- Hotez, P.J.; Alvarado, M.; Basáñez, M.-G.; Bolliger, I.; Bourne, R.; Boussinesq, M.; Brooker, S.J.; Brown, A.S.; Buckle, G.; Budke, C.M.; et al. The Global Burden of Disease Study 2010: Interpretation and Implications for the Neglected Tropical Diseases. PLoS Neglected Trop. Dis. 2014, 8, e2865. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Schistosomiasis: Fact Sheet. 2020. Available online: https://www.who.int/news-room/fact-sheets/detail/schistosomiasis (accessed on 12 December 2024).
- Verjee, M.A. Schistosomiasis: Still a Cause of Significant Morbidity and Mortality. Res. Rep. Trop. Med. 2019, 10, 153–163. [Google Scholar] [CrossRef] [PubMed]
- LoVerde, P.T. Schistosomiasis. Adv. Exp. Med. Biol. 2019, 1154, 45–70. [Google Scholar]
- Ezeamama, A.E.; Bustinduy, A.L.; Nkwata, A.K.; Martinez, L.; Pabalan, N.; Boivin, M.J.; King, C.H. Cognitive deficits and educational loss in children with schistosome infection-A systematic review and meta-analysis. PLoS Neglected Trop. Dis. 2018, 12, e0005524. [Google Scholar] [CrossRef] [PubMed]
- Olliaro, P.L.; Coulibaly, J.T.; Garba, A.; Halleux, C.; Keiser, J.; King, C.H.; Mutapi, F.; N’goran, E.K.; Raso, G.; Scherrer, A.U.; et al. Efficacy and safety of single-dose 40 mg/kg oral praziquantel in the treatment of schistosomiasis in preschool-age versus school-age children: An individual participant data meta-analysis. PLoS Neglected Trop. Dis. 2020, 14, e0008277. [Google Scholar] [CrossRef]
- World Health Organization. Fifty-Fourth World Health Assembly, Geneva, 14–22 May 2001: Resolutions and Decisions; World Health Organization: Geneva, Switzerland, 2001. [Google Scholar]
- World Health Organization. GUIDELINE on Control and Elimination of Human Schistosomiasis; World Health Organization: Geneva, Switzerland, 2022; ISBN 978-92-4-004160-8. [Google Scholar]
- Oluwole, A.S.; Ekpo, U.F.; Nebe, O.J.; Akpan, N.M.; Jacob, S.M.; Amazigo, U.V.; Stothard, J.R. The new WHO guideline for control and elimination of human Schistosomiasis: Implication for the Schistosomiasis elimination programme in Nigeria. Infect. Dis. Poverty 2022, 10, 111. [Google Scholar] [CrossRef]
- FMOH. Report on Epidemiological Mapping of Schistosomiasis and Soil Transmitted Helminthiasis in 19 States and the FCT; Federal Ministry of Health: Abuja Nigeria, 2015; Volume 76.
- Nduka, F.; Nebe, O.J.; Njepuome, N.; Akpan, N.M.; Jacob, S.M.; Amazigo, U.V.; Stothard, J.R. Epidemiological mapping of schistosomiasis and soil-transmitted helminthiasis for intervention strategies in Nigeria. Nig J. Parasitol. 2019, 40, 124–131. [Google Scholar] [CrossRef]
- World Health Organization. ESPEN Joint Reporting Form for Selected Medicines; WHO: Geneva, Switzerland, 2023. [Google Scholar]
- National Population Commission. Nigerian Population Census Report; National Population Commission: Abuja, Nigeria, 2006; pp. 21–27. [Google Scholar]
- Bartlett, J.E.; Kotrlik, J.W.; Higgins, C.C. Organizational Research: Determining Appropriate Sample Size in Survey Research. Inform. Technol. Learn Perform. J. 2001, 19, 43–50. [Google Scholar]
- Lengeler, C.; Mshinda, H.; Morona, D.; Desavigny, D. Urinary schistosomiasis: Testing with urine filtration and reagent sticks for haematuria provides a comparable prevalence estimate. Acta Trop. 1993, 53, 39–50. [Google Scholar] [CrossRef]
- World Health Organization. Bench Aids for the Diagnosis of Intestinal Parasites (Original Version 1994, Corrected in 2012); World Health Organization: Geneva, Switzerland, 2012. [Google Scholar]
- Harris-Roxas, B.; Viliani, F.; Bond, A.; Cave, B.; Divall, M.; Furu, P.; Harris, P.; Soeberg, M.; Wernham, A.; Winkler, M. Health impact assessment: The state of the art. Impact Assess. Proj. Apprais. 2012, 30, 43–52. [Google Scholar] [CrossRef]
- Mushi, V.; Zacharia, A.; Shao, M.; Mubi, M.; Tarimo, D. Persistence of Schistosoma haematobium transmission among school children and its implication for the control of urogenital schistosomiasis in Lindi, Tanzania. PLoS ONE 2022, 17, e0263929. [Google Scholar] [CrossRef] [PubMed]
- Malibiche, D.; Mushi, V.; Justine, N.C.; Silvestri, V.; Mhamilawa, L.E.; Tarimo, D. Prevalence and factors associated with ongoing transmission of Schistosoma haematobium after 12 rounds of Praziquantel Mass Drug Administration among school-aged children in Southern Tanzania. Parasite Epidemiol. Control 2023, 23, e00323. [Google Scholar] [CrossRef] [PubMed]
- Oluwole, A.S.; Adeniran, A.A.; Mogaji, H.O.; Olabinke, D.B.; Abe, E.M.; Bankole, S.O.; Sam-Wobo, S.O.; Ekpo, U.F. Prevalence, intensity and spatial co-distribution of schistosomiasis and soil transmitted helminths infections in Ogun state, Nigeria. Parasitol. Open 2018, 4, e8. [Google Scholar] [CrossRef]
- Alabi, P.; Oladejo, S.O.; Odaibo, A.B. Prevalence and intensity of urinary schistosomiasis in Ogun state, Southwest, Nigeria. J. Public Health Epidemiol. 2018, 10, 413–417. [Google Scholar] [CrossRef]
- Oyeyemi, O.T.; Jeremias, W.J.; Grenfell, R.F.Q. Schistosomiasis in Nigeria: Gleaning from the past to improve current efforts towards control. One Health 2020, 14, 100183. [Google Scholar] [CrossRef]
- Dawaki, S.; Al-Mekhlafi, H.M.; Ithoi, I.; Ibrahim, J.; Abdulsalam, A.M.; Ahmed, A.; Sady, H.; Nasr, N.A.; Atroosh, W.M. The Menace of Schistosomiasis in Nigeria: Knowledge, Attitude, and Practices Regarding Schistosomiasis among Rural Communities in Kano State. PLoS ONE 2015, 10, e0143667. [Google Scholar] [CrossRef]
- Ajakaye, O.G.; Olusi, T.A.; Oniya, M.O. Environmental factors and the risk of urinary schistosomiasis in Ile Oluji/Oke Igbo local government area of Ondo State. Parasite Epidemiol. Control 2016, 1, 98–104. [Google Scholar] [CrossRef][Green Version]
- Schafer, T.W.; Hale, B.R. Gastrointestinal Complications of Schistosomiasis. Curr. Gastroenterol. Rep. 2001, 3, 293–303. [Google Scholar] [CrossRef]
- Faust, C.L.; Osakunor, D.N.M.; Downs, J.A.; Kayuni, S.; Stothard, J.R.; Lamberton, P.H.; Reinhard-Rupp, J.; Rollinson, D. Schistosomiasis Control: Leave No Age Group Behind. Trends Parasitol. 2020, 36, 582–592. [Google Scholar] [CrossRef]
- Woldeyohannes, D.; Sahiledengle, B.; Tekalegnb, Y.; Hailemariam, Z. Prevalence of Schistosomiasis (S. mansoni and S. haematobium) and its association with gender of school age children in Ethiopia: A systematic review and meta-analysis. Parasite Epidemiol. Control 2021, 13, 210. [Google Scholar] [CrossRef] [PubMed]
- Balogun, J.B.; Adewale, B.; Balogun, S.U.; Lawan, A.; Haladu, I.S.; Dogara, M.M.; Aminu, A.U.; Caffrey, C.R.; De Koning, H.P.; Watanabe, Y.; et al. Prevalence and Associated Risk Factors of Urinary Schistosomiasis among Primary School Pupils in the Jidawa and Zobiya Communities of Jigawa State, Nigeria. Ann. Glob. Health 2022, 88, 71. [Google Scholar] [CrossRef] [PubMed]
- Oluwole, A.S.; Bettee, A.K.; Nganda, M.M.; Piotrowski, H.L.; O Fapohunda, V.; Adejobi, J.B.; Soneye, I.Y.; A Kafil-Emiola, M.; O Soyinka, F.; Nebe, O.J.; et al. A quality improvement approach in co-developing a primary healthcare package for raising awareness and managing female genital schistosomiasis in Nigeria and Liberia. Intern. Health 2023, 15, 30–42. [Google Scholar] [CrossRef]
- Masong, M.; Ozano, K.; Tagne, M.S.; Tchoffo, M.N.; Ngang, S.; Thomson, R.; Theobald, S.; Tchuenté, L.A.; Kouokam, E. Achieving equity in UHC interventions: Who is left behind by neglected tropical disease programs in Cameroon? Glob. Health Action 2021, 14, 1886457. [Google Scholar] [CrossRef]
- Phillips, A.E.; Ower, A.K.; Mekete, K.; Liyew, E.F.; Maddren, R.; Belay, H.; Chernet, M.; Anjulo, U.; Mengistu, B.; Salasibew, M.; et al. Association between water, sanitation, and hygiene access and the prevalence of soil-transmitted helminth and schistosome infections in Wolayita, Ethiopia. Parasites Vectors 2022, 15, 410. [Google Scholar] [CrossRef]
- Evan, S.W. Water-based interventions for schistosomiasis control. Path Global Health 2014, 108, 246–254. [Google Scholar] [CrossRef]
- Grimes, J.E.; Croll, D.; Harrison, W.E.; Utzinger, J.; Freeman, M.C.; Templeton, M.R. The relationship between water, sanitation and schistosomiasis: A systematic review and meta-analysis. PLoS Neglected Trop. Dis. 2014, 8, e3296. [Google Scholar] [CrossRef]
- Campbell, S.J.; Biritwum, N.K.; Woods, G.; Velleman, Y.; Fleming, F.; Stothard, J.R. Tailoring water, sanitation, and hygiene (WASH) targets for soil-transmitted helminthiasis and schistosomiasis control. Trends Parasitol. 2018, 34, 53–63. [Google Scholar] [CrossRef]
- Grimes, J.E.; Croll, D.; Harrison, W.E.; Utzinger, J.; Freeman, M.C.; Templeton, M.R. The roles of water, sanitation and hygiene in reducing schistosomiasis: A review. Parasites Vectors 2015, 8, 156. [Google Scholar] [CrossRef]
- Kosinski, K.C.; Kulinkina, A.V.; Abrah, A.F.A.; Adjei, M.N.; Breen, K.M.; Chaudhry, H.M.; Nevin, P.E.; Warner, S.H.; Tendulkar, S.A. A mixed-methods approach to understanding water use and water infrastructure in a schistosomiasis-endemic community: Case study of Asamama, Ghana. BMC Public Health 2016, 16, 322. [Google Scholar] [CrossRef]

| Abaji n (%) | AMAC n (%) | BWARI n (%) | Gwagwalada n (%) | Total | |
|---|---|---|---|---|---|
| Sex | |||||
| Male | 121 (48.99%) | 158 (51.63) | 148 (41.34) | 240 (52.29) | 667 (48.67) |
| Female | 126 (51.01%) | 148 (48.37) | 210 (58.66) | 219 (47.71) | 703 (51.31) |
| Total | 247 (100%) | 306 (100) | 358 (100) | 459 (100) | 1370 (100) |
| Age | |||||
| SAC 5–15 years | 123 (49.8%) | 149 (48.7) | 180 (50.3) | 245 (53.4) | 697 (50.9) |
| Adult > 15 years | 124 (50.2%) | 157 (51.3) | 178 (49.7) | 214 (46.6) | 673 (49.1) |
| Total | 247 (100%) | 306 (100) | 358 (100) | 459 (100) | 247 (100) |
| Source of water for domestic use | |||||
| Well/Rain | 89 (36.0%) | 25 (8.2%) | 0 (0.0%) | 173 (37.7%) | 287 (20.9%) |
| Borehole | 18 (7.3%) | 73 (23.9%) | 98 (27.4%) | 111 (24.2%) | 300 (21.9%) |
| Tap water | 0 (0%) | 0 (0%) | 238 (66.5%) | 0 (0%) | 238 (17.4%) |
| River | 113 (45.7%) | 67 (21.9%) | 15 (4.2%) | 129 (28.1%) | 324 (23.6%) |
| Well/Rain/River | 8 (3.2%) | 69 (22.5%) | 3 (0.8%) | 13 (2.8%) | 93 (6.8%) |
| Well/Rain/Borehole/River | 15 (6.1%) | 43 (14.1%) | 0 (0%) | 20 (4.4%) | 78 (5.7%) |
| Borehole and Rivers | 4 (1.6%) | 29 (9.5%) | 4 (1.1%) | 13 (2.8%) | 50 (3.6%) |
| Total | 247 (100%) | 306 (100%) | 358 (100%) | 459 (100%) | 1370 (100%) |
| Activities in the river | |||||
| Fetching water | 27 (19.3%) | 10 (4.8%) | 1 (4.5%) | 8 (4.6%) | 46 (8.4%) |
| Swimming | 44 (31.4%) | 37 (17.8%) | 21 (95.5%) | 57 (32.6%) | 159 (29.2%) |
| Bathing | 15 (10.7%) | 40 (19.2%) | - | 39 (22.3%) | 94 (17.2%) |
| Washing | 30 (21.4%) | 68 (32.7%) | - | 43 (24.6%) | 141 (25.9%) |
| Crossing river | 24 (7.1%) | 41 (19.7%) | - | 18 (10.3%) | 83 (15.2%) |
| Fishing | - | 12 (5.8%) | - | 10 (5.7%) | 22 (4.0%) |
| Total | 140 (100%) | 208 (100%) | 22 (100%) | 175 (100%) | 545 (100%) |
| LAC | No. Tested | No. Positive (%) | p-Value | WHO Prevalence Categories |
|---|---|---|---|---|
| Abaji | 247 | 64 (25.9) | <0.0001 | Moderate |
| AMAC | 306 | 150 (49) | Moderate | |
| Bwari | 358 | 22 (6.1) | Low | |
| Gwagwalada | 459 | 141 (30.7) | Moderate |
| Community | No. Tested | No. Positive | Prevalence of Schistosomiasis (%) | p-Value | Prevalence Level |
|---|---|---|---|---|---|
| Abaji LCA | |||||
| Abaji Central | 48 | 24 | 50.0 | High | |
| Dogon Ruwa | 51 | 22 | 43.1 | Moderate | |
| Gawu | 50 | 4 | 8.0 | Low | |
| Rimba gwari | 48 | 2 | 4.2 | Low | |
| Yaba | 50 | 12 | 24.0 | Moderate | |
| Total | 247 | 64 | 25.9 | <0.0001 * | Moderate |
| AMAC LCA | |||||
| Bassan Jiwa | 50 | 35 | 70.0 | High | |
| Gwagwa | 50 | 44 | 88.0 | High | |
| Karmo | 49 | 17 | 34.7 | Moderate | |
| Kpaipai | 55 | 15 | 27.3 | Moderate | |
| Rugan Fulani Dunamis | 50 | 23 | 46.0 | Moderate | |
| Toge Sabo | 52 | 16 | 30.8 | Moderate | |
| Total | 306 | 150 | 49.0 | <0.0001 * | Moderate |
| BWARI LCA | |||||
| Byazhin | 51 | 7 | 13.7 | Moderate | |
| Dutse Alhaji | 50 | 4 | 8.0 | Low | |
| Jigo | 54 | 3 | 5.6 | Low | |
| Katampe | 52 | 5 | 9.6 | Low | |
| Kogo | 50 | 0 | 0.0 | Low | |
| Shere | 49 | 1 | 2.0 | Low | |
| War college Camp, Ushafa | 52 | 2 | 3.8 | Low | |
| Total | 358 | 22 | 6.1 | 0.0549 | Moderate |
| GWAGWALADA LCA | |||||
| Angwan Bassa | 50 | 36 | 72.0 | High | |
| Angwan Dodo | 50 | 14 | 28.0 | Moderate | |
| Dagiri | 54 | 22 | 40.7 | Moderate | |
| Dobi | 50 | 16 | 32.0 | Moderate | |
| Dukpa | 50 | 4 | 8.0 | Low | |
| Ibwa | 50 | 7 | 14.0 | Moderate | |
| Kpakuru | 51 | 14 | 27.5 | Moderate | |
| Kpakuru Sarki | 50 | 6 | 12.0 | Moderate | |
| Paiko | 54 | 22 | 40.7 | Moderate | |
| Total | 459 | 141 | 30.7 | 0.00053 * | Moderate |
| LAC | No. Tested | No. Positive (%) | Light Infection (%) | Heavy Infection (%) | Total Egg Count | Mean Intensity | WHO Prevalence Categories |
|---|---|---|---|---|---|---|---|
| Abaji | 247 | 33 (13.4) | 30 (30.9) | 3 (1.2) | 621 | 19 | LI |
| AMAC | 306 | 138 (45.1) | 92 (66.7) | 46 (33.3) | 14,838 | 108 | HI |
| Bwari | 358 | 11 (3.1) | 10 (90.9) | 1 (9.1) | 212 | 19 | LI |
| Gwagwalada | 459 | 103 (22.4) | 85 (82.5) | 18 (17.5) | 3237 | 31 | LI |
| LAC | No. Tested | No. Positive (%) | LI (%) | MI (%) | HI (%) | Total Egg Count | Overall Mean Intensity | WHO Intensity Categories |
|---|---|---|---|---|---|---|---|---|
| Abaji | 247 | 36 (14.6) | 22 (61.1) | 11 (30.6) | 3 (8.3) | 6024 | 167 | MI |
| AMAC | 306 | 33 (10.8) | 6 (18.2) | 20 (60.6) | 7 (21.2) | 8736 | 265 | MI |
| Bwari | 358 | 12 (3.4) | 6 (50) | 5 (41.7) | 1 (8.3) | 2304 | 192 | MI |
| Gwagwalada | 459 | 53 (11.5) | 12 (22.6) | 30 (56.6) | 11 (20.8) | 13,104 | 247 | MI |
| LGA | Total Sampled | Male | Female | ||
|---|---|---|---|---|---|
| Sampled | Number Positive (Prevalence) | Sampled | Number Positive (Prevalence) | ||
| Abaji | 247 | 121 | 37 (30.6%) | 126 | 27 (21.4%) |
| AMAC | 306 | 158 | 82 (51.9%) | 148 | 68 (45.9%) |
| Bwari | 358 | 148 | 12 (8.1%) | 210 | 10 (4.8%) |
| Gwagwalada | 459 | 240 | 90 (37.5%) | 219 | 51 (23.3%) |
| Grand Total | 1370 | 667 | 221 (33.1%) | 703 | 156 (22.2%) |
| LAC | No. Examined | School-Aged Children | Adult | ||
|---|---|---|---|---|---|
| No. Tested | Number Positive (Prevalence) | No. Tested | Number Positive (Prevalence) | ||
| Abaji | 247 | 123 | 38 (30.9%) | 124 | 26 (21%) |
| AMAC | 306 | 149 | 87 (58.4%) | 157 | 63 (40.1%) |
| Bwari | 358 | 180 | 12 (6.7%) | 178 | 10 (5.6%) |
| Gwagwalada | 459 | 245 | 79 (32.2%) | 214 | 62 (29%) |
| Grand Total | 1370 | 697 | 216 (31.0%) | 673 | 161 (23.9%) |
| LAC | No. Examined | School-Aged Children | Adult | ||||
|---|---|---|---|---|---|---|---|
| No. Positive | Light Infection (<50 Eggs per mL) | Heavy Infection (>50 Eggs per mL) | No. Positive | Light Infection (<50 Eggs per mL) | Heavy Infection (>50 Eggs per mL) | ||
| Abaji | 247 | 123 | 121 (98.4) | 2 (1.6) | 124 | 123 (99.2) | 1 (0.8) |
| AMAC | 306 | 149 | 244 (98.8) | 3 (1.2) | 157 | 133 (84.70) | 24 (15.3) |
| Bwari | 358 | 180 | 179 (99.4) | 1 (0.6) | 178 | 178 (100.0) | 0 (0.0) |
| Gwagwalada | 459 | 245 | 234 (95.5) | 11 (4.5) | 214 | 207 (96.7) | 7 (3.3) |
| Grand Total | 1370 | 697 | 661 (94.8) | 36 (5.2) | 673 | 641 (95.2) | 32 (4.8) |
| LAC | No. Examined | School-Aged Children | Adult | ||||||
|---|---|---|---|---|---|---|---|---|---|
| No. Positive | Light Infection (1–99 epg) | Moderate Infection (100–399 epg) | Heavy Infection (≥400 epg) | No. Positive | Light Infection (1–99 epg) | Moderate Infection (100–399 epg) | Heavy Infection (≥400 epg) | ||
| Abaji | 247 | 21 | 14 (66.7%) | 4 (19.0%) | 3 (14.3%) | 15 | 8 (53.3%) | 7 (46.7%) | 0 (0%) |
| AMAC | 306 | 20 | 3 (15.0%) | 13 (65.0%) | 4 (20.0%) | 13 | 3 (23.1%) | 7 (53.8%) | 3 (23.1%) |
| Bwari | 358 | 7 | 3 (42.9%) | 4 (57.1%) | 0 (%) | 5 | 3 (60.0%) | 1 (20.0%) | 1 (20.0%) |
| Gwagwalada | 459 | 30 | 6 (20.0%) | 16 (53.3%) | 8 (26.7%) | 33 | 6 (26.1%) | 14 (60.9%) | 3 (13.0%) |
| Grand Total | 1370 | 78 | 26 (33.3%) | 37 (47.4%) | 15 (19.2%) | 66 | 20 (30.3%) | 29 (43.9) | 7 (10.6) |
| Source of Water | No. Examined | No. Positive (%) | p Value |
|---|---|---|---|
| Well/Rain | 287 | 0 (0%) | <0.0001 |
| Borehole | 300 | 0 (0%) | |
| Tap water | 238 | 0 (0%) | |
| River | 324 | 203 (62.7%) | |
| Well/Rain/River | 93 | 66 (71%) | |
| Well/Rain/borehole/Rivers | 78 | 63 (80.8%) | |
| Borehole and Rivers | 50 | 45 (90%) | |
| Total | 1370 | 377 (27.5%) |
| Activities in the River | Total Number Examined | No. Infected (%) | p-Value |
|---|---|---|---|
| Fetching | 46 | 31 (67.4%) | <0.0001 |
| Swimming | 159 | 132 (83%) | |
| Bathing | 94 | 58(61.7%) | |
| Washing | 141 | 90 (63.8%) | |
| Crossing water | 83 | 46 (55.4%) | |
| Fishing | 22 | 20(90.9%) | |
| Total | 545 | 377(69.2%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jacob, S.M.; Akinbo, S.Y.; Oluwole, A.S.; Agbana, T.; Omoruyi, Z.; Okungbowa, M.A.; Diehl, J.-C.; Akinbo, F.O. Impact of Praziquantel Mass Drug Administration on Schistosomiasis: A Comparison of Prevalence and Risk Factors Between Treated School Aged Children and Untreated Adults in Abuja, Nigeria. Int. J. Environ. Res. Public Health 2025, 22, 672. https://doi.org/10.3390/ijerph22050672
Jacob SM, Akinbo SY, Oluwole AS, Agbana T, Omoruyi Z, Okungbowa MA, Diehl J-C, Akinbo FO. Impact of Praziquantel Mass Drug Administration on Schistosomiasis: A Comparison of Prevalence and Risk Factors Between Treated School Aged Children and Untreated Adults in Abuja, Nigeria. International Journal of Environmental Research and Public Health. 2025; 22(5):672. https://doi.org/10.3390/ijerph22050672
Chicago/Turabian StyleJacob, Solomon M., Sophie Y. Akinbo, Akinola S. Oluwole, Temitope Agbana, Zainab Omoruyi, Michael A. Okungbowa, Jan-Carel Diehl, and Fredrick O. Akinbo. 2025. "Impact of Praziquantel Mass Drug Administration on Schistosomiasis: A Comparison of Prevalence and Risk Factors Between Treated School Aged Children and Untreated Adults in Abuja, Nigeria" International Journal of Environmental Research and Public Health 22, no. 5: 672. https://doi.org/10.3390/ijerph22050672
APA StyleJacob, S. M., Akinbo, S. Y., Oluwole, A. S., Agbana, T., Omoruyi, Z., Okungbowa, M. A., Diehl, J.-C., & Akinbo, F. O. (2025). Impact of Praziquantel Mass Drug Administration on Schistosomiasis: A Comparison of Prevalence and Risk Factors Between Treated School Aged Children and Untreated Adults in Abuja, Nigeria. International Journal of Environmental Research and Public Health, 22(5), 672. https://doi.org/10.3390/ijerph22050672





