Investigation of Influences on Indoor and Outdoor SVOC Exposure
Abstract
1. Introduction
2. Materials and Methods
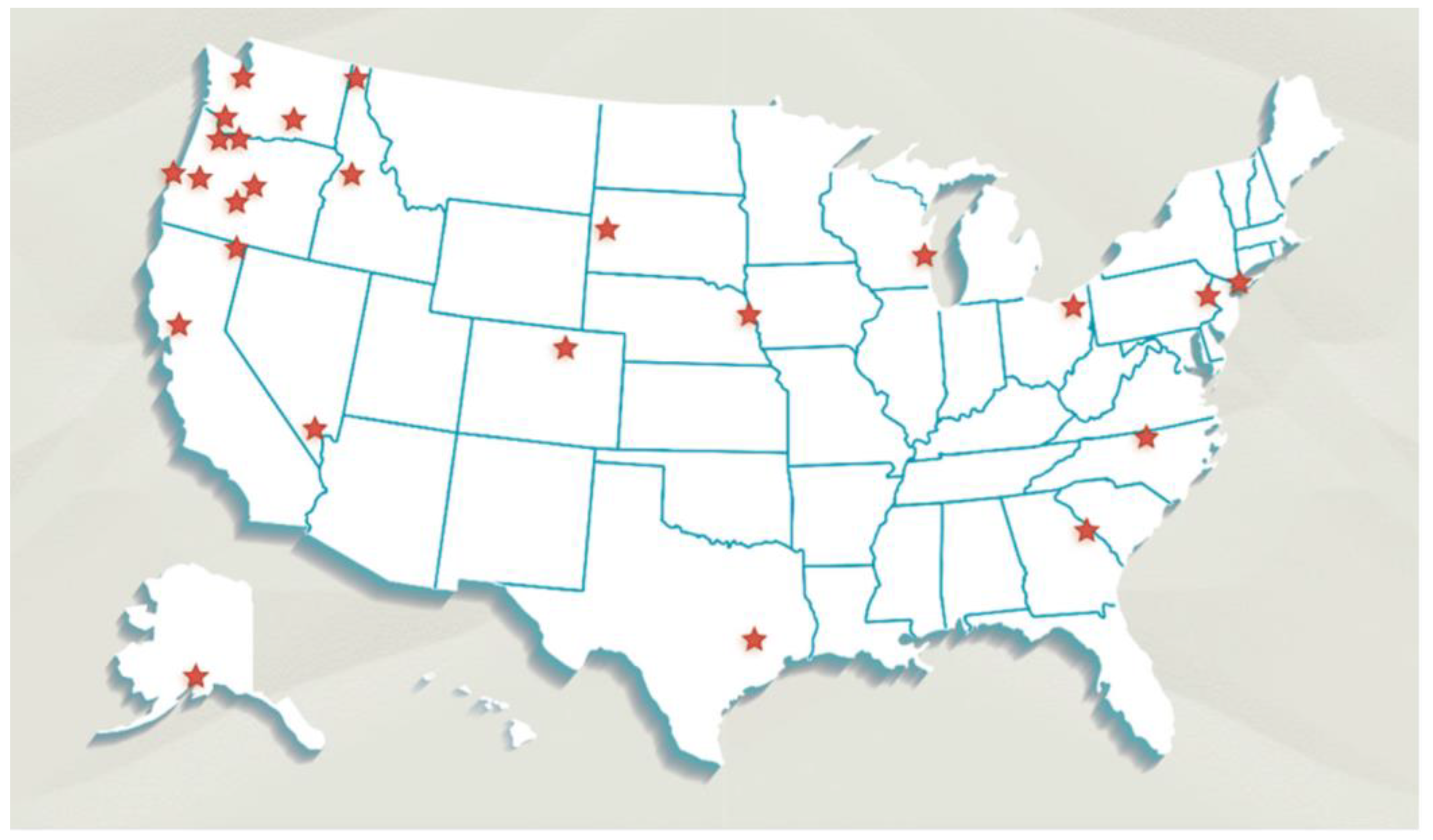
2.1. Study Design
2.2. Sampler Preparation and Deployment
2.3. LDPE Cleaning and Extraction
2.4. Instrumental Analysis and Chemical Classifications
2.5. Quality Control
2.6. Chemical Classifications
2.7. Environmental and Demographic Metadata
2.8. Statistical Analysis
2.8.1. Univariate Statistics
2.8.2. Multivariate Statistics
3. Results and Discussion
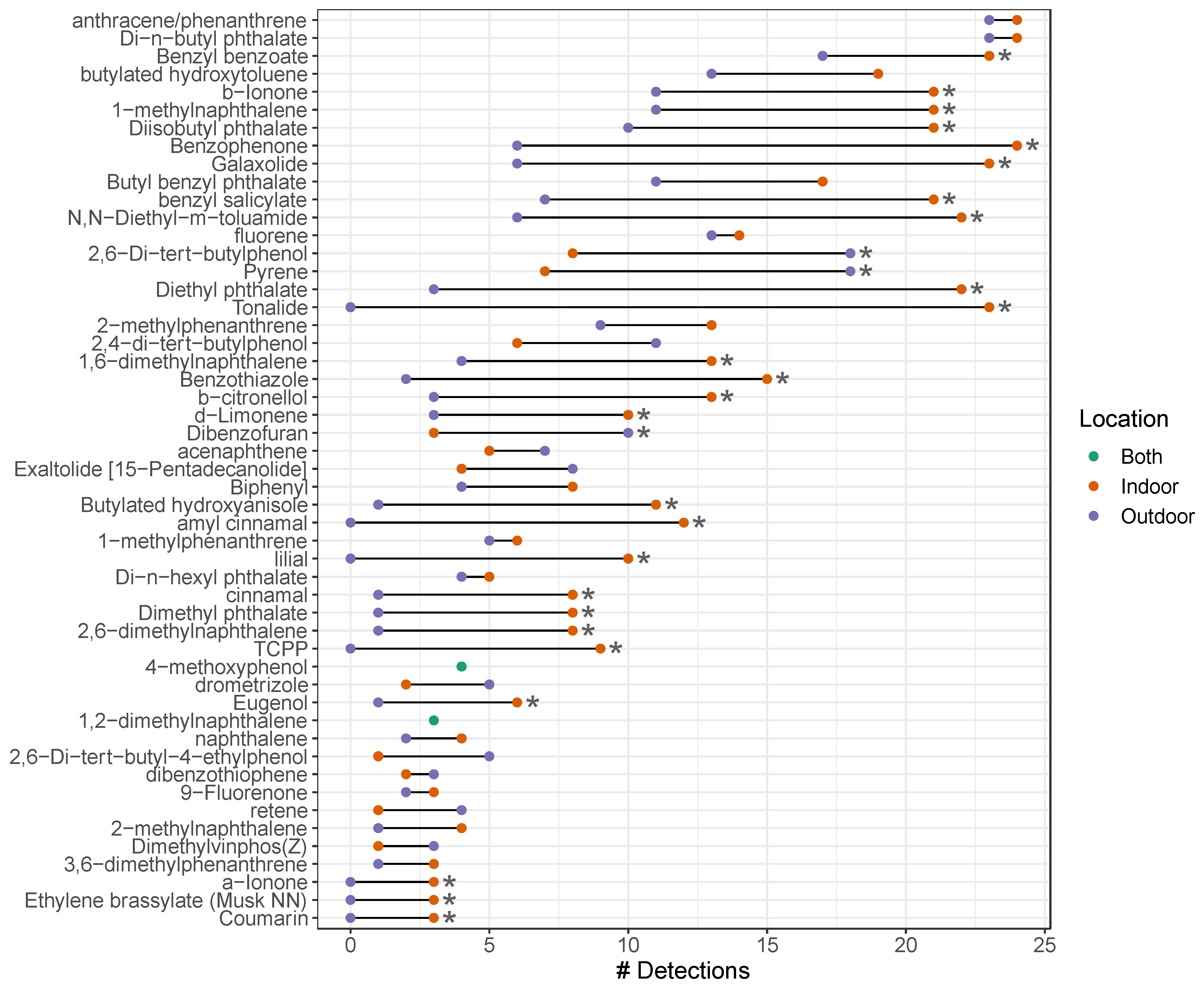
3.1. Chemical Detection and Concentration
3.2. Influences on Chemical Profiles Univariate
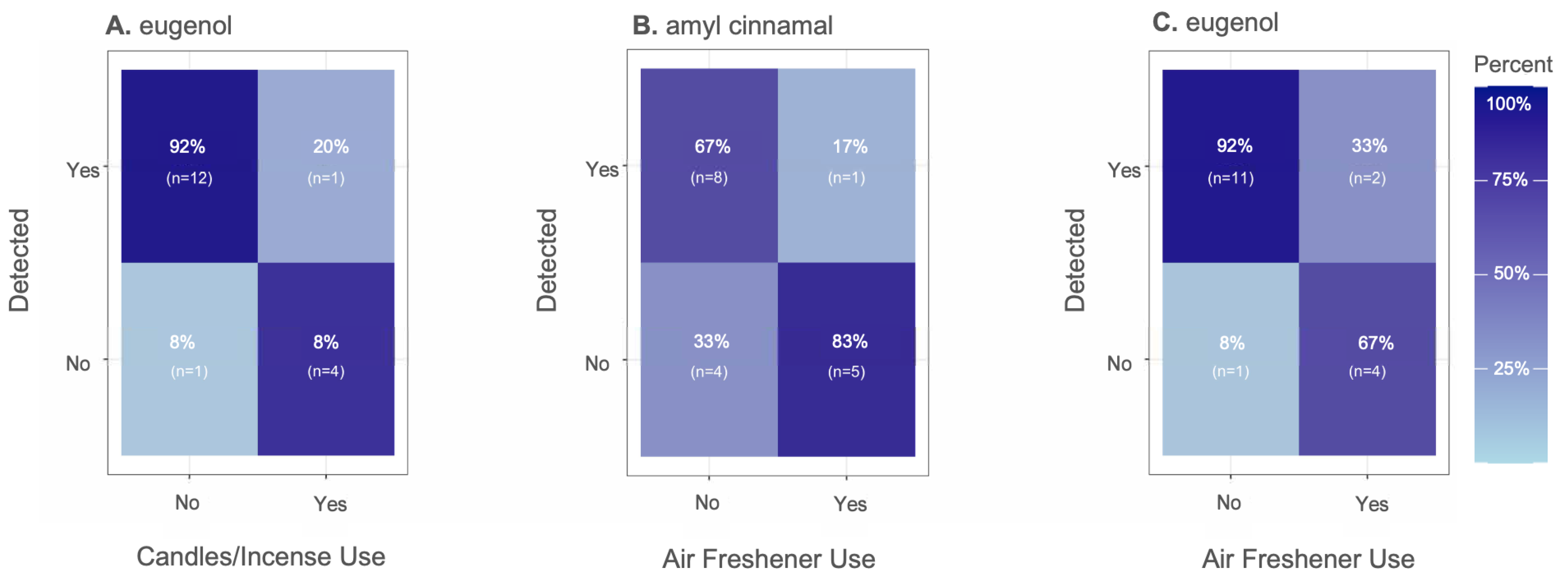
Household Behaviors, Environmental and Demographic Influences
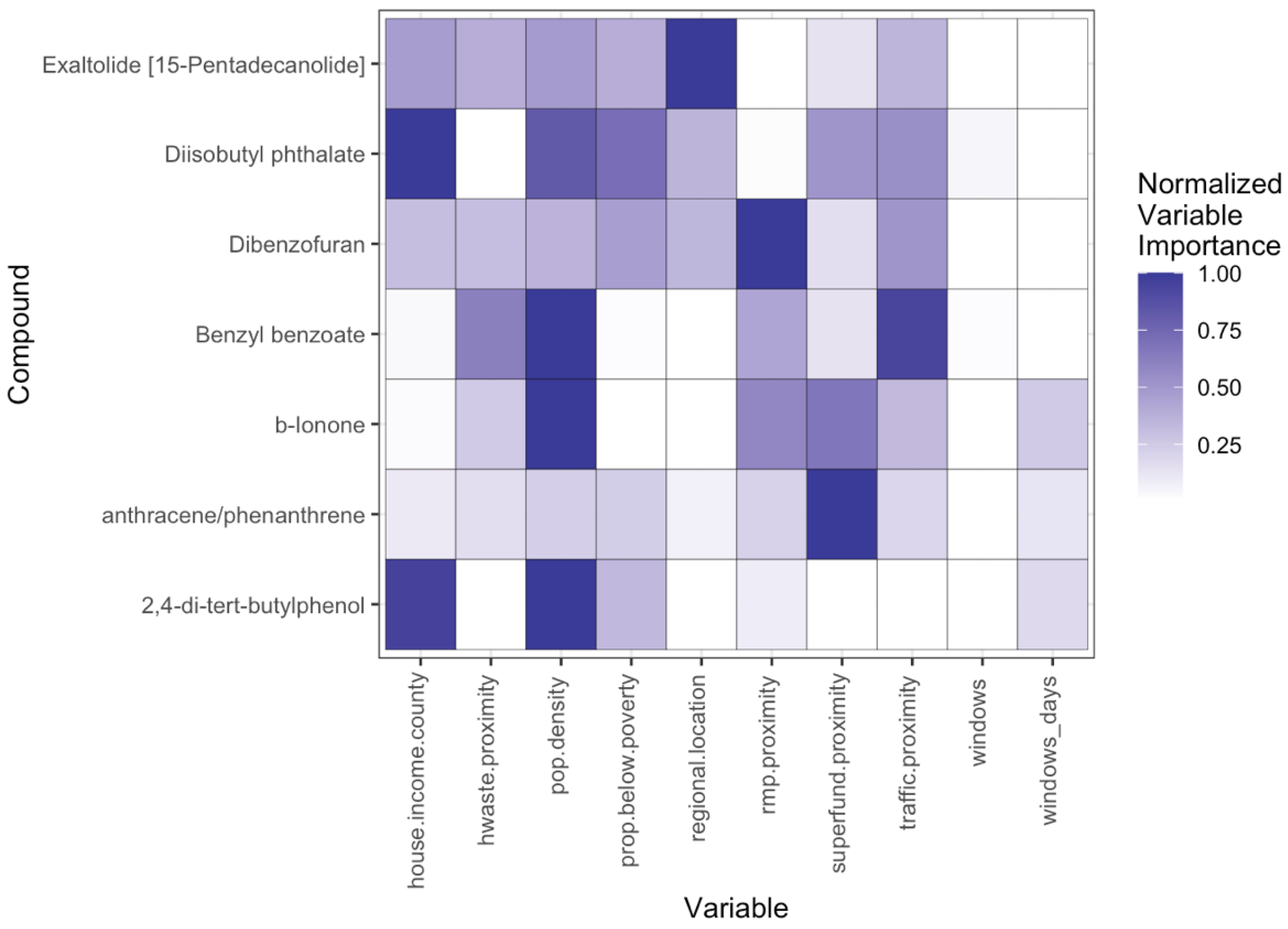
3.3. Influences on Chemical Profiles Multivariate
3.3.1. Household Behaviors, Environmental and Demographic Influences on Indoor Exposure Profiles
3.3.2. Household Behaviors, Environmental and Demographic Influences on Outdoor Exposure Profiles
3.3.3. Household Behaviors, Environmental and Demographic Influences on Indoor/Outdoor Exposure Ratios
4. Conclusions
5. Limitations
Sampling Locations and Sample Size
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bornehag, C.G.; Nanberg, E. Phthalate Exposure and Asthma in Children. Int. J. Androl. 2010, 33, 333–345. [Google Scholar] [CrossRef] [PubMed]
- Jaakkola, J.J.K.; Gissler, M. Maternal Smoking in Pregnancy, Fetal Development, and Childhood Asthma. Am. J. Public Health 2004, 94, 136–140. [Google Scholar] [CrossRef] [PubMed]
- Maragkidou, A.; Arar, S.; Al-Hunaiti, A.; Ma, Y.; Harrad, S.; Jaghbeir, O.; Faouri, D.; Hämeri, K.; Hussein, T. Occupational Health Risk Assessment and Exposure to Floor Dust PAHs inside an Educational Building. Sci. Total Environ. 2017, 579, 1050–1056. [Google Scholar] [CrossRef]
- Ataei, Y.; Sun, Y.; Liu, W.; Ellie, A.S.; Dong, H.; Ahmad, U.M. Health Effects of Exposure to Indoor Semi-Volatile Organic Compounds in Chinese Building Environment: A Systematic Review. Int. J. Environ. Res. Public Health 2023, 20, 678. [Google Scholar] [CrossRef]
- Rudel, R.A.; Perovich, L.J. Endocrine Disrupting Chemicals in Indoor and Outdoor Air. Atmos. Environ. 2009, 43, 170–181. [Google Scholar] [CrossRef]
- Swan, S.H.; Main, K.M.; Liu, F.; Stewart, S.L.; Kruse, R.L.; Calafat, A.M.; Mao, C.S.; Redmon, J.B.; Ternand, C.L.; Sullivan, S.; et al. Decrease in Anogenital Distance among Male Infants with Prenatal Phthalate Exposure. Environ. Health Perspect. 2005, 113, 1056–1061. [Google Scholar] [CrossRef]
- David, O. Carpenter. Polychlorinated Biphenyls (PCBs): Routes of Exposure and Effects on Human Health. Rev. Environ. Health 2006, 21, 1–24. [Google Scholar] [CrossRef]
- Gascon, M.; Vrijheid, M.; Martínez, D.; Forns, J.; Grimalt, J.O.; Torrent, M.; Sunyer, J. Effects of Pre and Postnatal Exposure to Low Levels of Polybromodiphenyl Ethers on Neurodevelopment and Thyroid Hormone Levels at 4years of Age. Environ. Int. 2011, 37, 605–611. [Google Scholar] [CrossRef]
- Herbstman, J.B.; Mall, J.K. Developmental Exposure to Polybrominated Diphenyl Ethers and Neurodevelopment. Curr. Environ. Health Report 2014, 1, 101–112. [Google Scholar] [CrossRef]
- Boffetta, P.; Jourenkova, N.; Gustavsson, P. Cancer Risk from Occupational and Environmental Exposure to Polycyclic Aromatic Hydrocarbons. Cancer Causes Control 1997, 8, 444–472. [Google Scholar] [CrossRef]
- Melymuk, L.; Bohlin-Nizzetto, P.; Vojta, Š.; Krátká, M.; Kukučka, P.; Audy, O.; Přibylová, P.; Klánová, J. Distribution of Legacy and Emerging Semivolatile Organic Compounds in Five Indoor Matrices in a Residential Environment. Chemosphere 2016, 153, 179–186. [Google Scholar] [CrossRef]
- Addington, C.K.; Phillips, K.A.; Isaacs, K.K. Estimation of the Emission Characteristics of SVOCs from Household Articles Using Group Contribution Methods. Environ. Sci. Technol. 2020, 54, 110–119. [Google Scholar] [CrossRef] [PubMed]
- Garrido, J.A.; Parthasarathy, S.; Moschet, C.; Young, T.M.; McKone, T.E.; Bennett, D.H. Exposure Assessment For Air-To-Skin Uptake of Semivolatile Organic Compounds (SVOCs) Indoors. Environ. Sci. Technol. 2019, 53, 1608–1616. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Zhang, J. Understanding SVOCs. ASHRAE J. 2011, 6, 121–125. [Google Scholar]
- Weschler, C.J.; Nazaroff, W.W. Semivolatile Organic Compounds in Indoor Environments. Atmos. Environ. 2008, 42, 9018–9040. [Google Scholar] [CrossRef]
- Demirtepe, H.; Melymuk, L.; Diamond, M.L.; Bajard, L.; Vojta, Š.; Prokeš, R.; Sáňka, O.; Klánová, J.; Palkovičová Murínová, Ľ.; Richterová, D.; et al. Linking Past Uses of Legacy SVOCs with Today’s Indoor Levels and Human Exposure. Environ. Int. 2019, 127, 653–663. [Google Scholar] [CrossRef]
- Rudel, R.A.; Camann, D.E.; Spengler, J.D.; Korn, L.R.; Brody, J.G. Phthalates, Alkylphenols, Pesticides, Polybrominated Diphenyl Ethers, and Other Endocrine-Disrupting Compounds in Indoor Air and Dust. Environ. Sci. Technol. 2003, 37, 4543–4553. [Google Scholar] [CrossRef]
- Lucattini, L.; Poma, G.; Covaci, A.; de Boer, J.; Lamoree, M.H.; Leonards, P.E.G. A Review of Semi-Volatile Organic Compounds (SVOCs) in the Indoor Environment: Occurrence in Consumer Products, Indoor Air and Dust. Chemosphere 2018, 201, 466–482. [Google Scholar] [CrossRef]
- Petry, T.; Vitale, D.; Joachim, F.J.; Smith, B.; Cruse, L.; Mascarenhas, R.; Schneider, S.; Singal, M. Human Health Risk Evaluation of Selected VOC, SVOC and Particulate Emissions from Scented Candles. Regul. Toxicol. Pharmacol. 2014, 69, 55–70. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, H.; Xu, B.; Wang, H.; Wang, Y.; Yang, T.; Tan, Y.; Xiong, J.; Liu, X. Predicting the Emissions of VOCs/SVOCs in Source and Sink Materials: Development of Analytical Model and Determination of the Key Parameters. Environ. Int. 2022, 160, 107064. [Google Scholar] [CrossRef]
- Weschler, C.J. Changes in Indoor Pollutants since the 1950s. Atmos. Environ. 2009, 43, 153–169. [Google Scholar] [CrossRef]
- Uhde, E.; Varol, D.; Mull, B.; Salthammer, T. Distribution of Five SVOCs in a Model Room: Effect of Vacuuming and Air Cleaning Measures. Environ. Sci. Process. Impacts 2019, 21, 1353–1363. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, Q.; Hou, J.; Wang, P.; Sundell, J. Exposure of Phthalates in Residential Buildings and Its Health Effects. Procedia Eng. 2017, 205, 1901–1904. [Google Scholar] [CrossRef]
- Eichler, C.M.A.; Hubal, E.A.C.; Xu, Y.; Cao, J.; Bi, C.; Weschler, C.J.; Salthammer, T.; Morrison, G.C.; Koivisto, A.J.; Zhang, Y.; et al. Assessing Human Exposure to SVOCs in Materials, Products, and Articles: A Modular Mechanistic Framework. Environ. Sci. Technol. 2021, 55, 25–43. [Google Scholar] [CrossRef] [PubMed]
- Mage, D.; Ozolins, G.; Peterson, P.; Webster, A.; Orthofer, R.; Vandeweerd, V.; Gwynne, M. Urban Air Pollution in Megacities of the World. Atmos. Environ. 1996, 30, 681–686. [Google Scholar] [CrossRef]
- Lawson, S.J.; Galbally, I.E.; Powell, J.C.; Keywood, M.D.; Molloy, S.B.; Cheng, M.; Selleck, P.W. The Effect of Proximity to Major Roads on Indoor Air Quality in Typical Australian Dwellings. Atmos. Environ. 2011, 45, 2252–2259. [Google Scholar] [CrossRef]
- Tong, Z.; Chen, Y.; Malkawi, A.; Adamkiewicz, G.; Spengler, J.D. Quantifying the Impact of Traffic-Related Air Pollution on the Indoor Air Quality of a Naturally Ventilated Building. Environ. Int. 2016, 89–90, 138–146. [Google Scholar] [CrossRef]
- Majd, E.; McCormack, M.; Davis, M.; Curriero, F.; Berman, J.; Connolly, F.; Leaf, P.; Rule, A.; Green, T.; Clemons-Erby, D.; et al. Indoor Air Quality in Inner-City Schools and Its Associations with Building Characteristics and Environmental Factors. Environ. Res. 2019, 170, 83–91. [Google Scholar] [CrossRef]
- Vorhees, D.J.; Cullen, A.C.; Altshul, L.M. Exposure to Polychlorinated Biphenyls in Residential Indoor Air and Outdoor Air near a Superfund Site. Environ. Sci. Technol. 1997, 31, 3612–3618. [Google Scholar] [CrossRef]
- Burwell-Naney, K.; Zhang, H.; Samantapudi, A.; Jiang, C.; Dalemarre, L.; Rice, L.; Williams, E.; Wilson, S. Spatial Disparity in the Distribution of Superfund Sites in South Carolina: An Ecological Study. Environ. Health 2013, 12, 96. [Google Scholar] [CrossRef]
- Kramar, D.E.; Anderson, A.; Hilfer, H.; Branden, K.; Gutrich, J.J. A Spatially Informed Analysis of Environmental Justice: Analyzing the Effects of Gerrymandering and the Proximity of Minority Populations to U.S. Superfund Sites. Environ. Justice 2018, 11, 29–39. [Google Scholar] [CrossRef]
- Perlin, S.A.; Wong, D.; Sexton, K. Residential Proximity to Industrial Sources of Air Pollution: Interrelationships among Race, Poverty, and Age. J. Air Waste Manag. Assoc. 2001, 51, 406–421. [Google Scholar] [CrossRef]
- Ferro, A.R.; Kopperud, R.J.; Hildemann, L.M. Source Strengths for Indoor Human Activities That Resuspend Particulate Matter. Environ. Sci. Technol. 2004, 38, 1759–1764. [Google Scholar] [CrossRef]
- Huckins, J.N.; Petty, J.D.; Booij, K. Monitors of Organic Chemicals in the Environment: Semipermeable Membrane Devices; Springer: New York, NY, USA, 2006. [Google Scholar]
- Dixon, H.M.; Poutasse, C.M.; Anderson, K.A. Silicone Wristbands and Wearables to Assess Chemical Exposures. In Total Exposure Health; CRC Press: Boca Raton, FL, USA, 2020. [Google Scholar]
- Greenberg, M.S.; Chapman, P.M.; Allan, I.J.; Anderson, K.A.; Apitz, S.E.; Beegan, C.; Bridges, T.S.; Brown, S.S.; Cargill IV, J.G.; McCulloch, M.C.; et al. Passive Sampling Methods for Contaminated Sediments: Risk Assessment and Management. Integr. Environ. Assess. Manag. 2014, 10, 224–236. [Google Scholar] [CrossRef]
- Ghetu, C.C.; Rohlman, D.; Smith, B.W.; Scott, R.P.; Adams, K.A.; Hoffman, P.D.; Anderson, K.A. Wildfire Impact on Indoor and Outdoor PAH Air Quality. Environ. Sci. Technol. 2022, 56, 10042–10052. [Google Scholar] [CrossRef]
- Allan, S.E.; Smith, B.W.; Anderson, K.A. Impact of the Deepwater Horizon Oil Spill on Bioavailable Polycyclic Aromatic Hydrocarbons in Gulf of Mexico Coastal Waters. Environ. Sci. Technol. 2012, 46, 2033–2039. [Google Scholar] [CrossRef]
- Donald, C.E.; Elie, M.R.; Smith, B.W.; Hoffman, P.D.; Anderson, K.A. Transport Stability of Pesticides and PAHs Sequestered in Polyethylene Passive Sampling Devices. Env. Sci. Pollut. Res. 2016, 23, 12392–12399. [Google Scholar]
- Paulik, L.B.; Donald, C.E.; Smith, B.W.; Tidwell, L.G.; Hobbie, K.A.; Kincl, L.; Haynes, E.N.; Anderson, K.A. Emissions of Polycyclic Aromatic Hydrocarbons from Natural Gas Extraction into Air. Environ. Sci. Technol. 2016, 50, 7921–7929. [Google Scholar] [CrossRef] [PubMed]
- Poutasse, C.M.; Poston, W.S.C.; Jahnke, S.A.; Haddock, C.K.; Tidwell, L.G.; Hoffman, P.D.; Anderson, K.A. Discovery of Firefighter Chemical Exposures Using Military-Style Silicone Dog Tags. Environ. Int. 2020, 142, 105818. [Google Scholar] [CrossRef]
- Minick, D.J.; Anderson, K.A. Diffusive Flux of PAHs across Sediment–Water and Water–Air Interfaces at Urban Superfund Sites. Environ. Toxicol. Chem. 2017, 36, 2281–2289. [Google Scholar] [CrossRef]
- Bergmann, A.J.; Points, G.L.; Scott, R.P.; Wilson, G.; Anderson, K.A. Development of quantitative screen for 1550 chemicals with GC-MS. Anal. Bioanal. Chem. 2018, 410, 3101–3110. [Google Scholar] [PubMed]
- Petty, J.D.; Huckins, J.N.; Martin, D.B.; Adornato, T.G. Use of semipermeable membrane devices (SPMDS) to determine bioavailable organochlorine pesticide residues in streams receiving irrigation drainwater. Chemosphere 1995, 30, 1891–1903. [Google Scholar] [CrossRef]
- Tidwell, L.G.; Allan, S.E.; O’Connell, S.G.; Hobbie, K.A.; Smith, B.W.; Anderson, K.A. PAH and OPAH Flux during the Deepwater Horizon Incident. Environ. Sci. Technol. 2016, 50, 7489–7497. [Google Scholar] [CrossRef] [PubMed]
- Donald, C.E.; Anderson, K.A. Assessing Soil-Air Partitioning of PAHs and PCBs with a New Fugacity Passive Sampler. Sci. Total Environ. 2017, 596–597, 293–302. [Google Scholar]
- Williams, A.J.; Grulke, C.M.; Edwards, J.; McEachran, A.D.; Mansouri, K.; Baker, N.C.; Patlewicz, G.; Shah, I.; Wambaugh, J.F.; Judson, R.S.; et al. The CompTox Chemistry Dashboard: A Community Data Resource for Environmental Chemistry. J. Cheminform 2017, 9, 61. [Google Scholar] [CrossRef]
- Homepage—ECHA. Available online: https://echa.europa.eu/ (accessed on 25 May 2022).
- The Good Scents Company. 1,2-Dimethyl Naphthalene, 573-98-8. Available online: http://www.thegoodscentscompany.com/data/rw1145701.html (accessed on 2 October 2021).
- U.S. Census Bureau. Geographic Division or Region—Health, United States. Available online: https://www.cdc.gov/nchs/hus/sources-definitions/geographic-region.htm (accessed on 2 October 2021).
- US EPA, O. EJScreen: Environmental Justice Screening and Mapping Tool. Available online: https://19january2021snapshot.epa.gov/ejscreen_.html (accessed on 25 May 2022).
- Benjamini, Y.; Hochberg, Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J. R. Stat. Soc. Ser. B (Methodol.) 1995, 57, 289–300. [Google Scholar]
- Webb-Robertson, B.-J.M.; Matzke, M.M.; Metz, T.O.; McDermott, J.E.; Walker, H.; Rodland, K.D.; Pounds, J.G.; Waters, K.M. Sequential Projection Pursuit Principal Component Analysis—Dealing with Missing Data Associated with New -Omics Technologies. BioTechniques 2013, 54, 165–168. [Google Scholar] [CrossRef]
- Breiman, L. Classification and Regression Trees; Routledge: New York, NY, USA, 2017. [Google Scholar]
- Therneau, T.; Atkinson, B.; Port, B.R. (Producer of the Initial R.; Maintainer 1999–2017). Rpart: Recursive Partitioning and Regression Trees. 2022; Available online: https://cran.r-project.org/web/packages/rpart/index.html (accessed on 2 October 2023).
- Lisa Bramer, Brianna Rivera. Univariate Analysis. Available online: https://pnnl-superfund-research-center.github.io/Rivera_etal_Supplemental/ (accessed on 2 July 2024).
- Nieuwenhuijsen, M. Design of Exposure Questionnaires for Epidemiological Studies. Occup. Environ. Med. 2005, 62, 272–280. [Google Scholar] [CrossRef]
- Gaydos, R.M. Kirk-Othmer Encycl Chem Tech. Wiley N. Y. 1981, 15, 698–719. [Google Scholar]
- Center for Disease Control. Biomonitoring Summary | CDC. Available online: https://www.cdc.gov/biomonitoring/about/index.html (accessed on 2 October 2023).
- Thakore, K.N.; Mehendale, H.M. Dibenzofuran. In Encyclopedia of Toxicology (Third Edition); Wexler, P., Ed.; Academic Press: Oxford, UK, 2014; pp. 67–69. [Google Scholar] [CrossRef]
- Zillow: Real Estate, Apartments, Mortgages & Home Values. Available online: https://www.zillow.com/ (accessed on 11 October 2023).
- Dixon, H.M.; Armstrong, G.; Barton, M.; Bergmann, A.J.; Bondy, M.; Halbleib, M.L.; Hamilton, W.; Haynes, E.; Herbstman, J.; Hoffman, P.; et al. Discovery of Common Chemical Exposures across Three Continents Using Silicone Wristbands. R. Soc. Open Sci. 2019, 6. [Google Scholar] [CrossRef]
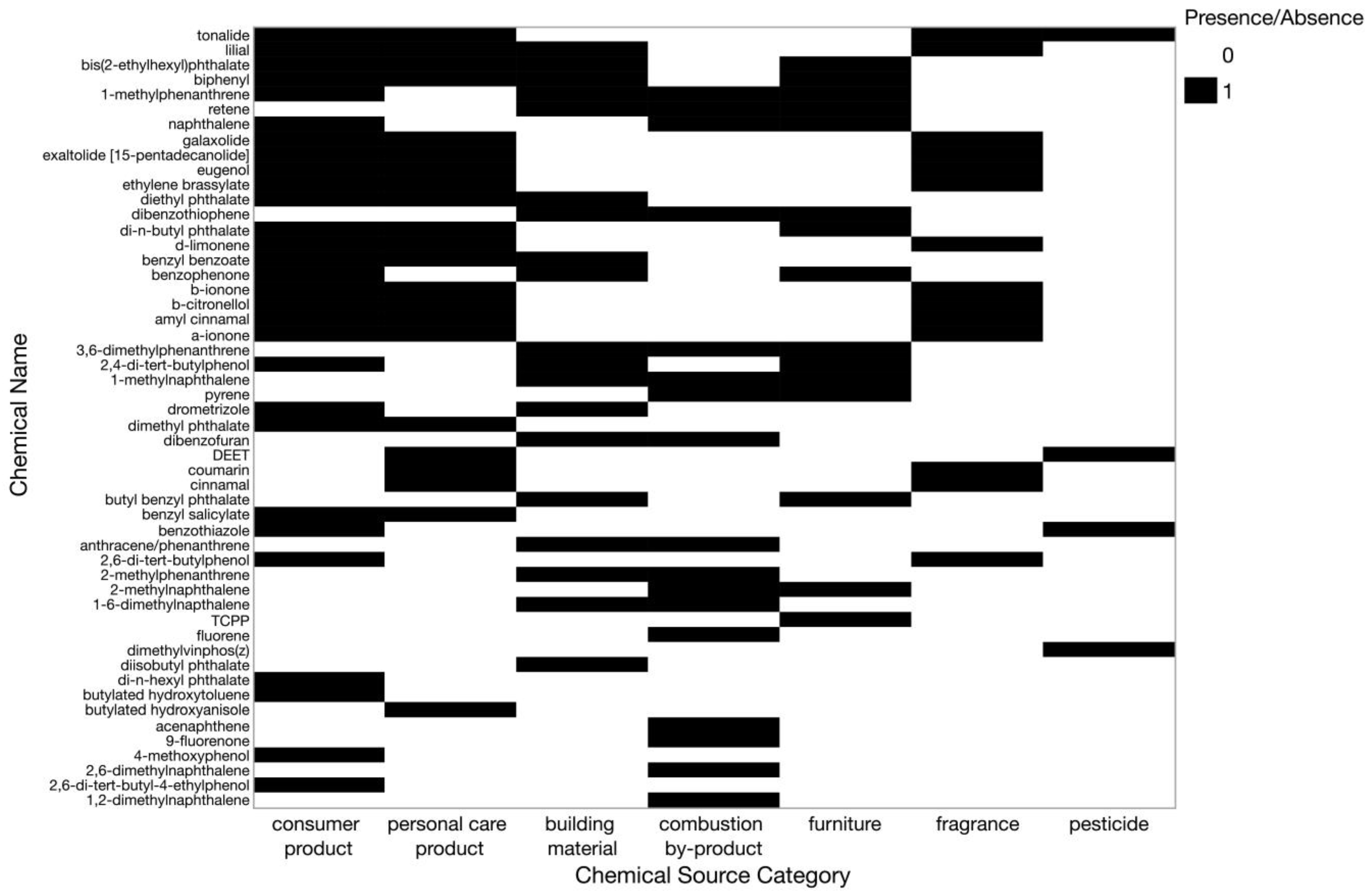


| Chemical Source Category | Associated Sources |
|---|---|
| building material | paints, paints, coatings, adhesives, carpet, carpet padding, vinyl flooring |
| combustion by-product | vehicle exhaust, biomass burning, burning candles/incense, cooking or grilling |
| consumer product | children’s toys, shower curtains, cleaning products, detergents, air fresheners, lubricants, greases, clothing, polishes and waxes |
| fragrance | perfume, fragrance |
| furniture | fabric/vinyl upholstery |
| personal care product | sunscreen, shaving cream, hand soap, perfumes, cosmetics, toothpaste, deodorant, lipstick, shampoo, baby soap |
| pesticide | inert pesticide ingredients, pesticide |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rivera, B.N.; Bramer, L.M.; Ghetu, C.C.; Rohlman, D.; Adams, K.; Waters, K.M.; Anderson, K.A. Investigation of Influences on Indoor and Outdoor SVOC Exposure. Int. J. Environ. Res. Public Health 2025, 22, 556. https://doi.org/10.3390/ijerph22040556
Rivera BN, Bramer LM, Ghetu CC, Rohlman D, Adams K, Waters KM, Anderson KA. Investigation of Influences on Indoor and Outdoor SVOC Exposure. International Journal of Environmental Research and Public Health. 2025; 22(4):556. https://doi.org/10.3390/ijerph22040556
Chicago/Turabian StyleRivera, Brianna N., Lisa M. Bramer, Christine C. Ghetu, Diana Rohlman, Kaley Adams, Katrina M. Waters, and Kim A. Anderson. 2025. "Investigation of Influences on Indoor and Outdoor SVOC Exposure" International Journal of Environmental Research and Public Health 22, no. 4: 556. https://doi.org/10.3390/ijerph22040556
APA StyleRivera, B. N., Bramer, L. M., Ghetu, C. C., Rohlman, D., Adams, K., Waters, K. M., & Anderson, K. A. (2025). Investigation of Influences on Indoor and Outdoor SVOC Exposure. International Journal of Environmental Research and Public Health, 22(4), 556. https://doi.org/10.3390/ijerph22040556







