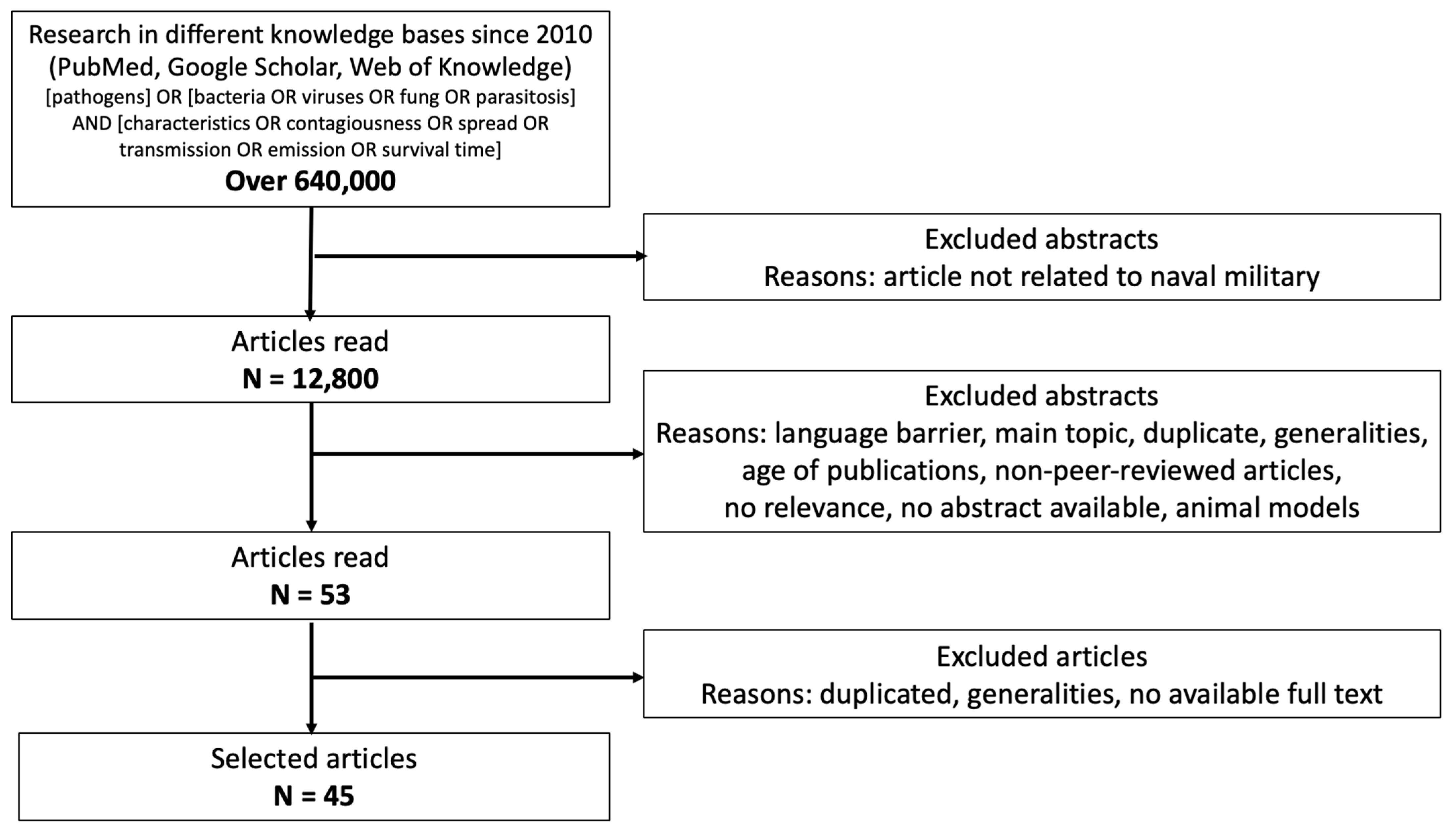
Methodology of Epidemic Risk Analysis in the Naval Military
Abstract
1. Introduction
2. Literature Review
2.1. Search Strategy for the Characterization of Pathogens
- It minimizes selection bias by following a predefined inclusion and exclusion criteria framework, ensuring that only relevant and high-quality studies are considered.
- It enhances transparency by providing a detailed flow diagram of the study selection process, allowing for better reproducibility of the research.
- PRISMA improves the reliability of findings by promoting a comprehensive and structured literature review process, facilitating the identification of key trends and gaps in the existing knowledge.
2.2. Characterization of Pathogens
2.2.1. Pathogen Transmission
2.2.2. Contagiousness
2.2.3. Environmental Conditions
2.2.4. Survival Time Outside the Host
2.2.5. Emission
2.2.6. Severity of Disease
3. Methodology
- A coefficient of 0.5 was applied to diseases with a vaccine effectiveness rate between 35% and 65% [47]; and
- A coefficient of 0.8 was applied to diseases with a vaccine effectiveness rate between 0 and 34%.
- Category 1 (10 ≤ RPI ≤ 25): used for diseases with a significant impact on the military ship’s mission;
- Category 2 (5 ≤ RPI ≤ 9): used for diseases with a moderate impact on the military ship’s mission; and
- Category 3 (1 ≤ RPI ≤ 4): used for diseases with a low impact on the military ship’s mission.
- Category A: high-priority agents, which include organisms that pose a significant risk to national security due to several factors:
- They are easily disseminated or transmitted from person to person;
- They have high mortality rates, with the potential for a major public health impact;
- They have the ability to cause public panic and social disruption; and
- They require specific enhancements in disease surveillance.
- Category B: second-highest priority agents, which include pathogens that:
- Are moderately easy to disseminate;
- Cause moderate morbidity rates and low mortality rates; and
- Require specific enhancements in disease surveillance.
- Category C: third-highest priority agents, which include emerging pathogens (such as Nipah virus and Hantavirus) that could potentially be engineered for mass dissemination in the future, due to:
- Their availability;
- Their ease of production and dissemination; and
- Their potential for high morbidity and mortality rates, and their significant health impact.
4. Results
5. Discussion
- Experimental trials in a controlled environment to test the effectiveness of detection, identification, containment, and elimination solutions adapted to the constraints of the maritime environment (humidity, salinity, and vibrations); and
- Numerical simulations (particularly computational fluid dynamics) to model the spread of pathogens and assess preventive and detection measures.
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fernandes, E.G.; de Souza, P.B.; de Oliveira, M.E.B.; Lima, G.D.; Pellini, A.C.G.; Ribeiro, M.C.S.; Sato, H.K.; Ribeiro, A.F.; Yu, A.L.F. Influenza B outbreak on a cruise ship off the São Paulo Coast, Brazil. J. Travel Med. 2014, 21, 298–303. [Google Scholar] [CrossRef] [PubMed]
- Vivancos, R.; Keenan, A.; Sopwith, W.; Smith, K.; Quigley, C.; Mutton, K.; Dardamissis, E.; Nichols, G.; Harris, J.; Gallimore, C.; et al. Norovirus outbreak in a cruise ship sailing around the British Isles: Investigation and multi-agency management of an international outbreak. J. Infect. 2010, 60, 478–485. [Google Scholar] [CrossRef] [PubMed]
- Qi, L.; Xiang, X.; Xiong, Y.; Ling, H.; Shen, H.; Deng, W.; Tang, W.; Shen, T.; Li, Q. Outbreak of acute gastroenteritis caused by norovirus genogroup II attributed to contaminated cold dishes on a cruise ship in Chongqing, China, 2017. Int. J. Environ. Res. Public Health 2018, 15, 2823. [Google Scholar] [CrossRef] [PubMed]
- Wikswo, M.E.; Cortes, J.; Hall, A.J.; Vaughan, G.; Howard, C.; Gregoricus, N.; Cramer, E.H. Disease transmission and passenger behaviors during a high morbidity norovirus outbreak on a cruise ship, January 2009. Clin. Infect. Dis. 2011, 52, 1116–1122. [Google Scholar] [CrossRef]
- Moira, P.; Mylonopoulos, D.; Terzoglou, E. Health Issues and Cruising in COVID-19 Era. Int. J. Res. Tour. Hosp. 2020, 6, 12–22. [Google Scholar]
- Brewster, R.K.; Chan, K.; Allen, H.; Sundermann, A.; Keane, S.; Boles, C. Future directions of infection control and risk management on military vessels: A narrative review. J. Public Health Emerg. 2022, 6, 33. [Google Scholar] [CrossRef]
- Kordsmeyer, A.-C.; Mojtahedzadeh, N.; Heidrich, J.; Militzer, K.; von Münster, T.; Belz, L.; Jensen, H.-J.; Bakir, S.; Henning, E.; Heuser, J.; et al. Systematic review on outbreaks of SARS-CoV-2 on cruise, navy and cargo ships. Int. J. Environ. Res. Public Health 2021, 18, 5195. [Google Scholar] [CrossRef]
- Sekizuka, T.; Itokawa, K.; Kageyama, T.; Saito, S.; Takayama, I.; Asanuma, H.; Nao, N.; Tanaka, R.; Hashino, M.; Takahashi, T.; et al. Haplotype networks of SARS-CoV-2 infections in the Diamond Princess cruise ship outbreak. Proc. Natl. Acad. Sci. USA 2020, 117, 20198–20201. [Google Scholar] [CrossRef]
- de Laval, F.; Chaudet, H.; Gorgé, O.; Marchi, J.; Lacrosse, C.; Dia, A.; Marbac, V.; Mrenda, B.M.; Texier, G.; Letois, F.; et al. Investigation of a COVID-19 outbreak on the Charles de Gaulle aircraft carrier, March to April 2020: A retrospective cohort study. Eurosurveillance 2022, 27, 2100612. [Google Scholar] [CrossRef]
- Kasper, M.R.; Geibe, J.R.; Sears, C.L.; Riegodedios, A.J.; Luse, T.; Von Thun, A.M.; McGinnis, M.B.; Olson, N.; Houskamp, D.; Fenequito, R.; et al. An outbreak of COVID-19 on an aircraft carrier. N. Engl. J. Med. 2020, 383, 2417–2426. [Google Scholar] [CrossRef]
- Malone, J.D. USS Theodore Roosevelt, COVID-19, and ships: Lessons learned. JAMA Netw. Open 2020, 3, e2022095. [Google Scholar] [CrossRef]
- Pourabdollahian, B.; Eslami, Y.; Chenouard, R.; da Cunha, C. (Chemnitz, Germany). A correlated redefinition of the concept of resilience in a production system. Advances in Production Management Systems, APMS2024. Personal communication, 2024. [Google Scholar]
- Patry, E.; Bourgeois, Q.; Bernaud, V.; de Wykerslooth, C. Résilience des Armées. DIA-3.4.1_Résilience 2022, N°23/ARM/CICDE/NP. Available online: https://www.defense.gouv.fr/sites/default/files/cicde/20220208-NP-DIA-3.4.1_RESILIENCE2022-VF.pdf (accessed on 29 March 2025).
- Bhamra, R.; Dani, S.; Burnard, K. Resilience: The concept, a literature review and future directions. Int. J. Prod. Res. 2011, 49, 5375–5393. [Google Scholar] [CrossRef]
- Leung, N.H.L. Transmissibility and transmission of respiratory viruses. Nat. Rev. Microbiol. 2021, 19, 528–545. [Google Scholar] [CrossRef] [PubMed]
- Seto, W.; Conly, J.; Silva, C.P.; Malik, M.; Eremin, S. Infection prevention and control measures for acute respiratory infections in healthcare settings: An update. East. Mediterr. Health J. 2013, 19, S39–S47. [Google Scholar] [CrossRef]
- Azimi, P.; Keshavarz, Z.; Laurent, J.G.C.; Stephens, B.; Allen, J.G. Mechanistic transmission modeling of COVID-19 on the Diamond Princess cruise ship demonstrates the importance of aerosol transmission. Proc. Natl. Acad. Sci. USA 2021, 118, e2015482118. [Google Scholar] [CrossRef]
- Diekmann, O.; Heesterbeek, J.A.P.; Metz, J.A.J. On the definition and the computation of the basic reproduction ratio R 0 in models for infectious diseases in heterogeneous populations. J. Math. Biol. 1990, 28, 365–382. [Google Scholar] [CrossRef] [PubMed]
- Delamater, P.L.; Street, E.J.; Leslie, T.F.; Yang, Y.T.; Jacobsen, K.H. Complexity of the Basic Reproduction Number (R0). Emerg. Infect. Dis. 2019, 25, 1–4. [Google Scholar] [CrossRef]
- Huang, L.-S.; Li, L.; Dunn, L.; He, M. Taking account of asymptomatic infections: A modeling study of the COVID-19 outbreak on the Diamond Princess cruise ship. PLoS ONE 2021, 16, e0248273. [Google Scholar] [CrossRef]
- Flahaut, A.; Slama, R.; Spira, A. Que dit la science à propos de l’épidémiologie des maladies infectieuses émergentes ? Inserm, From science to health, 2020, 12p. Available online: https://www.inserm.fr/wp-content/uploads/2021-05/inserm-miseaupointepidemiomalinfectieuses-majmai2021.pdf (accessed on 29 March 2025).
- Watanabe, T.; Bartrand, T.A.; Weir, M.H.; Omura, T.; Haas, C.N. Development of a Dose-Response Model for SARS Coronavirus. Risk Anal. 2010, 30, 1129–1138. [Google Scholar] [CrossRef]
- Buonanno, G.; Stabile, L.; Morawska, L. Estimation of airborne viral emission: Quanta emission rate of SARS-CoV-2 for infection risk assessment. Environ. Int. 2020, 141, 105794. [Google Scholar] [CrossRef]
- Prentiss, M.; Chu, A.; Berggren, K. Superspreading events without superspreaders: Using high attack rate events to estimate Nº for airborne transmission of COVID-19. MedRxiv 2020, 28p. [Google Scholar] [CrossRef]
- Bazant, M.Z.; Bush, J.W.M. A guideline to limit indoor airborne transmission of COVID-19. Proc. Natl. Acad. Sci. USA 2021, 118, e2018995118. [Google Scholar] [CrossRef]
- Miller, S.L.; Nazaroff, W.W.; Jimenez, J.L.; Boerstra, A.; Buonanno, G.; Dancer, S.J.; Kurnitski, J.; Marr, L.C.; Morawska, L.; Noakes, C. Transmission of SARS-CoV-2 by inhalation of respiratory aerosol in the Skagit Valley Chorale superspreading event. Indoor Air 2020, 31, 314–323. [Google Scholar] [CrossRef] [PubMed]
- Peng, Z.; Rojas, A.P.; Kropff, E.; Bahnfleth, W.; Buonanno, G.; Dancer, S.; Kurnitski, J.; Li, Y.; Loomans, M.; Marr, L.; et al. Practical indicators for Risk of Airborne Transmission in Shared Indoor Environments and their application to COVID-19 Outbreaks. Environ. Sci. Technol. 2022, 56, 1125–1137. [Google Scholar] [CrossRef] [PubMed]
- Poydenot, F.; Abdourahamane, I.; Caplain, E.; Der, S.; Haiech, J.; Jallon, A.; Khoutami, I.; Loucif, A.; Marinov, E.; Andreotti, B. Risk assessment for long- and short-range airborne transmission of SARS-CoV-2, indoors and outdoors. PNAS Nexus 2022, 1, pgac223. [Google Scholar] [CrossRef]
- Buonanno, G.; Morawska, L.; Stabile, L. Quantitative assessment of the risk of airborne transmission of SARS-CoV-2 infection: Prospective and retrospective applications. Environ. Int. 2020, 145, 106112. [Google Scholar] [CrossRef] [PubMed]
- Ho, C.K. Modeling airborne pathogen transport and transmission risks of SARS-CoV-2. Appl. Math. Model. 2021, 95, 297–319. [Google Scholar] [CrossRef]
- McMichael, A.J.; Campbell-Lendrum, D.H.; Corvalan, C.F.; Ebi, K.L.; Githeko, A.K.; Scheraga, J.D.; Woodward, A. International consensus on the science of climate and health: The IPCC Third Assessment Report. Clim. Chang. Hum. Health Risks Responses 2003, 2, 103–132. [Google Scholar]
- Pica, N.; Bouvier, N.M. Environmental factors affecting the transmission of respiratory viruses. Curr. Opin. Virol. 2012, 2, 90–95. [Google Scholar] [CrossRef]
- Fuster-Valls, N.; Hernández-Herrero, M.; Marín-De-Mateo, M.; Rodríguez-Jerez, J.J. Effect of different environmental conditions on the bacteria survival on stainless steel surfaces. Food Control. 2008, 19, 308–314. [Google Scholar] [CrossRef]
- Hanczvikkel, A.; Tóth, Á. Quantitative study about the role of environmental conditions in the survival capability of multidrug-resistant bacteria. J. Infect. Public Health 2018, 11, 801–806. [Google Scholar] [CrossRef] [PubMed]
- Van Doremalen, N.; Bushmaker, T.; Morris, D.H.; Holbrook, M.G.; Gamble, A.; Williamson, B.N.; Tamin, A.; Harcourt, J.L.; Thornburg, N.J.; Gerber, S.I.; et al. Aerosol and Surface Stability of SARS-CoV-2 as Compared with SARS-CoV-1. N. Engl. J. Med. 2020, 382, 1564–1567. [Google Scholar] [CrossRef]
- Wang, C.C.; Prather, K.A.; Sznitman, J.; Jimenez, J.L.; Lakdawala, S.S.; Tufekci, Z.; Marr, L.C. Airborne transmission of respiratory viruses. Science 2021, 373, eabd9149. [Google Scholar] [CrossRef] [PubMed]
- Service de Santé des Armées (SSA). Calendrier Vaccinal à L’incorporation. Available online: https://professionnels.vaccination-info-service.fr/var/vis/storage/original/application/download/20221222_NP_DCSSA-SDD-OS_annexes1_6_fiches_techniques_calendrier-vaccinal-2023%20(4).pdf (accessed on 29 March 2025).
- Boogaard, J.v.D.; de Gier, B.; Lima, P.d.O.B.; Desai, S.; de Melker, H.E.; Hahné, S.J.; Veldhuijzen, I.K. Immunogenicity, duration of protection, effectiveness and safety of rubella containing vaccines: A systematic literature review and meta-analysis. Vaccine 2001, 39, 889–900. [Google Scholar] [CrossRef] [PubMed]
- Chit, A.; Zivaripiran, H.; Shin, T.; Lee, J.K.H.; Tomovici, A.; Macina, D.; Johnson, D.R.; Decker, M.D.; Wu, J. Acellular pertussis vaccines effectiveness over time: A systematic review, meta-analysis and modeling study. PLoS ONE 2018, 13, e0197970. [Google Scholar] [CrossRef]
- Griffin, D.E. Measles Vaccine. Viral Immunol. 2018, 31, 86–95. [Google Scholar] [CrossRef]
- Langan, R.C.; Goodbred, A.J. Hepatitis A. Am. Fam. Physician 2021, 104, 368–374. [Google Scholar]
- Pillsbury, A.; Quinn, H. An assessment of measles vaccine effectiveness, Australia, 2006–2012. West. Pac. Surveill. Response 2015, 6, 43–50. [Google Scholar] [CrossRef]
- Deeks, S.L.; Lim, G.H.; Simpson, M.A.; Gagné, L.; Gubbay, J.; Kristjanson, E.; Fung, C.; Crowcroft, N.S. An assessment of mumps vaccine effectiveness by dose during an outbreak in Canada. Can. Med. Assoc. J. 2011, 183, 1014–1020. [Google Scholar] [CrossRef]
- A Truelove, S.; Keegan, L.T.; Moss, W.J.; Chaisson, L.H.; Macher, E.; Azman, A.S.; Lessler, J. Clinical and epidemiological aspects of diphtheria: A systematic review and pooled analysis. Clin. Infect. Dis. 2019, 71, 89–97. [Google Scholar] [CrossRef]
- Brisson, M.; Edmunds, W.; Gay, N. Varicella vaccination: Impact of vaccine efficacy on the epidemiology of VZV. J. Med. Virol. 2003, 70, S31–S37. [Google Scholar] [CrossRef] [PubMed]
- Quiambao, B.; Peyrani, P.; Li, P.; Cutler, M.W.; Van Der Wielen, M.; Perez, J.L.; Webber, C. Efficacy and safety of a booster dose of the meningococcal A, C, W, Y-tetanus toxoid conjugate vaccine administered 10 years after primary vaccination and long-term persistence of tetanus toxoid conjugate or polysaccharide vaccine. Hum. Vaccines Immunother. 2020, 16, 1272–1279. [Google Scholar] [CrossRef]
- Milligan, R.; Paul, M.; Richardson, M.; Neuberger, A. Vaccines for preventing typhoid fever. Cochrane Database Syst. Rev. 2018, 2018, CD001261. [Google Scholar] [CrossRef]
- Luo, Y.; Li, Y.; Xiao, S.; Lei, H. Comparative analysis of inflight transmission of SARS-CoV-2, influenza, and SARS-CoV-1. Epidemiol. Infect. 2023, 151, 1–22. [Google Scholar] [CrossRef]
- Rocklöv, J.; Sjödin, H.; Wilder-Smith, A. COVID-19 outbreak on the Diamond Princess cruise ship: Estimating the epidemic potential and effectiveness of public health countermeasures. J. Travel Med. 2020, 27, taaa030. [Google Scholar] [CrossRef]
- Whittaker, D.R.; Callan, J.E.; Campbell, J.T.; McCarten, M.D. Viral Gastroenteritis: The USS THEODORE ROOSEVELT Experience. Mil. Med. 2004, 169, 747–750. [Google Scholar] [CrossRef]
- Salmonsmith, J.; Ducci, A.; Guo, L.; Torii, R.; Balachandran, R.; Houlihan, C.; Epstein, R.; Rubin, J.; Tiwari, M.K.; Lovat, L.B. The influence of mechanical ventilation and portable air cleaners upon aerosol spread in a hospital outpatients clinic. Aerosol Sci. Technol. 2024, 1–12. [Google Scholar] [CrossRef]
- Berry, G.; Parsons, A.; Morgan, M.; Rickert, J.; Cho, H. A review of methods to reduce the probability of the airborne spread of COVID-19 in ventilation systems and enclosed spaces. Environ. Res. 2021, 203, 111765. [Google Scholar] [CrossRef] [PubMed]
- Harvard University Institute fo Disease Modeling. Available online: https://hsph.harvard.edu/research/communicable-disease-ccdd/ (accessed on 29 March 2025).
- Abbas, M.; Nunes, T.R.; Martischang, R.; Zingg, W.; Iten, A.; Pittet, D.; Harbarth, S. Nosocomial transmission and outbreaks of coronavirus disease 2019: The need to protect both patients and healthcare workers. Antimicrob. Resist. Infect. Control. 2021, 10, 1–13. [Google Scholar] [CrossRef]
- Sy, K.T.L.; White, L.F.; Nichols, B.E. Population density and basic reproductive number of COVID-19 across United States counties. PLoS ONE 2021, 16, e0249271. [Google Scholar] [CrossRef]
- Hsu, C.-Y.; Chen, J.-K.; Wikramaratna, P.S.; Yen, A.M.-F.; Chen, S.L.-S.; Chen, H.-H.; Lai, C.-C. Can ship travel contain COVID-19 outbreak after re-opening: A Bayesian meta-analysis. Epidemiology Infect. 2023, 151, e99. [Google Scholar] [CrossRef]
- Xi, Z.; Meng, D.; Zhao, J. The Analysis of COVID-19 Transmission on Diamond Princess Cruise Ship. In Proceedings of the 2021 IEEE 10th Data Driven Control and Learning Systems Conference (DDCLS), Suzhou, China, 14–16 May 2021; pp. 1224–1229. [Google Scholar]
- Inghels, C.; Da Cunha, C.; Billon-Denis, E.; Duclos, A.; Beghin, C. (Strasbourg, France). Methods and tools for biological risk assessment of naval vessels. International conference CBRNE Research & Innovation. Personal communication, 2024. [Google Scholar]
- Narayan, R.; Kundu, D.; Ghatak, A.; Tripathi, S.; Datta, S. Efficient elimination of airborne pathogens: A study on aerosolized Mycobacterium tuberculosis and SARS-CoV-2 using ZeBox technology. J. Hosp. Infect. 2022, 129, 17–21. [Google Scholar] [CrossRef] [PubMed]
| Clinical Severity | |
|---|---|
| 5—Catastrophic | Frequent mortality |
| 4—Critical | Immediate morbidity with severity |
| 3—Major | Immediate morbidity without serious but with distant consequences |
| 2—Moderate | Immediate morbidity without serious or long-term consequences |
| 1—Minor | No clinical impact (ambulatory without impact on daily life) |
| Contagiousness | |
|---|---|
| 5—Extreme | R0 between 12.1 and 20 |
| 4—Very high | R0 between 7.1 and 12 |
| 3—Significant | R0 between 5.1 and 7 |
| 2—Moderate | R0 between 1.1 and 5 |
| 1—Minor | R0 between 0 and 1 |
| Contagiousness | ||||||
|---|---|---|---|---|---|---|
| Criteria | 5 | 4 | 3 | 2 | 1 | |
| Clinical severity | 5 | 25 | 20 | 15 | 10 | 5 |
| 4 | 20 | 16 | 12 | 8 | 4 | |
| 3 | 15 | 12 | 9 | 6 | 3 | |
| 2 | 10 | 8 | 6 | 4 | 2 | |
| 1 | 5 | 4 | 3 | 2 | 1 | |
| (a) | ||||||||
| Disease | Transmission | Probability of Occurrence in the Naval Military | Severity | Contagiousness | Moderating Coefficient | Risk Prioritization Index | ||
| Symptoms | Rated | Description | Rated | |||||
| Chikungunya | Transmission by mosquito | The disease is not very widespread in metropolitan France, but is regularly encountered in epidemic episodes throughout the world. Contamination is by mosquito bite, as well as human-to-human transmission by blood transfusion; its moderate contagiousness limits the probability that the disease can be found on board a military ship. |
| 3 | It is not a contagious disease. Transmission of the virus occurs only through the mosquito bite. | 1 | 3 | |
| COVID-19 | Airborne transmission Hand-carried transmission | Very large number of cases and deaths worldwide. The varied and incapacitating symptoms, coupled with very simple modes of transmission, moderate contagiousness, short incubation period, and the history of numerous previous outbreaks in military buildings, suggest that new outbreaks are highly likely in the future. |
| 4 |
| 2 | 0.2 | 3.2 |
| Dengue | Transmission by mosquito Transmission from mother to child | Despite a very large number of cases worldwide each year and significant symptoms with a high mortality rate, human-to-human transmission can only occur through specific means (mosquito bite or the transplacental route). This strongly limits the probability of the emergence of this disease on board. |
| 3 | It is not a contagious disease. Transmission of the virus occurs only through the mosquito bite. | 1 | 3 | |
| Ebola | Animal transmission Hand-carried transmission | This virus, which has extremely severe symptoms and a very high mortality rate, occurs in epidemic episodes. The moderate contagiousness and hand-carried transmission, combined with an incubation period of several days, may suggest that an epidemic could occur, but this would mainly follow a stopover in a hard-hit area. |
| 5 | A person can contract the Ebola virus if he or she comes into contact with the bodily fluids of an infected person, or a surface or object contaminated with the bodily fluids of an infected person. | 2 | 10 (TBR A) | |
| Hepatitis A virus | Fecal–oral transmission | Despite moderate contagiousness, the disease is present throughout the world and can be spread between humans in a simple manner. Because symptoms take time to appear, the time it takes to detect the disease can allow for significant contamination in a ship’s crew. The disease is preventable with vaccination. |
| 5 |
| 2 | 0.2 | 2 |
| Hepatitis B virus | Sexual relations Blood transmission | Although the hepatitis B virus (HBV) is present in most of the biological fluids of infected people and is highly contagious, its probability of occurrence in a military naval environment is zero. (All sailors are vaccinated). | ||||||
| Hepatitis C virus | Blood transmission | Hepatitis C is an infectious liver disease that is transmitted primarily through the bloodstream. It is caused by the HCV virus. Acute hepatitis C becomes chronic in approximately 70% of cases. With treatment, chronic hepatitis C is cured in 99% of cases. In the absence of treatment, liver fibrosis eventually appears. The incubation period for hepatitis C is usually 2 to 12 weeks. Acute hepatitis C is usually asymptomatic and about 30% of infected people clear the virus within 6 months of infection and recover without any treatment. Approximately 70% of infected people do not clear the virus and develop chronic hepatitis C. Chronic hepatitis C is responsible, in the shorter term, for cancer of the liver and cirrhosis in 15 to 30% of cases after several years of development. | ||||||
| Hepatitis D virus | Blood transmission Skin lesion | Hepatitis D is an inflammation of the liver caused by HBD, which requires HBV to replicate. Hepatitis D cannot occur in the absence of hepatitis B. | ||||||
| Hepatitis E virus | Fecal–oral transmission (contaminated water) | This virus is excreted in the stool of infected people and enters the human body through the intestines. It is transmitted mainly through contaminated drinking water. HEV infection is found all over the world. The disease is common in low- and middle-income countries with limited access to clean water, sanitation, hygiene, and health services. |
| 5 |
| 2 | 10 | |
| Human immunodeficiency virus |
| A disease that is widespread throughout the world. The fact that transmission can occur through several modes and that symptoms take several weeks to appear increases the risk of multiple cases emerging within the naval forces. | ||||||
| Influenza A H1N1 | Animal transmission Airborne transmission Hand-carried transmission | This disease was widespread during the 2009 epidemic. The disease has a short incubation period and moderate contagiousness and it is preventable with the seasonal influenza vaccine, the effectiveness of which may vary, depending on the years. |
| 4 | Influenza A H1N1 is caught like other forms of influenza. People who are sick or who are developing the disease will cough and sneeze, which spreads droplets containing the virus around them. Healthy people become infected either by breathing in these microscopic particles (if they are in close proximity to sick people in an enclosed environment) or by touching a contaminated surface and then touching their eyes, mouth, or inside their nose (which seems to be a common mode of transmission). On a contaminated surface, the influenza virus remains active for a few hours at most (usually about two hours). | 2 | 8 | |
| Junin | Animal transmission Direct contact Inhalation Ingestion | The very low number of cases each year are associated with a very particular mode of human-to-human transmission and because of its minor contagiousness, the risk can be considered as negligible with respect to the military naval domain. |
| 4 | The R0 of the Junin virus is high, requiring strict measures of active surveillance, contact tracing, quarantine, and social distancing to stop transmission | 4 | 16 (TBR A) | |
| Lassa fever | Animal transmission Hand-carried transmission | This disease is not widespread worldwide and is of low contagiousness (R0 < 1), which leaves little room for the accidental contamination of a seafarer. Nevertheless, its symptoms are critical and the incubation period is long, which may allow the disease to incubate on several hosts before developing on board ship, or perhaps following an act of bioterrorism. |
| 5 | The virus can be transmitted from human to human, mainly in a hospital context, by skin–mucosal contact with a patient’s biological fluids. | 1 | 5 (TBR A) | |
| Marburg virus disease | Animal transmission Hand-carried transmission | The number of annual cases of this disease is extremely low. Although the symptoms are severe and human-to-human transmission is variable, the contagiousness remains moderate. Isolated cases could occur on board in a crew, but an epidemic seems unlikely. |
| 5 | Marburg virus disease is highly infectious but not very contagious. It spreads through direct contact with infected animals, particularly fruit bats, or through contact with the bodily fluids of infected individuals. The virus is not airborne and is considered not to be contagious before symptoms appear | 1 | 5 (TBR A) | |
| Measles | Airborne transmission | Measles is a disease that is still very present around the world and its clinical severity is catastrophic, with a high mortality rate. Moreover, it is transmitted between human beings by air, with a very high level of contagiousness. Its incubation period of a few days can, therefore, allow a large number of sailors to be contaminated before the first symptoms appear. The disease is preventable with a vaccine. |
| 5 | Measles is a highly contagious disease caused by a virus that is easily transmitted through coughing, sneezing, and nasal secretions. One person infected with measles can infect 15 to 20 people. | 4 | 0.2 | 4 |
| Meningitis (Neisseria meningitidis, Haemophilus influenzae, Streptococcus pneumoniae, Listeria monocytogenes) | Fecal–oral transmission | In this type of meningitis, symptoms known as meningeal syndrome (headache, photophobia, and vomiting) predominate, while the patient’s general condition remains unaltered. The disease is generally benign: in patients without immune deficiency, recovery is usually spontaneous. Recovery takes place within a few days, with no after-effects. It is usually caused by widespread viruses belonging to the enterovirus family. |
| 3 | Viral meningitis is the most common form, caused by a virus (enterovirus, which can spread throughout the body). | 2 | 0.2 | 1.2 |
| Mpox | Airborne transmission Hand-carried transmission | A global mpox outbreak began in May 2022, with cases increasing in the Democratic Republic of the Congo and spreading to previously unaffected areas. The outbreak is caused by an orthopox virus, primarily spreading through close contact, and has led to over 100,000 cases in 122 countries. During a 2022 outbreak, 146 military health system beneficiaries, including 118 active-duty personnel, were affected by confirmed or suspected mpox cases. | ||||||
| Mumps | Airborne transmission Hand-carried transmission | Although the disease causes few cases each year in France and the symptoms are moderate, it is easily transmitted by air and by hand, with very high contagiousness. Its long incubation period can lead to an epidemic. |
| 2 | A person can get mumps in the same way they can catch a cold: by coming into contact with saliva particles from someone who is coughing or sneezing, by sharing a drink with an infected person, by kissing an infected person, or by touching a surface that has been contaminated with the virus. Therefore, frequent hand-washing is important. | 1 | 0.2 | 0.4 |
| Rabies | Animal transmission | In spite of the important symptoms and a very long incubation period (which could leave time to contaminate many people), this disease can only be caught by animal contact or the transplant of contaminated organs. It is, therefore, logical to evaluate its severity in the maritime world as minor. |
| 5 | The disease is most prevalent in Africa Asia, and Latin America, where dogs are the main vector of transmission to humans. In Europe, bats can be infected by the lyssavirus, which is different from that in dogs. The disease can be contracted during stopovers. | 2 | 10 | |
| Rubella | Airborne transmission Manipulative transmission Transplacental transmission | The number of annual cases in France is extremely low. Nevertheless, its simple transmission, by air and by hand, its important contagiousness, and its long incubation period could be the origin of an epidemic on board a military ship. |
| 2 | Contamination occurs through droplets of saliva from the upper airways containing the virus:
| 3 | 0.2 | 1.2 |
| Sexually transmitted infection | Sexual relations Transmission from mother to child | Widespread infection worldwide, but with minor contagiousness. The incubation period and its simple transmission through sexual intercourse suggest that several sailors could become infected before the first symptoms appear. | ||||||
| Smallpox | Airborne transmission Hand-carried transmission | Smallpox is a disease considered to be eradicated to this day. Despite this, strains are kept in laboratories. The disease is very aggressive, relatively contagious, and is transmitted between humans in a simple way, by air or by hand. Its use as a bioterrorism weapon would be catastrophic for a crew. |
| 5 | Contagiousness rate R0 = 5 This disease has been eradicated in theory but could be a terrorist biological risk. | 2 | 10 (TBR A) | |
| Varicella | Airborne transmission Hand-carried transmission | The number of annual cases in France is significant, with relatively troublesome symptoms, even proving fatal in rare cases, with very high contagiousness and a short incubation period. A case on board a military ship could quickly lead to others in sailors who would never have contracted the disease in the past or in whom shingles could be triggered. |
| 2 | The varicella virus is transmitted by direct contact with the skin and mucous membrane vesicles, as well as by the respiratory route, by the inhalation of saliva droplets. | 4 | 0.2 | 1.6 |
| Yellow fever | Mosquito transmission | The number of annual cases worldwide is significant, as are the symptoms. However, since the mode of transmission is limited to mosquito bites, it is unlikely that a seafarer will become infected. If this does occur, symptoms appear quickly and there would be no transmission to another crew member. | ||||||
| (b) | ||||||||
| Disease | Transmission | Probability of Occurrence in the Naval Military | Severity | Contagiousness | Moderating Coefficient | Risk Prioritization Index | ||
| Symptoms | Rated | Description | Rated | |||||
| Anthrax | Animal transmission | Few cases occur per year around the world and there is no transmission from one human being to another. Most outbreaks occur as a result of malicious acts. The most feared scenario for a military vessel would, therefore, be the use of the pathogen as a weapon of bioterrorism. |
| 5 | Anthrax cannot be transmitted from person to person, but in rare cases, cutaneous anthrax can be spread from person to person through direct contact with an infected person or an object contaminated by an infected person. | 1 | 5 (TBR A) | |
| Botulism | Contaminated food Contaminated water | In spite of its very serious symptoms, there are few cases of the disease each year in France or in Europe, and human-to-human transmission is impossible. Therefore, only isolated cases could appear following the consumption of contaminated food or contaminated water during stopovers in more affected areas of the world. As the incubation period is very short, the disease’s diagnosis and treatment are made easier. |
| 5 | Botulism is not transmitted from person to person. Botulism develops when a person ingests the toxin (or, rarely, if the toxin is inhaled or injected) or when the bacteria grow in the intestines and release the toxin. | 1 | 5 (TBR A) | |
| Brucellosis | Animal transmission Contaminated food | The number of cases worldwide each year is low, with common symptoms despite some rare deaths. As human-to-human transmission is extremely rare, only isolated cases could appear, with a very low probability. |
| 3 | Brucellosis is not highly contagious among humans, as it primarily spreads from animals to humans rather than from person to person. However, brucellosis can be transmitted through the inhalation of aerosols. | 1 | 3 (TBR B) | |
| Cholera | Contaminated food Contaminated water | This disease is quite widespread around the world; its mode of contamination is simple, as is its mode of human-to-human transmission. Associated with high contagiousness and a relatively short period of onset of symptoms, the probability that a sailor could be a carrier becomes important. |
| 4 | It is a contagious disease that is transmitted by dirty hands and contaminated food or water. Less than 25% of infected people develop symptoms and 10 to 20% of them will develop severe disease. | 4 | 16 (TBR B) | |
| Diphtheria | Airborne transmission Hand-carried transmission | Despite the catastrophic clinical severity, the number of annual cases is too low for the disease to be of concern in our field. All sailors are vaccinated and as a result, this risk can be excluded. |
| 5 | Contagiousness index R0: 2.5 | 2 | 0.2 | 2 |
| Erysipelas | Hand-carried transmission | The number of cases in France each year is low, and although moderately contagious, the disease is only transmitted by direct contact with an injured person, which strongly limits the probability of an epidemic developing on board a French ship. |
| 2 | Erysipelas is contagious and is transmitted through skin-to-skin contact if the uninfected person also has an “entry point” (cut, wound, etc.). | 1 | 2 | |
| Glanders | Contaminated food Contaminated water Animal transmission | This disease develops very serious and even fatal symptoms. Despite this, human-to-human transmission is unlikely. Isolated cases are, nevertheless, possible following stopovers in areas where the disease has not been eradicated. Another case to consider is the use of this agent as a bioterrorism weapon. |
| 5 | The infecting dose of these bacteria is low, and aerosolized transmission is possible. This property has already enabled these agents to be used as a weapon of war in the last century. The use of these bacteria in acts of bioterrorism cannot be ruled out. European recommendations exist for the treatment and prophylaxis of glanders. Human-to-human transmission is unlikely. | 1 | 5 (TBR B) | |
| Legionellosis | Respiratory (inhalation of contaminated water aerosol) | Despite the troublesome symptoms, the number of annual cases is limited and the disease is not contagious. Nevertheless, contamination of drinking water on board a ship could result in the contamination of many of the crew members. |
| 2 | It is not a contagious disease from one person to another. Contamination is by the inhalation of contaminated water diffused in aerosol form. | 1 | 2 | |
| Leptospirosis | Waterborne transmission (dermal contact of injured skin or mucosa only, with water infested with bacteria) Animal transmission (infected animal or rat bite) | Given the number of cases per year at the national and global levels, it is unlikely that a French sailor would be exposed to this disease. Although its clinical severity is evaluated as critical, its contagiousness is null because it is not transmissible from one human to another. |
| 4 | The bacteria mainly enter through damaged skin or mucous membranes. | 1 | 4 | |
| Melioidosis | Skin contact (on burns or abrasions) Contaminated food Contaminated water Inhalation of contaminated aerosols | The clinical severity of this disease is catastrophic. Despite this, the number of annual cases worldwide is relatively low and the contagiousness is minor because human-to-human transmission is impossible. Rare individual cases may appear after visits to an affected area, but could not cause an epidemic. |
| 5 | Humans can contract melioidosis by contamination from scrapes or burns and by ingestion or inhalation, but not directly from infected animals or people. | 1 | 5 (TBR B) | |
| Meningitis (Neisseria meningitidis, Haemophilus influenzae, Streptococcus pneumoniae, Listeria monocytogenes) | Airborne transmission Contaminated food | Despite the troublesome symptoms and a rather simple mode of transmission, the number of annual cases is relatively limited worldwide and the contagiousness is moderate. One can easily conclude that the potential seriousness of such a disease on board a ship will also be moderate. |
| 5 | Meningococci are transmitted by close (at less than one meter), direct, and prolonged contact (more than one hour) with nasopharyngeal secretions. Promiscuity on board ship and lengthy missions can encourage disease transmission. | 2 | 0.2 | 2 |
| Pertussis | Airborne | Although the disease primarily affects children and the elderly, it can be contracted at any age and several times in a lifetime. Although the symptoms are mild, its airborne transmission and extreme contagiousness make it a potential concern in the naval context. |
| 2 | Pertussis is highly contagious and it is estimated that one sick person can infect an average of 15 to 17 people. This contamination is via airborne contact with the sick person through droplets from the nose or mouth when coughing. | 5 | 0.2 | 2 |
| Plague | Airborne transmission Hand-carried transmission | Few cases occur worldwide each year. Despite this, the proportion of deaths is high, human-to-human transmission is simple, and the incubation period is short. The disease, therefore, is rapidly detectable. Nevertheless, a case on board a military ship could cause an epidemic. |
| 5 | Infection can be transmitted from human to human via respiratory droplets. Handling the bodies of people who have died of plague is another possible mode of contamination. | 2 | 10 (TBR A) | |
| Q Fever | Animal transmission | Given the number of cases per year at the national and global levels, it is unlikely that a French sailor would be exposed to this disease. Although its clinical severity is evaluated as being major, its contagiousness is null because it is not transmissible from one human to another. |
| 3 | Q fever is highly contagious, but it does not spread easily from person to person. Instead, it is primarily transmitted from animals to humans through environmental exposure:
| 1 | 3 (TBR B) | |
| Salmonellosis | Fecal–oral transmission | Food contamination on a French military ship cannot be ruled out. The rapid emergence of relatively incapacitating symptoms can even be fatal in rare cases, and its human-to-human transmission by a simple mode could lead to an epidemic on board. |
| 4 | Contamination is strictly human-to-human for Salmonella. It occurs either directly, through dirty hands, or indirectly, through the ingestion of water or food soiled by the stool of a healthy carrier or a patient. A contaminated meal could infect many people. | 1 | 4 (TBR B) | |
| Scarlet fever | Airborne transmission Manipulative transmission | Scarlet fever has become a rare disease, affecting few individuals worldwide each year. Although moderately contagious and transmissible by air and by hand, the disease rarely affects adults, who are usually already immune. |
| 3 | R0 contagiousness index: 4 | 2 | 6 | |
| Sexually transmitted infection | Sexual relations Transmission from mother to child | Widespread infection worldwide, but with minor contagiousness. The incubation period and its simple transmission through sexual intercourse suggest that several sailors could become infected before the first symptoms appear. | ||||||
| Shiga toxin-producing E. coli (STEC) | Fecal–oral transmission | Few cases of this disease occur each year, but the clinical severity in case of infection is high. Although human-to-human transmission is rare, it occurs through a simple route, which is facilitated in communal spaces. It is therefore possible that a small outbreak of the disease could occur on board a military ship. |
| 5 | Human-to-human transmission is possible, but rare. In the majority of cases, it is observed in the family environment or in communities (such as in nurseries). | 2 | 10 (TBR B) | |
| Shigellosis | Contaminated food Contaminated water Fecal–oral transmission | At the French Navy level, the probability of an epidemic of this disease is low. The number of annual cases worldwide is low and the contagiousness is moderate. Although the incubation period is short and the symptoms very severe, only isolated cases could eventually appear. |
| 4 | Shigella is transmitted by the fecal-–oral route (through the stool of infected patients or convalescent carriers): 10 to 100 bacilli are sufficient to cause the disease. Humans are the only reservoir and can eliminate these bacteria in their stool for weeks after a dysenteric episode. | 3 | 12 (TBR B) | |
| Tuberculosis | Airborne transmission | The disease is fairly widespread throughout the world and its airborne transmission makes it relatively easy to spread. Although moderately contagious, it can take several months to incubate, which may be enough time to infect a large number of sailors, eventually leading to the emergence of serious symptoms. This has already occurred in 2001 on aircraft carriers. |
| 5 | Tuberculosis is transmitted by airborne microsecretions from a person with tuberculosis, especially when coughing, talking, singing, or sneezing. Only the respiratory forms (pulmonary, bronchial, and laryngeal forms) of tuberculosis disease are contagious. Extra-respiratory localizations are not contagious. Latent infection is not contagious. | 2 | 10 | |
| Tularemia | Animal transmission | Very few cases of the disease occur each year, and there is no human-to-human transmission. Despite the troublesome symptoms and the long incubation period, even the probability of isolated cases is very limited. |
| 4 | Tularemia is highly infectious but is not easily contagious between humans. It primarily spreads from animals to humans through direct contact, insect bites, ingestion, or inhalation of contaminated materials. | 1 | 4 (TBR A) | |
| Typhoid fever | Fecal–oral transmission | Numerous cases are recorded each year around the world. As the disease can be incapacitating and even fatal (and, given its easy human-to-human transmission, simple, with a moderate R0 and a long incubation period), it is possible that an epidemic could occur following a stopover in a region where the disease is endemic. |
| 5 | Typhoid is spread by ingesting water or food contaminated with the stool of an infected person (fecal–oral transmission). The infected person remains contagious as long as he or she is excreting the bacteria in their stool. Two to five percent of infected people become chronic carriers. | 3 | 0.5 | 7.5 (TBR B) |
| Typhus | Arthropod vectors (fleas, lice, and mites) | Typhus is transmitted simply by contact and its symptoms can be relatively severe. Nevertheless, the disease has been very rare in humans since the 2000s, and is, therefore, of little concern. |
| 4 | Typhus is not directly contagious between humans in most cases. Instead, it is transmitted through arthropod vectors (lice, fleas, mites, or ticks) | 1 | 4 (TBR B) | |
| (c) | ||||||||
| Disease | Transmission | Probability of Occurrence in Naval Military | Severity | Contagiousness | Moderating Coefficient | Risk Prioritization Index | ||
| Symptoms | Rated | Description | Rated | |||||
| Bedbugs | Hand-carried transmission | Increasingly frequent cases in the national territory, but contagiousness is moderate. |
| 1 | Due to the speed of reproduction, the bedbug can quickly infect a boat. Unlike ticks or mosquitoes, bedbugs present no risk of transmitting infectious agents (viruses, bacteria, parasites, etc.). | 1 | 1 (TBR B) | |
| Bilharzia | Water transmission | Despite a large number of cases worldwide and critical clinical severity, the transmission of this disease can only occur through contaminated water. This greatly limits the likelihood of an outbreak on board a military vessel. | ||||||
| Cryptosporidiosis | Fecal–oral transmission | The number of cases of the disease is very low and its symptoms are limited, although troublesome. R0 contagiousness is minor, despite a common mode of transmission. A few isolated cases could eventually appear, but not enough to create an epidemic. |
| 2 | Cryptosporidiosis is primarily transmitted through the fecal–oral route. The infection is highly contagious, particularly in environments where hygiene is poor or sanitation is inadequate. | 2 | 2 (TBR B) | |
| Integumentary leishmaniasis | Blood transmission Transmission from mother to child | It is unlikely that a contamination event will occur on board a French military vessel. The disease is not widespread worldwide, its transmission is carried out by particular vectors, and the incubation period varies from several weeks to several years. | ||||||
| Malaria | Transmission by mosquito Blood transmission Transmission from mother to child | The probability of a sailor being affected is limited and contamination of other sailors is even more limited, due to the very specific modes of human-to-human transmission. | ||||||
| Plasmodium falciparum malaria | Transmission by mosquito Blood transmission Transmission from mother to child | The probability of a sailor being affected is limited and the contamination of other sailors is even more limited, due to the very specific modes of human-to-human transmission. | ||||||
| Scabies | Manipulative transmission Sexual intercourse | The number of annual cases worldwide is very high, but slightly less at the national level. Despite this, the disease is very contagious, its transmission is via simple vectors, and the incubation period of several weeks suggests that a large number of sailors could become infected before the first symptoms emerge. |
| 1 | 4 | 4 | ||
| Sexually transmitted infection | Sexual relations Transmission from mother to child | Widespread infection worldwide, but with minor contagiousness. The incubation period and simple transmission through sexual intercourse suggest that several sailors could become infected before the first symptoms appear. | ||||||
| (d) | ||||||||
| Disease | Transmission | Probability of Occurrence in Naval Military | Severity | Contagiousness | Moderating Coefficient | Risk Prioritization Index | ||
| Symptoms | Rated | Description | Rated | |||||
| Meningitis (Cryptococcus neoformans, Histoplasma capsulatum) | Animal transmission | Fungal meningitis occurs mainly in immunocompromised individuals and is caused by yeasts (microscopic fungi) such as cryptococci. |
| 5 | This type of meningitis generally occurs in people with serious illnesses or weakened immune systems. | 1 | 5 | |
| Ringworm | Fungi Animal transmission Direct contact Hand-carried transmission | Cases of ringworm are very rare and mostly affect children. Although it is easily transmitted by skin-to-skin contact, symptoms are mild. Its rarity makes it a disease of minor concern in our field. | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peultier-Celli, L.; Gérard, A.; Letourneur, F.; Inghels, C.; Duclos, A.; Perrin, P. Methodology of Epidemic Risk Analysis in the Naval Military. Int. J. Environ. Res. Public Health 2025, 22, 572. https://doi.org/10.3390/ijerph22040572
Peultier-Celli L, Gérard A, Letourneur F, Inghels C, Duclos A, Perrin P. Methodology of Epidemic Risk Analysis in the Naval Military. International Journal of Environmental Research and Public Health. 2025; 22(4):572. https://doi.org/10.3390/ijerph22040572
Chicago/Turabian StylePeultier-Celli, Laetitia, Alain Gérard, Franck Letourneur, Clara Inghels, Audrey Duclos, and Philippe Perrin. 2025. "Methodology of Epidemic Risk Analysis in the Naval Military" International Journal of Environmental Research and Public Health 22, no. 4: 572. https://doi.org/10.3390/ijerph22040572
APA StylePeultier-Celli, L., Gérard, A., Letourneur, F., Inghels, C., Duclos, A., & Perrin, P. (2025). Methodology of Epidemic Risk Analysis in the Naval Military. International Journal of Environmental Research and Public Health, 22(4), 572. https://doi.org/10.3390/ijerph22040572







