COVID-19 Disease and Economic Burden to Healthcare Systems in Adults in Six Latin American Countries Before Nationwide Vaccination Program: Ministry of Health Database Assessment and Literature Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Inclusion and Exclusion Criteria
2.2. Design of Studies Included
2.3. Risk of Bias
2.4. Epidemiological and Cost Outcomes
2.5. Statistical Analysis
3. Results
3.1. Hospitalizations
3.2. Mortality
3.3. General Characteristics and Risk of Bias of the Included Studies
3.4. Years of Life Lost and Excess Mortality
3.5. Economic Burden of COVID-19
3.6. Other Vaccine-Preventable Diseases
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- ECLAC United Nations. The Sociodemographic Impacts of the COVID-19 Pandemic in Latin America and the Caribbean. 2022. Available online: https://repositorio.cepal.org/server/api/core/bitstreams/10c71b97-a147-4349-b7ee-7fb952f950c6/content (accessed on 1 March 2025).
- Mathieu, E.; Ritchie, H.; Rodés-Guirao, L.; Appel, C.; Giattino, C.; Hasell, J.; Macdonald, B.; Dattani, S.; Beltekian, D.; Ortiz-Ospina, E.; et al. Our World in Data. Available online: https://ourworldindata.org/coronavirus (accessed on 1 March 2025).
- Schwalb, A.; Armyra, E.; Méndez-Aranda, M.; Ugarte-Gil, C. COVID-19 in Latin America and the Caribbean: Two years of the pandemic. J. Intern. Med. 2022, 292, 409–427. [Google Scholar] [CrossRef] [PubMed]
- United Nations. Economic Commission for Latin America and the Caribbean. Social panorama of Latin America 2021; United Nations: New York, NY, USA, 2023. [Google Scholar]
- COVID-19 in Latin America and the Caribbean: An Overview of Government Responses to the Crisis. In COVID-19 in Latin America and the Caribbean: An Overview of Government Responses to the Crisis; OECD Publishing: Paris, France, 2020.
- Vargas, E.D.; Sanchez, G.R. COVID-19 is having a devastating impact on the economic well-being of Latino families. J. Econ. Race Pol. 2020, 3, 262–269. [Google Scholar] [CrossRef]
- Excler, J.-L.; Saville, M.; Privor-Dumm, L.; Gilbert, S.; Hotez, P.J.; Thompson, D.; Abdool-Karim, S.; Kim, J.H. Factors, enablers and challenges for COVID-19 vaccine development. BMJ Glob. Health 2023, 8, e011879. [Google Scholar] [CrossRef]
- Pennisi, F.; Genovese, C.; Gianfredi, V. Lessons from the COVID-19 pandemic: Promoting vaccination and public health resilience, a narrative review. Vaccines 2024, 12, 891. [Google Scholar] [CrossRef]
- Titball, R.W.; Bernstein, D.I.; Fanget, N.V.J.; Hall, R.A.; Longet, S.; MacAry, P.A.; Rupp, R.E.; van Gils, M.; von Messling, V.; Walker, D.H.; et al. Progress with COVID vaccine development and implementation. NPJ Vaccines 2024, 9, 69. [Google Scholar] [CrossRef] [PubMed]
- Taborda, A.; Murillo, D.A.; Moreno, C.; Taborda, P.A.; Fuquen, M.; Díaz, P.A.; Londoño, D. Analysis of budgetary impact of COVID-19 vaccination in Latin America. Análise do impacto orçamentário da vacinação contra a COVID-19 na América Latina. Rev. Panam. Salud Publica 2022, 46, e5. [Google Scholar] [CrossRef]
- Golan, A.; Mumladze, T.; Perloff, J.M.; Wilson, D. An information-theoretic method for identifying effective treatments and policies at the beginning of a pandemic. Entropy 2024, 26, 1021. [Google Scholar] [CrossRef]
- Prieto, A.; Huang, R.; Eusebi, C.A.; Shostak, M. A Brief Overview of Emerging Vaccine Technologies for Pandemic Preparedness; Rand Corporation: Santa Monica, CA, USA, 2023; Volume 11, p. 6. Available online: https://www.ncbi.nlm.nih.gov/pubmed/38264321 (accessed on 1 March 2025).
- UNESCO Office Montevideo and Regional Bureau for Science in Latin America and the Caribbean; Vélez, C. COVID-19 and vaccination in Latin America and the Caribbean: Challenges, needs and opportunities; UNESCO: Montevideo, Uruguay, 2021; p. 88. [Google Scholar]
- Covidence: Software for Systematic Review Management. Available online: https://www.covidence.org (accessed on 1 February 2024).
- Quality Assessment Tool for Observational Cohort and Cross-Sectional Studies. 2017. Available online: https://www.researchgate.net/profile/Irismar-Encarnacao/post/How-can-I-rate-as-good-fair-or-poor-using-Quality-Assessment-Tool-for-Observational-Cohort-and-Cross-Sectional-Studies/attachment/5db20e84cfe4a777d4e9b51d/AS%3A817632605376513%401571950212542/download/Quality-Assessment-Tool-for-Observational-Cohort-and-Cross-Sectional-Studies-NHLBI-NIH+%283%29.pdf (accessed on 1 February 2024).
- Manejo Clínico de la COVID-19: Orientaciones Evolutivas. 25 January 2021. Available online: https://www.who.int/es/publications/i/item/WHO-2019-nCoV-clinical-2021-1 (accessed on 6 March 2025).
- Martinez, R.; Soliz, P.; Caixeta, R.; Ordunez, P. Reflection on modern methods: Years of life lost due to premature mortality-a versatile and comprehensive measure for monitoring non-communicable disease mortality. Int. J. Epidemiol. 2019, 48, 1367–1376. [Google Scholar] [CrossRef]
- Msemburi, W.; Karlinsky, A.; Knutson, V.; Aleshin-Guendel, S.; Chatterji, S.; Wakefield, J. The WHO estimates of excess mortality associated with the COVID-19 pandemic. Nature 2023, 613, 130–137. [Google Scholar] [CrossRef]
- Mogyorosy, Z.; Smith, P.C. The Main Methodological Issues in Costing Health Care Services—A Literature Review. (CHE Research Paper). Centre for Health Economics, University of York. 2005; Available online: https://www.york.ac.uk/media/che/documents/papers/researchpapers/rp7_Methodological_issues_in_costing_health_care_services.pdf (accessed on 6 March 2025).
- World Health Organization. Living Guidance for Clinical Management of COVID-19. WHO/2019-nCoV/clinical/2021.2. Available online: https://apps.who.int/iris/bitstream/handle/10665/349321/WHO-2019-nCoV-clinical-2021.2-eng.pdf (accessed on 6 March 2025).
- Johnston, R.J.; Wainger, L.A. Benefit transfer for ecosystem service valuation: An introduction to theory and methods. In Benefit Transfer of Environmental and Resource Values; Springer: Dordrecht, Netherlands, 2015; pp. 237–273. [Google Scholar] [CrossRef]
- Rosenberger, R.S.; Stanley, T.D. Measurement, generalization, and publication: Sources of error in benefit transfers and their management. Ecol. Econ. 2006, 60, 372–378. [Google Scholar] [CrossRef]
- Navrud, S.; Ready, R. Review of methods for value transfer. In Environmental Value Transfer: Issues and Methods; Springer: Dordrecht, Netherlands, 2007; pp. 1–10. [Google Scholar] [CrossRef]
- SIGTAP—Sistema de Gerenciamento da Tabela de Procedimentos. Medicamentos e OPM do SUS. Available online: http://sigtap.datasus.gov.br/tabela-unificada/app/sec/inicio.jsp?first=10 (accessed on 6 March 2025).
- Muñoz, C.F. Manual Tarifario de Salud SOAT 2023—Versión PDF; CONSULTORSALUD: Bogotá, Colombia, 2023; Available online: https://consultorsalud.com/manual-tarifario-de-salud-soat-2023-version-pdf/ (accessed on 6 March 2025).
- Manual Tarifario SOAT 2001. Available online: https://lexsaludcolombia.files.wordpress.com/2010/10/tarifas-iss-2001.pdf (accessed on 6 March 2025).
- Ochisap CGC. Eventual Reforma de FONASA Hacia Una Particular ISAPRE Pública, Con dos Categorías Más FONASA PLUS. Available online: https://www.ochisap.cl/wp-content/uploads/2023/04/Reforma-de-FONASA-2023-C.-Gattini-OCHISAP.pdf (accessed on 6 March 2025).
- Oficial Web Page Kairos—El Portal Farmacéutico. Available online: https://kairosweb.com/ (accessed on 6 March 2025).
- Ministerio de Salud y Protección Social. Termómetro de Precios de Medicamentos. Available online: https://www.minsalud.gov.co/salud/MT/Paginas/termometro-de-precios.aspx (accessed on 6 March 2025).
- OPPF. Plataforma del Estado Peruano. Available online: https://opm-digemid.minsa.gob.pe/#/consulta-producto (accessed on 6 March 2025).
- Extended National Consumer Price Index. Available online: https://www.ibge.gov.br/en/statistics/economic/prices-and-costs/17129-extended-national-consumer-price-index.html?=&t=series-historicas (accessed on 25 October 2023).
- INDEC (Instituto Nacional de Estadistica y Censos). de la REPUBLICA ARGENTINA. INDEC: Instituto Nacional de Estadística y Censos de la República Argentina. Available online: https://www.indec.gob.ar/ (accessed on 30 October 2023).
- DANE (Departamentp Administrativo Nacional de Estadística)—IPC Información Técnica. Available online: https://www.dane.gov.co/index.php/estadisticas-por-tema/precios-y-costos/indice-de-precios-al-consumidor-ipc/ipc-informacion-tecnica (accessed on 25 October 2023).
- Chile INE. Calculadora IPC; Instituto Nacional de Estadística-INE: Santiago, Chile; Available online: https://calculadoraipc.ine.cl/ (accessed on 26 October 2023).
- Banco Central de Reserva del Perú. Available online: https://www.bcrp.gob.pe/ (accessed on 30 October 2023).
- Banco Central de la República Argentina. Available online: https://www.bcra.gob.ar/ (accessed on 13 March 2024).
- Banco Central do Brasil. Available online: https://www.bcb.gov.br/conversao (accessed on 13 March 2024).
- Banco Central de Chile. Available online: https://www.bcentral.cl/inicio (accessed on 13 March 2024).
- Banco de la República de Colombia. Available online: https://www.banrep.gov.co/es (accessed on 13 March 2024).
- Instituto Nacional de Salud Colombia. Coronavirus Colombia. Power BI Report. Available online: https://www.ins.gov.co/Noticias/Paginas/Coronavirus.aspx (accessed on 3 March 2024).
- Power BI Report Casos Oficiales Chile. Seguimiento COVID-19 en Chile. In Datos COVID-19 de Chile en el repositorio del Ministerio de Ciencia, Tecnología, Conocimieinto e Innovación en GitHub. Available online: https://app.powerbi.com/view?r=eyJrIjoiMTc0ODBlNjYtNmUyYS00ZmU2LWE2NjMtZmNmOGFlYzA4YWNhIiwidCI6IjBlZGVkN2QyLWMwNGMtNGRjMi05YWFjLTYzZjlkNDY1ODliOCIsImMiOjF9 (accessed on 3 March 2025).
- Giglio, N.D.; Castellano, V.E.; Mizrahi, P.; Micone, P.V. Cost-Effectiveness of Pneumococcal Vaccines for Adults Aged 65 Years and Older in Argentina. Value Health Reg. Issues 2022, 28, 76–81. [Google Scholar] [CrossRef]
- Michelin, L.; Weber, F.M.; Scolari, B.W.; Menezes, B.K.; Gullo, M.C. Mortality and costs of pneumococcal pneumonia in adults: A cross-sectional study. J. Bras. Pneumol. 2019, 45, e20180374. [Google Scholar] [CrossRef]
- Castañeda-Orjuela, C.; Alvis-Guzmán, N.; Paternina, Á.J.; De la Hoz-Restrepo, F. Cost-effectiveness of the introduction of the pneumococcal polysaccharide vaccine in elderly Colombian population. Vaccine 2011, 29, 7644–7650. [Google Scholar] [CrossRef]
- Pugh, S.; Wasserman, M.; Moffatt, M.; Marques, S.; Reyes, J.M.; Prieto, V.A.; Reijnders, D.; Rozenbaum, M.H.; Laine, J.; Åhman, H.; et al. Estimating the Impact of Switching from a Lower to Higher Valent Pneumococcal Conjugate Vaccine in Colombia, Finland, and The Netherlands: A Cost-Effectiveness Analysis. Infect. Dis. Ther. 2020, 9, 305–324. [Google Scholar] [CrossRef]
- Ordóñez, J.E.; Ordóñez, A. A cost-effectiveness analysis of pneumococcal conjugate vaccines in infants and herd protection in older adults in Colombia. Expert. Rev. Vaccines 2023, 22, 2184090. [Google Scholar] [CrossRef]
- Urueña, A.; Micone, P.; Magneres, C.; Mould-Quevedo, J.; Giglio, N. Cost-Effectiveness Analysis of Switching from Trivalent to Quadrivalent Seasonal Influenza Vaccine in Argentina. Vaccines 2021, 9, 335. [Google Scholar] [CrossRef]
- Nguyen, V.H.; Vizzotti, C.; Uruena, A.; Giglio, N.; Magneres, C.; Richmond, H. Cost-effectiveness of introducing an MF59-adjuvanted trivalent influenza vaccine for older adults in Argentina. Vaccine 2020, 38, 3682–3689. [Google Scholar] [CrossRef]
- Tinoco, Y.O.; Azziz-Baumgartner, E.; Rázuri, H.; Kasper, M.R.; Romero, C.; Ortiz, E.; Gomez, J.; Widdowson, M.; Uyeki, T.M.; Gilman, R.H.; et al. A population-based estimate of the economic burden of influenza in Peru, 2009–2010. Influenza Other Respi Viruses 2016, 10, 301–309. [Google Scholar] [CrossRef]
- Burki, T. COVID-19 in Latin America. Lancet Infect. Dis. 2020, 20, 547–548. [Google Scholar] [CrossRef]
- Diseases, C. Living Guidance for Clinical Management of COVID-19; World Health Organization: Geneva, Switzerland, 2021; Available online: https://www.who.int/publications/i/item/WHO-2019-nCoV-clinical-2021-2 (accessed on 13 March 2024).
- Kim, L.; Garg, S.; O’halloran, A.; Whitaker, M.; Pham, H.; Anderson, E.J.; Armistead, I.; Bennett, N.M.; Billing, L.; Como-Sabetti, K.; et al. Risk factors for intensive care unit admission and in-hospital mortality among hospitalized adults identified through the US Coronavirus Disease 2019 (COVID-19)-Associated Hospitalization Surveillance Network (COVID-NET). Clin. Infect. Dis. 2021, 72, e206–e214. [Google Scholar] [CrossRef]
- Vardavas, C.I.; Mathioudakis, A.G.; Nikitara, K.; Stamatelopoulos, K.; Georgiopoulos, G.; Phalkey, R.; Leonardi-Bee, J.; Fernandez, E.; Carnicer-Pont, D.; Vestbo, J.; et al. Prognostic factors for mortality, intensive care unit and hospital admission due to SARS-CoV-2: A systematic review and meta-analysis of cohort studies in Europe. Eur. Respir. Rev. 2022, 31, 220098. [Google Scholar] [CrossRef] [PubMed]
- Harris, E. COVID-19 Hospitalizations Up Among Older Adults. JAMA 2023, 330, 1611. [Google Scholar] [CrossRef] [PubMed]
- Olivas-Martínez, A.; Cárdenas-Fragoso, J.L.; Jiménez, J.V.; Lozano-Cruz, O.A.; Ortiz-Brizuela, E.; Tovar-Méndez, V.H.; Medrano-Borromeo, C.; Martínez-Valenzuela, A.; Román-Montes, C.M.; Martínez-Guerra, B.; et al. In-hospital mortality from severe COVID-19 in a tertiary care center in Mexico City; causes of death, risk factors and the impact of hospital saturation. PLoS ONE 2021, 16, e0245772. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Soto, M.C.; Ortega-Cáceres, G. Analysis of Excess All-Cause Mortality and COVID-19 Mortality in Peru: Observational Study. Trop. Med. Infect. Dis. 2022, 7, 44. [Google Scholar] [CrossRef]
- Merlo, F.; Lepori, M.; Malacrida, R.; Albanese, E.; Fadda, M. Physicians’ acceptance of the Swiss Academy of Medical Sciences guidelines “COVID-19 pandemic: Triage for intensive-care treatment under resource scarcity”. Swiss Med. Wkly. 2021, 151, w20472. [Google Scholar] [CrossRef]
- Liu, X.; Li, X.; Sun, T.; Qin, H.; Zhou, Y.; Zou, C.; Cao, J.; Zhang, H. East-West differences in clinical manifestations of COVID-19 patients: A systematic literature review and meta-analysis. J. Med. Virol. 2021, 93, 2683–2693. [Google Scholar] [CrossRef]
- Valdez Huarcaya, W.; Miranda Monzón, J.A.; Napanga Saldaña, E.O.; Driver, C.R. Impacto de la COVID-19 en la mortalidad en Perú mediante la triangulación de múltiples fuentes de datos. Mortal. Perú Mediante Triangulación Múltiples Fuentes Datos Rev. Panam. Salud Publica 2022, 2022, e53. [Google Scholar] [CrossRef]
- Gianino, M.M.; Savatteri, A.; Politano, G.; Nurchis, M.C.; Pascucci, D.; Damiani, G. Burden of COVID-19: Disability-Adjusted Life Years (DALYs) across 16 European countries. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 5529–5541. [Google Scholar] [CrossRef]
- dos Santos, A.M.; de Souza, B.F.; de Carvalho, C.A.; Campos, M.A.G.; de Oliveira, B.L.C.A.; Diniz, E.M.; Branco, M.d.R.F.C.; Queiroz, R.C.d.S.; de Carvalho, V.A.; Araújo, W.R.M.; et al. Excess deaths from all causes and by COVID-19 in Brazil in 2020. Rev. Saude Publica 2021, 55, 71. [Google Scholar] [CrossRef]
- Mejía, L.S.P.; Fernández, J.L.W.; Hernández, I.O.; Ridaura, R.L.; Ramirez, H.L.-G.; Avila, M.H.; Ávila, J.E.H.; Mortalidad, G.I.P.l.E.d.E.d. Estimación del exceso de mortalidad por todas las causas durante la pandemia del Covid-19 en México. Salud Publica De Mex. 2021, 63, 211–224. [Google Scholar] [CrossRef]
- Alenzi, K.A.; Al-Malky, H.S.; Altebainawi, A.F.; Abushomi, H.Q.; Alatawi, F.O.; Atwadi, M.H.; Khobrani, M.A.; Almazrou, D.A.; Alrubeh, N.; Alsoliabi, Z.A.; et al. Health economic burden of COVID-19 in Saudi Arabia. Front. Public Health 2022, 10, 927494. [Google Scholar] [CrossRef] [PubMed]
- Perlaza, C.L.; Mosquera, F.E.C.; Reyes, S.P.M.; Salazar, S.M.T.; Rojas, A.F.C.; Serna, J.D.E.; Liscano, Y. Sociodemographic, Clinical, and Ventilatory Factors Influencing COVID-19 Mortality in the ICU of a Hospital in Colombia. Healthcare 2024, 12, 2294. [Google Scholar] [CrossRef] [PubMed]
- Páginas—Ministry of Health and Social Protection. Available online: https://www.minsalud.gov.co/English/Paginas/inicio.aspx (accessed on 5 March 2025).
- Pifarré I Arolas, H.; Acosta, E.; López-Casasnovas, G.; Lo, A.; Nicodemo, C.; Riffe, T.; Myrskylä, M. Years of Life Lost to COVID-19 in 81 Countries. Sci. Rep. 2021, 11, 3504. [Google Scholar] [CrossRef]
- de Castro, A.P.B.; Moreira, M.F.; de Souza Bermejo, P.H.; Rodrigues, W.; Prata, D.N. Mortality and Years of Potential Life Lost Due to COVID-19 in Brazil. Int. J. Environ. Res. Public Health 2021, 18, 7626. [Google Scholar] [CrossRef]
- Islam, N.; Jdanov, D.A.; Shkolnikov, V.M.; Khunti, K.; Kawachi, I.; White, M.; Lewington, S.; Lacey, B. Effects of Covid-19 Pandemic on Life Expectancy and Premature Mortality in 2020: Time Series Analysis in 37 Countries. BMJ 2021, 375, e066768. [Google Scholar] [CrossRef]
- Ugarte, M.P.; Achilleos, S.; Quattrocchi, A.; Gabel, J.; Kolokotroni, O.; Constantinou, C.; Nicolaou, N.; Rodriguez-Llanes, J.M.; Huang, Q.; Verstiuk, O.; et al. Premature Mortality Attributable to COVID-19: Potential Years of Life Lost in 17 Countries around the World, January–August 2020. BMC Public Health 2022, 22, 1–13. [Google Scholar] [CrossRef]
- Salinas-Escudero, G.; Toledano-Toledano, F.; García-Peña, C.; Parra-Rodríguez, L.; Granados-García, V.; Carrillo-Vega, M.F. Disability-Adjusted Life Years for the COVID-19 Pandemic in the Mexican Population. Front. Public Health 2021, 9, 686700. [Google Scholar] [CrossRef]
- Rearte, A.; Moisés, M.S.; Rueda, D.V.; Laurora, M.A.; Marucco, A.F.; Pennini, V.A.; Giovacchini, C.M.; Guevel, C.; Vizzoti, C. All-cause excess mortality during the COVID-19 pandemic in Argentina, 2020. Rev. Argent. Salud Publica 2021, 13, 18. [Google Scholar]
- Quapper, D.D. Excess deaths due to the COVID-19 pandemic in Chile. Rev. Med Chil. 2021, 149, 1525–1531. [Google Scholar] [CrossRef]
- Benavides, F.G.; Vives, A.; Zimmerman, M.; Silva-Peñaherrera, M. Excess Mortality in 2020 in the Working-Age Population of Nine Latin American countriesExcesso de Mortalidade Na População Em Idade Ativa Em Nove Países Da América Latina No Ano de 2020. Rev. Panam. De Salud Publica (Pan Am. J. Public Health) 2022, 46, e75. [Google Scholar] [CrossRef]
- Araujo, M.; Ossandón, P.; Abarca, A.M.; Menjiba, A.M.; Muñoz, A.M. Prognosis of patients with COVID-19 admitted to a tertiary center in Chile: A cohort study. Medwave 2020, 20, e8066. [Google Scholar] [CrossRef] [PubMed]
- Lima, E.E.C.; Vilela, E.A.; Peralta, A.; Rocha, M.; Queiroz, B.L.; Gonzaga, M.R.; Piscoya-Díaz, M.; Martinez-Folgar, K.; García-Guerrero, V.M.; Freire, F.H.M.A. Investigating Regional Excess Mortality during 2020 COVID-19 Pandemic in Selected Latin American Countries. Genus 2021, 77, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Macchia, A.; Ferrante, D.; Battistella, G.; Mariani, J.; González Bernaldo de Quirós, F. COVID-19 among the Inhabitants of the Slums in the City of Buenos Aires: A Population-Based Study. BMJ Open 2021, 11, e044592. [Google Scholar] [CrossRef]
- Machado-Alba, J.E.; Valladales-Restrepo, L.F.; Machado-Duque, M.E.; Gaviria-Mendoza, A.; Sánchez-Ramírez, N.; Usma-Valencia, A.F.; Rodríguez-Martínez, E.; Rengifo-Franco, E.; Forero-Supelano, V.H.; Gómez-Ramirez, D.M.; et al. Factors Associated with Admission to the Intensive Care Unit and Mortality in Patients with COVID-19, Colombia. PLOS ONE 2021, 16, e0260169. [Google Scholar] [CrossRef]
- Arias Ramos, D.; Restrepo Rueda, D.L.; Rios Quintero, E.V.; Olaya Gómez, J.C.; Cortés Bonilla, I. Severe and Critical COVID-19 in a Tertiary Center in Colombia, a Retrospective Cross-Sectional Study. BMC Infect. Dis. 2022, 22, 247. [Google Scholar] [CrossRef]
- Schönfeld, D.; Arias, S.; Bossio, J.C.; Fernández, H.; Gozal, D.; Pérez-Chada, D. Clinical Presentation and Outcomes of the First Patients with COVID-19 in Argentina: Results of 207079 Cases from a National Database. PLOS ONE 2021, 16, e0246793. [Google Scholar] [CrossRef]
- COVID-19 Excess Mortality Collaborators Estimating Excess Mortality due to the COVID-19 Pandemic: A Systematic Analysis of COVID-19-Related Mortality, 2020-21. Lancet 2022, 399, 1513–1536. [CrossRef]
- Javier, R.D.; Daniela, O.M.; Daniela, G.A.; Julián, B.J.; Camila, B.O.; Marieliz, R.C.H.; María Luisa, R.Z.; Loreto, R.W.; Cristian, M.A.; Carlos, I.P.; et al. COVID-19 en adultos en el Hospital de Puerto Montt en la primera etapa de la pandemia. Rev. méd. Chile 2022, 150, 465–472. [Google Scholar] [CrossRef]
- Reyes, L.F.; Bastidas, A.; Narváez, P.O.; Parra-Tanoux, D.; Fuentes, Y.V.; Serrano-Mayorga, C.C.; Ortíz, V.; Caceres, E.L.; Ospina-Tascon, G.; Díaz, A.M.; et al. Clinical Characteristics, Systemic Complications, and in-Hospital Outcomes for Patients with COVID-19 in Latin America. LIVEN-Covid-19 Study: A Prospective, Multicenter, Multinational, Cohort Study. PLOS ONE 2022, 17, e0265529. [Google Scholar] [CrossRef]
- González, F.J.; Miranda, F.A.; Chávez, S.M.; Gajardo, A.I.; Hernández, A.R.; Guiñez, D.V.; Díaz, G.A.; Sarmiento, N.V.; Ihl, F.E.; Cerda, M.A.; et al. Clinical Characteristics and in-Hospital Mortality of Patients with COVID-19 in Chile: A Prospective Cohort Study. Int. J. Clin. Pract. 2021, 75, e14919. [Google Scholar] [CrossRef]
- Ñamendys-Silva, S.A.; Alvarado-Ávila, P.E.; Domínguez-Cherit, G.; Rivero-Sigarroa, E.; Sánchez-Hurtado, L.A.; Gutiérrez-Villaseñor, A.; Romero-González, J.P.; Rodríguez-Bautista, H.; García-Briones, A.; Garnica-Camacho, C.E.; et al. Outcomes of Patients with COVID-19 in the Intensive Care Unit in Mexico: A Multicenter Observational Study. Heart Lung 2021, 50, 28–32. [Google Scholar] [CrossRef]
- Andrade, A.L.; Afonso, E.T.; Minamisava, R.; Bierrenbach, A.L.; Cristo, E.B.; Morais-Neto, O.L.; Policena, G.M.; Cmas, D.; Toscano, C.M. Direct and Indirect Impact of 10-Valent Pneumococcal Conjugate Vaccine Introduction on Pneumonia Hospitalizations and Economic Burden in All Age-Groups in Brazil: A Time-Series Analysis. PloS ONE 2017, 12, e0184204. [Google Scholar] [CrossRef] [PubMed]
- Biagini, L.; Pezzani, M.; Rojas, R.; Fuentealba, F. Cost-Utility Study of PCV13 Versus PPSV23 in Adults in Chile. Value Health Reg. Issues 2018, 17, 194–201. [Google Scholar] [CrossRef]
- Cáceres, R.C.; Aj, L.M.; La, P.G.; Ortegón, V.V.; Posso, P.M.; Flórez, E.V.; Gómez, L.E.; Sj, V.F.; Mc, R.L.; Pa, C.L. General Hospitalization and Intensive Care Unit-Related Factors of COVID-19 Patients in Northeastern Colombia: Baseline Characteristics of a Cohort Study. Cureus 2023, 15, e43888. [Google Scholar] [CrossRef] [PubMed]
- Soto-Cabezas, M.G.; Reyes-Vega, M.F.; Soriano-Moreno, A.N.; Ordoñez-Ibargüen, L.; Martel, K.S.; Flores-Jaime, N.; Chirinos-Saire, J.; Velásquez, J.P.; Munayco, C.V. Comorbilidades Asociadas a La Mortalidad Por COVID-19 En Adultos En Lima, Perú: Un Estudio de Cohorte Retrospectiva. Rev. Peru. De Med. Exp. Y Salud Publica 2023, 40, 132–140. [Google Scholar] [CrossRef]
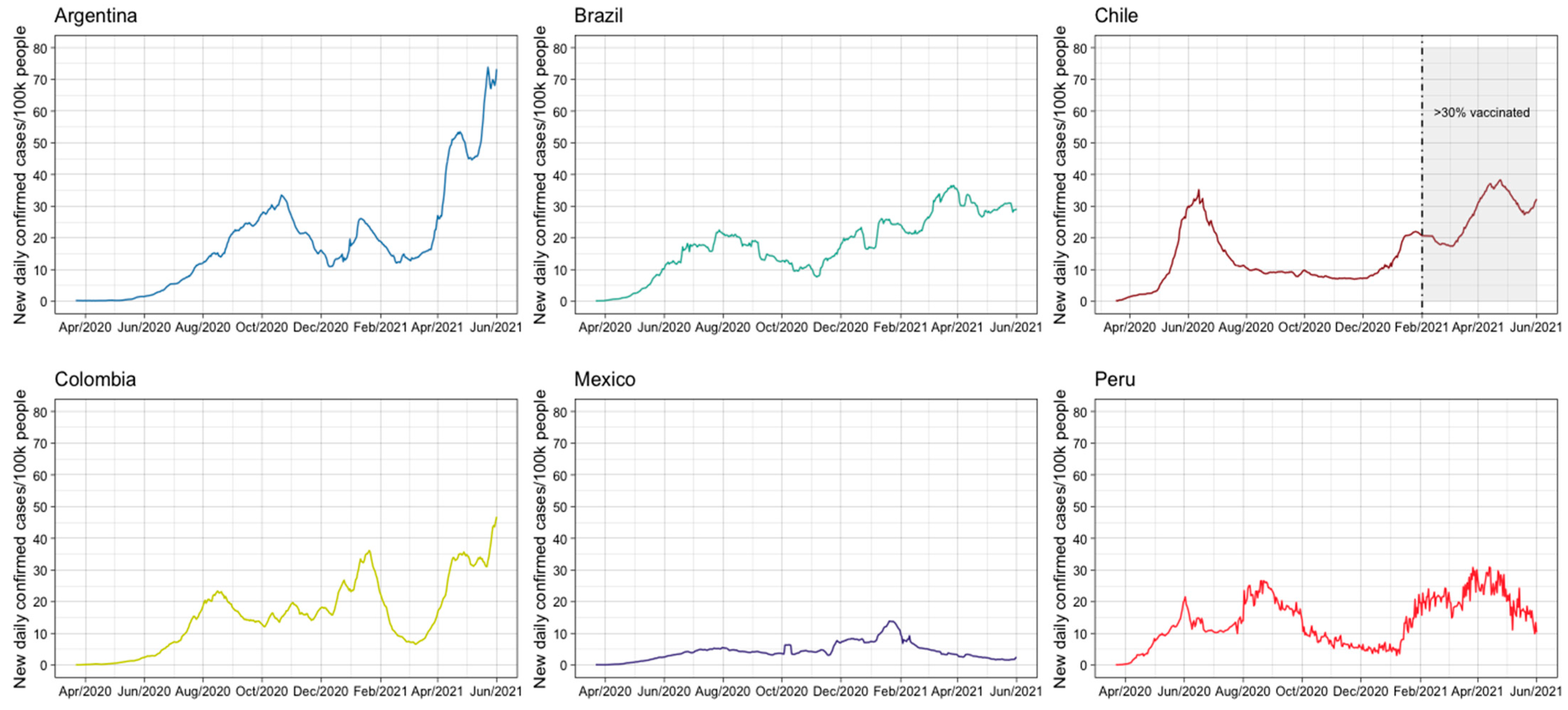
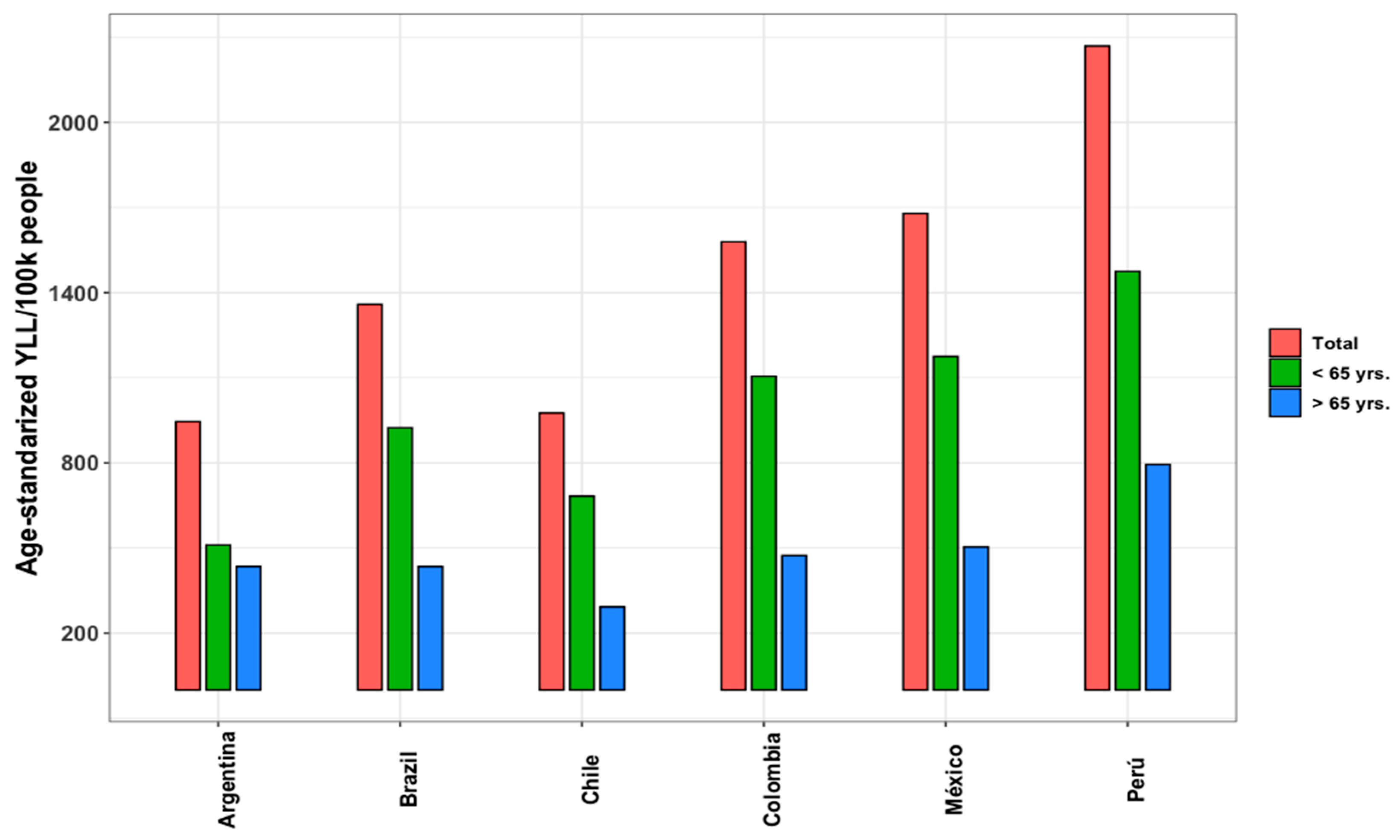
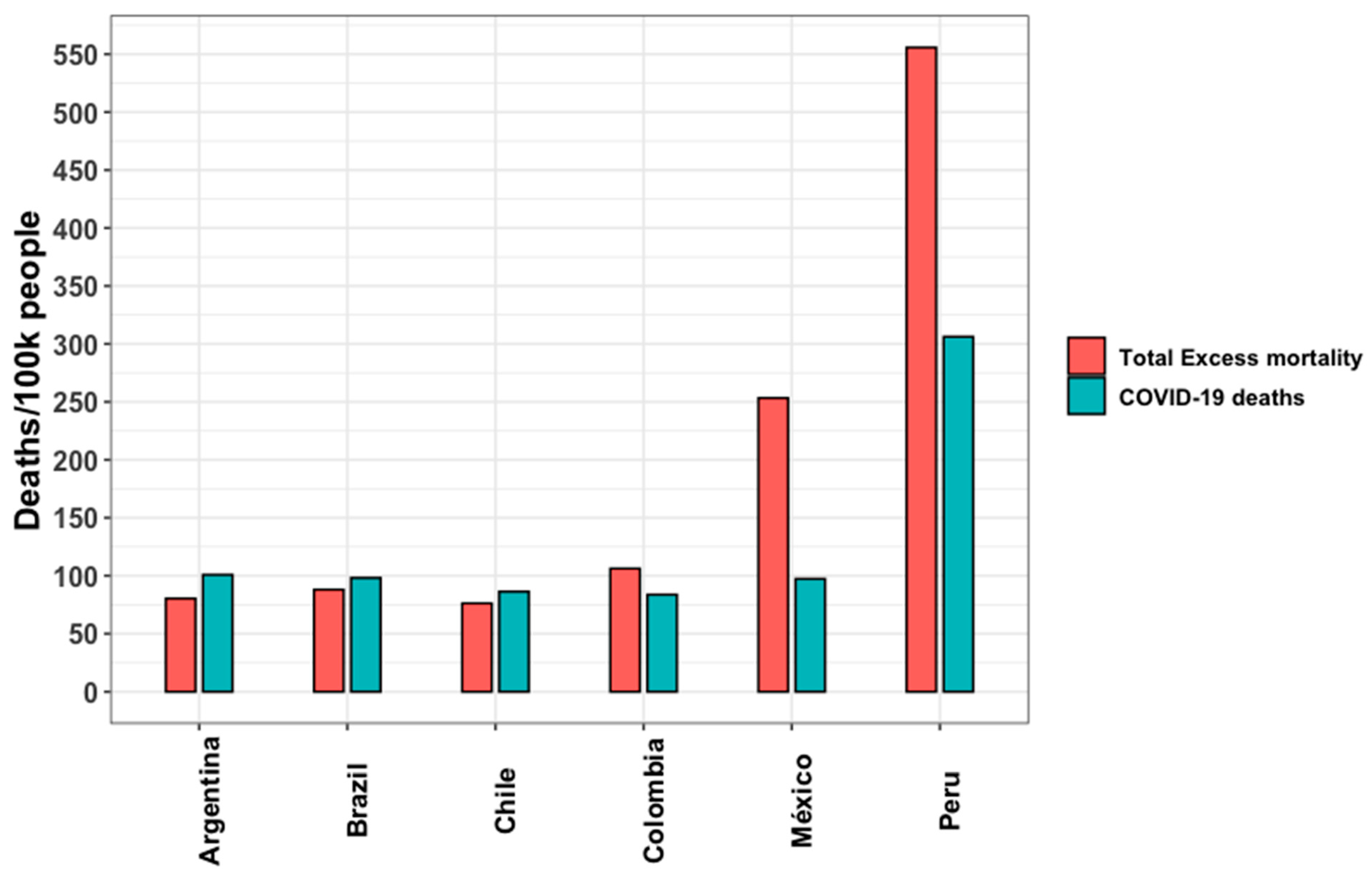
| Indicators | Argentina | Brazil | Chile | Colombia | Mexico | Peru |
|---|---|---|---|---|---|---|
| COVID-19 All cases, N (%) | 3,711,250 (100) | 11,956,157 (100) | 728,812 (100) | 3,240,355 (100) | 2,363,214 (100) | 1,867,034 (100) |
| Mild n (%) | 3,544,077 (95) | 10,459,717 (88) | 675,039 * (93) | 3,133,423 * (97) | 1,898,410 (80) | 1,768,939 (94) |
| Moderate and Severe n (%) | 133,339 (4) | 836,398 (7) | 35,236 * (5) | 69,900 * (2) | 427,694 (18) | 86,796 (5) |
| Critical n (%) | 33,834 (1) | 478,171 (4) | 18,537 (2) | 33,503 * (1) | 37,110 (2) | 11,299 (1) |
| Missing data | 0 | 181,871 (1) | 0 | 0 | 0 | 0 |
| Incidence (both sex) | 11,490 | 7507 | 4865 | 8684 | 2700 | 8225 |
| 18–49 years | 12,362 | 9635 | 5122 | 8712 | 2486 | 7719 |
| 50–64 years | 11,648 | 3559 | 4969 | 8680 | 3248 | 9789 |
| >=65 years | 7886 | 2968 | 3724 | 8527 | 3039 | 8696 |
| Incidence Female | 11,210 | 7154 | 4767 | 8852 | 2597 | 7821 |
| 18–49 years | 12,520 | 9483 | 5102 | 9029 | 2466 | 7471 |
| 50–64 years | 11,318 | 3220 | 4802 | 8814 | 3044 | 9154 |
| >=65 years | 6815 | 2518 | 3564 | 7985 | 2589 | 7754 |
| Incidence Male | 11,594 | 7874 | 4965 | 8507 | 2811 | 8647 |
| 18–49 years | 12,035 | 9779 | 5143 | 8391 | 2506 | 7974 |
| 50–64 years | 11,849 | 3933 | 4973 | 8534 | 3478 | 10,445 |
| >=65 years | 9092 | 3569 | 3922 | 9204 | 3575 | 9781 |
| Indicators | Argentina | Brazil | Chile ** | Colombia ** | Mexico | Peru |
|---|---|---|---|---|---|---|
| COVID-19 All cases, N | 3,711,250 | 11,956,157 | 728,812 | 3,240,355 | 2,363,214 | 1,867,034 |
| Hospital admission (%) | 4.5 | 11 | 7.4 | 3.2 | 19.7 | 5.3 |
| Hospitalization rate (per 100,000 cases) | 3562 | 7362 | 9380 | 469 | 18,096 | 5230 |
| Hospitalizations both sex n (%) | 167,173 (100) | 1,314,569 (100) | 53,773 (100) | 103,402 (100) | 464,804 (100) | 98,095 (100) |
| 18–49 years | 50,899 (30) | 388,030 (20) | 11,509 (21) | 29,262 (28) | 127,446 (28) | 36,081 (37) |
| 50–64 years | 43,678 (26) | 414,813 (31) | 22,426 (42) | 27,768 (27) | 163,738 (35) | 28,063 (28) |
| >=65 years | 72,596 (44) | 511,726 (39) | 19,838 (37) | 46,372 (45) | 173,620 (37) | 33,951 (35) |
| Hospitalizations Female | 73,366 (44) | 582,555 (44.3) | 28,520 (53) | 44,375 (43) | 187,952 (40) | 44,168 (45) |
| 18–49 years | 23,766 (32) | 156,044 (27) | 6695 (23) | 13,616 (30) | 49,432 (26) | 20,801 (47) |
| 50–64 years | 17,108 (23) | 179,109 (31) | 12,307 (43) | 10,536 (24) | 65,800 (35) | 9988 (23) |
| >=65 years | 32,492 (44) | 247,402 (42) | 9518 (34) | 20,223 (46) | 72,720 (39) | 13,379 (30) |
| Hospitalizations Male | 90,999 (54) | 731,910 (54.4) | 25,253 (47) | 59,024 (57) | 276,852 (60) | 53,677 (55) |
| 18–49 years | 26,590 (29) | 231,955 (32) | 4814 (19) | 15,851 (27) | 78,014 (28) | 15,117 (28) |
| 50–64 years | 26,214 (29) | 235,675 (32) | 10,120 (40) | 17,514 (29) | 97,938 (35) | 18,018 (34) |
| >=65 years | 38,195 (42) | 264,280 (36) | 10,319 (41) | 25,659 (44) | 100,900 (37) | 20,542 (38) |
| Critical care admission both sex n (%) | 33,834 (20) | 478,171 (36) | 18,537 (34) | 33,503 (32) | 37,113 (8) | 11,299 (11) |
| Hospitalized cases with mechanical ventilation both sex n (%) | 19,387(12) | 268,411 (20) | 8,386 (16) | 18,174 (18) | 62,640 (14) | 8,576 (9) |
| Indicators n (%) | Argentina | Brazil | Chile | Colombia | Mexico | Peru |
|---|---|---|---|---|---|---|
| Death both sex | 92,434 (100) | 521,577 (100) | 18,480 (100) | 89,137 (100) | 237,947 (100) | 184,969 (100) |
| 18–49 years | 6,816 (7) | 88,035 (16.9) | 1,041 (5.6) | 8,928 (10) | 37,678 (16) | 23,947 (13) |
| 50–64 years | 19,257 (21) | 140,505 (26.9) | 5679 (30.7) | 22,104 (25) | 82,553 (35) | 54,055 (29) |
| >=65 years | 66,361 (72) | 293,037 (56.2) | 11,760 (63.6) | 58,105 (65) | 117,716 (49) | 106,967 (58) |
| Death Female | 37,714 (41) | 228,360 (43.8) | 6,542 (35.4) | 33,978 (38) | 89,116 (37) | 66,805 (36) |
| 18–49 years | 2,531 (7) | 35,620 (15.6) | 262 (4) | 2,912 (9) | 12,074 (14) | 7,729 (12) |
| 50–64 years | 6,507 (17) | 58,070 (25.4) | 2,682 (40.9) | 7,834 (23) | 30,249 (34) | 18,360 (27) |
| >=65 years | 28,676 (76) | 134,670 (58.9) | 3,598 (54.9) | 23,232 (68) | 46,793 (52) | 40,716 (61) |
| Death Male | 52,728 (57) | 293,159 (50) | 11,938 (64.6) | 55,159 (62) | 148,831 (63) | 118,164 (64) |
| 18–49 years | 4,201 (8) | 52,402 (18) | 477(4) | 6,016 (11) | 25,604 (17) | 16,218 (14) |
| 50–64 years | 12,604 (24) | 82,419 (28) | 4,895 (41) | 14,270 (26) | 52,304 (35) | 35,695 (30) |
| >=65 years | 35,923 (68) | 158,338 (54) | 6,566 (55) | 34,873 (63) | 70,923 (48) | 66,251 (56) |
| Mortality rate per 100,000 | 276.1 | 327.5 | 123.4 | 238.8 | 271.8 | 814.9 |
| Case fatality rate | 2.5 | 4.4 | 2.5 | 2.8 | 10.1 | 9.9 |
| Country | YLLs | YLDs | DALYs | DALYs/100,000 |
|---|---|---|---|---|
| Argentina (min-max) | 510,222 - | 9235 (3418–69,900) | 519,457 (513,640–580,122) | 1680.6 (1661–1876) |
| Brazil (min-max) | 3,312,346 - | 59,953 (22,192–453,791) | 3,372,299 (3,334,538–3,766,137) | 2209 (2184–2467) |
| Chile (min-max) | 241,089 - | 4363 (1615–33,029) | 245,452 (242,704–274,118) | 1697.3 (1678–1895) |
| Colombia (min-max) | 885,793 - | 16,033 (5934–121,353) | 901,826 (891,727–1,007,146) | 2532.7 (2504–2828) |
| Mexico (min-max) | 2,097,504 - | 37,761 - | 2,135,265 - | 2549.5 - |
| Peru (min-max) | 744,331 - | 13,472 (4987–101,973) | 757,803 (749,318–846,304) | 3510.7 (3471–3920) |
| Argentina | Brazil | Chile | Colombia | Mexico | Peru | |
|---|---|---|---|---|---|---|
| Mild | USD 68.9 (100.0%) | USD 26.6 (100.0%) | USD 56.3 (100.0%) | USD 28.9 (100.0%) | USD 44.2 (100.0%) | USD 27.6 (100.0%) |
| Consultations | USD 20.0 (29.0%) | USD 7.2 (27.1%) | USD 46.5 (82.5%) | USD 13.9 (48.2%) | USD 20.5 (46.5%) | USD 13.2 (47.7%) |
| Diagnostic and laboratory tests | USD 45.5 (66.0%) | USD 17.6 (66.1%) | USD 9.6 (17.0%) | USD 13.1 (45.3%) | USD 21.9 (49.6%) | USD 14.1 (50.9%) |
| Hospitalizations | USD 0.0 (0.0%) | USD 0.0 (0.0%) | USD 0.0 (0.0%) | USD 0.0 (0.0%) | USD 0.0 (0.0%) | USD 0.0 (0.0%) |
| Drugs | USD 3.4 (5.0%) | USD 1.8 (6.8%) | USD 0.3 (0.6%) | USD 1.9 (6.5%) | USD 1.8 (4.0%) | USD 0.4 (1.4%) |
| Moderate and severe | USD 2510.0 (100.0%) | USD 1059.4 (100.0%) | USD 2971.3 (100.0%) | USD 1721.8 (100.0%) | USD 1936.6 (100.0%) | USD 1357.7 (100.0%) |
| Consultations | USD 9.1 (0.4%) | USD 2.8 (0.3%) | USD 24.3 (0.8%) | USD 12.2 (0.7%) | USD 12.2 (0.6%) | USD 7.8 (0.6%) |
| Diagnostic and laboratory tests | USD 242.3 (9.7%) | USD 77.0 (7.3%) | USD 279.4 (9.4%) | USD 448.3 (26.0%) | USD 311.0 (16.1%) | USD 199.8 (14.7%) |
| Hospitalizations | USD 2253.0 (89.8%) | USD 976.6 (92.2%) | USD 2667.1 (89.8%) | USD 1258.2 (73.1%) | USD 1610.0 (83.1%) | USD 1149.5 (84.7%) |
| Drugs | USD 5.7 (0.2%) | USD 3.0 (0.3%) | USD 0.5 (0.0%) | USD 3.1 (0.2%) | USD 3.5 (0.2%) | USD 0.6 (0.0%) |
| Critical | USD 23,384.2 (100.0%) | USD 19,391.5 (100.0%) | USD 19,839.8 (100.0%) | USD 7147.2 (100.0%) | USD 30,040.9 (100.0%) | USD 8053.8 (100.0%) |
| Consultations | USD 9.2 (0.0%) | USD 2.8 (0.0%) | USD 25.2 (0.1%) | USD 12.7 (0.2%) | USD 12.6 (0.0%) | USD 8.1 (0.1%) |
| Diagnostic and laboratory tests | USD 1207.7 (5.2%) | USD 351.4 (1.8%) | USD 1654.2 (8.3%) | USD 2280.8 (31.9%) | USD 1606.5 (5.3%) | USD 1032.1 (12.8%) |
| Hospitalizations | USD 8611.8 (36.8%) | USD 5217.6 (26.9%) | USD 9211.8 (46.4%) | USD 3806.7 (53.3%) | USD 5936.1 (19.8%) | USD 4911.5 (61.0%) |
| Drugs | USD 13,555.5 (58.0%) | USD 13,819.7 (71.3%) | USD 8948.7 (45.1%) | USD 1047.1 (14.7%) | USD 22,485.8 (74.9%) | USD 2102.1 (26.1%) |
| Argentina | Brazil | Chile | Colombia | Mexico | Peru | |
|---|---|---|---|---|---|---|
| Mild COVID cases | USD 244.2 (17.8%) | USD 278.5 (2.7%) | USD 38.0 (7.4%) | USD 90.6 (20.1%) | USD 83.8 (4.1%) | USD 48.9 (19.0%) |
| Moderate and severe COVID cases | USD 334.7 (24.4%) | USD 886.1 (8.5%) | USD 104.7 (20.5%) | USD 120.4 (26.7%) | USD 828.3 (40.9%) | USD 117.8 (45.7%) |
| Critical COVID cases | USD 791.2 (57.7%) | USD 9272.4 (88.8%) | USD 367.8 (72.0%) | USD 239.5 (53.2%) | USD 1114.8 (55.0%) | USD 91.0 (35.3%) |
| Total cost for all COVID cases | USD 1370.1 (100.0%) | USD 10,437.1 (100.0%) | USD 510.5 (100.0%) | USD 450.4 (100.0%) | USD 2026.9 (100.0%) | USD 257.7 (100.0%) |
| % of health expenditure | 2.2% | 5.3% | 1.7% | 1.5% | 2.3% | 1.7% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Espinola, N.; Loudet, C.I.; Luxardo, R.; Moreno, C.; Kyaw, M.H.; Spinardi, J.; Mendoza, C.F.; Carballo, C.M.; Dantas, A.C.; Abalos, M.G.; et al. COVID-19 Disease and Economic Burden to Healthcare Systems in Adults in Six Latin American Countries Before Nationwide Vaccination Program: Ministry of Health Database Assessment and Literature Review. Int. J. Environ. Res. Public Health 2025, 22, 669. https://doi.org/10.3390/ijerph22050669
Espinola N, Loudet CI, Luxardo R, Moreno C, Kyaw MH, Spinardi J, Mendoza CF, Carballo CM, Dantas AC, Abalos MG, et al. COVID-19 Disease and Economic Burden to Healthcare Systems in Adults in Six Latin American Countries Before Nationwide Vaccination Program: Ministry of Health Database Assessment and Literature Review. International Journal of Environmental Research and Public Health. 2025; 22(5):669. https://doi.org/10.3390/ijerph22050669
Chicago/Turabian StyleEspinola, Natalia, Cecilia I. Loudet, Rosario Luxardo, Carolina Moreno, Moe H. Kyaw, Julia Spinardi, Carlos Fernando Mendoza, Carolina M. Carballo, Ana Carolina Dantas, Maria Gabriela Abalos, and et al. 2025. "COVID-19 Disease and Economic Burden to Healthcare Systems in Adults in Six Latin American Countries Before Nationwide Vaccination Program: Ministry of Health Database Assessment and Literature Review" International Journal of Environmental Research and Public Health 22, no. 5: 669. https://doi.org/10.3390/ijerph22050669
APA StyleEspinola, N., Loudet, C. I., Luxardo, R., Moreno, C., Kyaw, M. H., Spinardi, J., Mendoza, C. F., Carballo, C. M., Dantas, A. C., Abalos, M. G., Ballivian, J., Navarro, E., & Bardach, A. (2025). COVID-19 Disease and Economic Burden to Healthcare Systems in Adults in Six Latin American Countries Before Nationwide Vaccination Program: Ministry of Health Database Assessment and Literature Review. International Journal of Environmental Research and Public Health, 22(5), 669. https://doi.org/10.3390/ijerph22050669








