Internet-Based Psycho-Physical Exercise Intervention Program in Mild-to-Moderate Depression: The Study Protocol of the SONRIE Randomized Controlled Trial
Abstract
1. Introduction
2. Materials and Methods
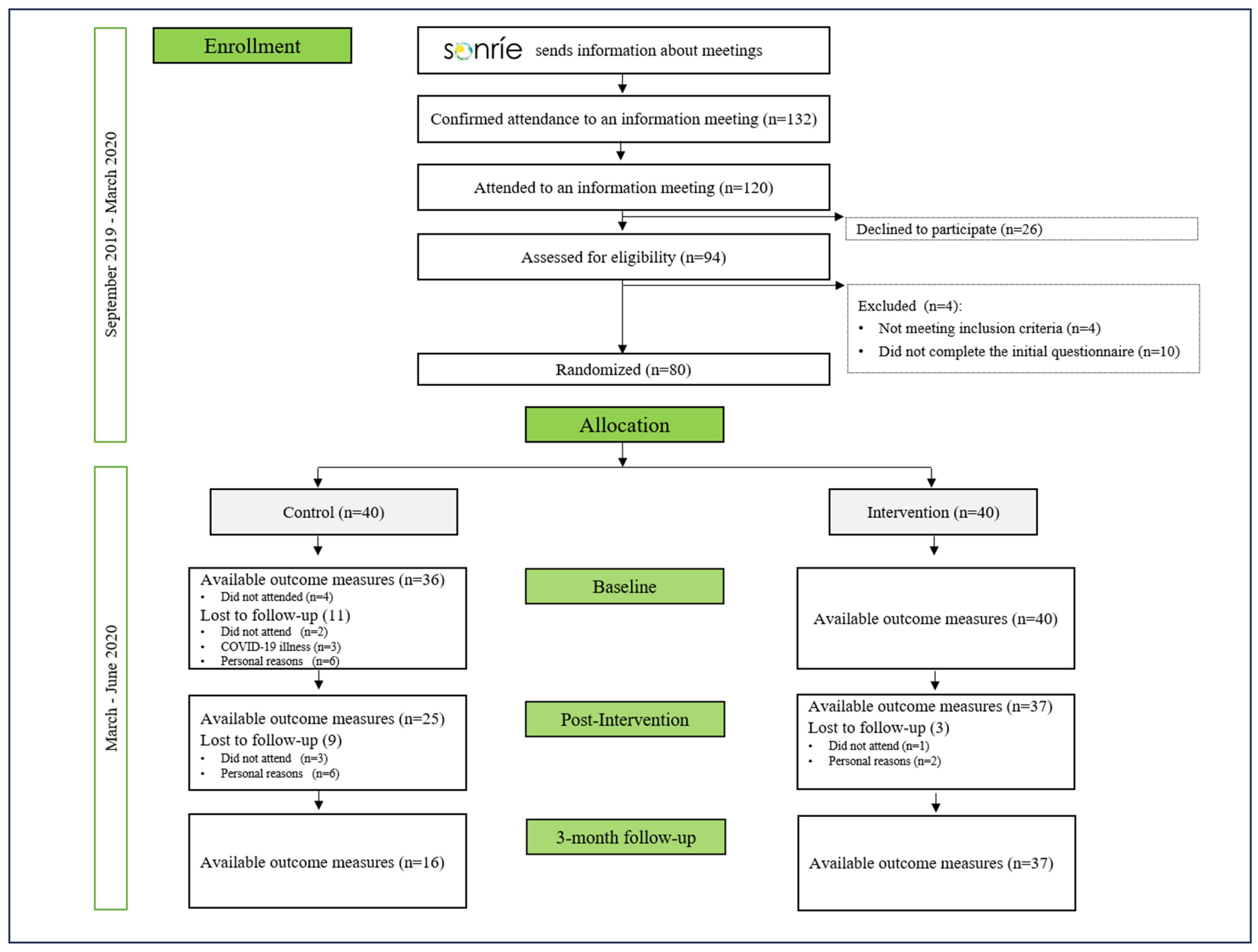
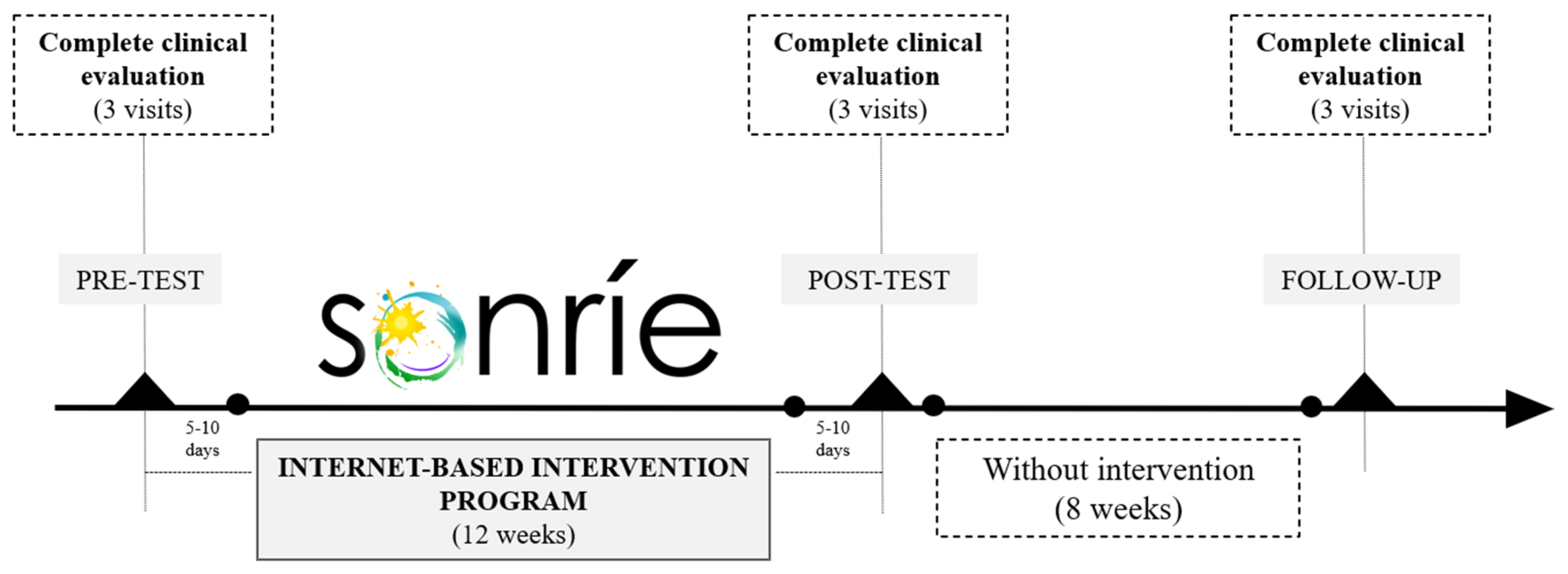
2.1. Study Design and Protocol Registration
2.2. Recruitment and Eligibility Criteria
2.3. Sample Size and Randomization
2.4. Data Collection
2.5. Primary Outcome
2.6. Secondary Outcomes
2.6.1. Physical Domain
2.6.2. Psychological Domain
2.6.3. Control Variables
2.7. Internet-Based Psycho-Physical Exercise Intervention Program
2.7.1. Control Group (CG, Usual Care)
2.7.2. Experimental Group (EG, Enhanced Care with Physical Exercise and iCBT)
2.8. Statistical Analysis
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 2-AG | 2-Arachidonoylglycerol |
| 2-LG | linoleoyl glycerol |
| 2-OG | oleoyl glycerol |
| AEA | Anandamide |
| ANCOVA | Analysis of Covariance |
| BDI | Beck Depression Inventory |
| BDNF | Brain-Derived Neurotrophic Factor |
| CERT | Consensus on Exercise Reporting Template |
| CG | Control Group |
| CRF | Cardiorespiratory Fitness |
| eCBs | Plasma levels of endocannabinoids |
| EG | Experimental Group |
| ELISA | Enzyme-Linked Immunosorbent Assay |
| ES | Endocannabinoid System |
| FFQ | Food Frequency Questionnaire |
| GAD-7 | Generalized Anxiety Disorder 7-item |
| GPAQ | Global Physical Activity Questionnaire |
| iCBT | Internet-Based Cognitive Behavioral Therapy |
| ICD-10 | International Classification of Diseases, 10th Revision |
| LC-MS/MS | Liquid Chromatography–Tandem Mass Spectrometry |
| LEA | Linoleoylethanolamide |
| DEA | Dihomo-γ-linolenoyl ethanolamide |
| DHEA | Docosahexaenoylethanolamide |
| MAIA | Multidimensional Assessment of Interoceptive Awareness |
| OEA | Oleoylethanolamide |
| PANAS | Positive and Negative Affect Scale |
| PA | Physical Activity |
| RCT | Randomized Controlled Trial |
| RPE | Rate of Perceived Exertion |
| SEA | Stearoylethanolamide |
| SF-36 | 36-Item Short-Form Health Survey |
Appendix A
| Week | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Warm-Up | Objective | Prepare the body for physical activity by increasing heart rate, enhancing blood flow to muscles, and improving flexibility, which reduces the risk of injury | |||||||||||||
| Exercise | Low-intensity aerobic activity involving dynamic stretches and mobility exercises | ||||||||||||||
| Total time | 7–10′ | ||||||||||||||
| Main Exercise Segment | Balance Training | Objective | Improve coordination and postural control | Increase balance and stability | Enhance core stability and proprioception | Develop advanced balance skills and functional coordination | |||||||||
| Exercises | 4 Static Exercises, e.g., Tandem Stand, Single-Leg Stand, and Hip Flexion Exercise | 4 Dynamic Exercises e.g., Tandem Stand with Arm Movements, Dynamic Hip Movements, Single-Leg Stand with Dynamic Movements | 4 Complex Exercises, e.g., Eyes Closed Balance Exercises, Combined Upper and Lower Body Movements, Dynamic Balance with Sensory Deprivation | 4 Advanced Balance and Coordination Exercises, e.g., Balance Exercises with Object Manipulation, Heel-to-Toe Walking, Single-Leg Stand with Object Manipulation | |||||||||||
| Volume (Sets/Rest) | |||||||||||||||
| Sets | 3 | 3 | |||||||||||||
| Work | 10″ | 15″ | |||||||||||||
| /Rest between sets | /30″ | /30″ | |||||||||||||
| Total time | 10′ | 10′ | |||||||||||||
| Intensity (RPE) | 1–3 | ||||||||||||||
| RESISTANCE TRAINING | Objective | Focus on learning movement patterns and improving motor control using bodyweight exercises | Increase strength and core stability by working with time under tension | Strength training with time under tension and progression to light-to-moderate weights | |||||||||||
| Exercises | 7 Bodyweight Squats, Lunges (Lateral and Fronts), Biceps Curls, Push-ups, Bent-Over Rows, Shoulder Presses | ||||||||||||||
| Volume (Sets/rest) | |||||||||||||||
| Sets | 3 | 3 | 3 | 3 | 4 | 3 | 4 | 3 | 3 | 3 | 3 | 3 | |||
| Work | 8 reps | 10 reps | 12 reps | 40″ | 30″ | 40 | 20″ | 35″ | 30″ | 30″ | 25″ | 25″ | |||
| Rest | 20″ | 20″ | 30″ | 30″ | 20″ | 20″ | 15″ | 15″ | 15″ | ||||||
| /Rest between sets | /45″ | /45″ | /45″ | /60″ | /60″ | /60″ | /60″ | /60″ | /60″ | /60″ | /60″ | /45″ | |||
| Total time | 25′ | 25′ | 25′ | 35′ | 35′ | 35′ | 35′ | 35′ | 30′ | 30′ | 30′ | 30′ | |||
| Intensity (RPE) | 3–4 | 4–5 | 5–6 | 6–7 | 7–8 | ||||||||||
| CRF TRAINING | Objective | Emphasizes building endurance | Increasing CRF endurance | Challenges CRF further | |||||||||||
| Exercises | Light-intensity aerobic exercises, such as marching, side-to-side steps, bringing foot to opposite ankle and glute | Moderate-intensity aerobic activity, such as marching, V-step with knee lifts, V-step with heel to glute, and combinations of these with arm movements | Moderate to vigorous-intensity aerobic activity, combining steps like V-step with knee lifts and heels to glute with faster pace and arm movements | ||||||||||||
| Volume (Sets/rest) | |||||||||||||||
| Sets | 3 | 3 | 2 | 2 | 2 | ||||||||||
| Work | 3′30″ | 6′-3′-2′ | 8′-4′ | 9′-3′ | 9′-4′ | ||||||||||
| /Rest between sets | /60″ | /60″ | /60″ | /60″ | /45″ | ||||||||||
| Total time | 13′ | 13′ | 13′ | 13′ | 14′ | ||||||||||
| Intensity (RPE) | 3–4 | 4–5 | 5–6 | 6–7 | 7–8 | ||||||||||
| Cool-Down | Objective | Promote muscle recovery, and aid in the removal of metabolic waste, helping to prevent muscle stiffness and reduce the risk of injury | |||||||||||||
| Exercise | Static and dynamic stretches for major muscle groups, postural adjustments, and guided breathing | ||||||||||||||
| Total time | 8–10′ | ||||||||||||||
| Week | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | |||
| Week | Module | Tittle | Content |
|---|---|---|---|
| 1 | Module 1 | Welcome and introduction | Explanation of the relationship between PA, mood, and emotional states, as well as the significance of PA for overall well-being. Introduction to the subjective perception of effort (RPE). |
| 2 | Module 2 | Anxiety | Psycho-education about what is anxiety; cognitive, somatic, and behavioral anxiety responses; physical and psychological symptoms; and how it affects us. |
| Module 3 | PA to cope with anxiety | Learn to identify cognitive and somatic anxiety and how we can use PA to reduce anxiety symptoms. | |
| 3 | Module 4 | Emotions | Psycho-education about what are basic and social emotions, different functions of each emotion, how emotions work, and how they manifest. |
| Module 5 | Managing emotions | How to regulate our emotions: various approaches to emotional regulation. | |
| 4 | Module 6 | Bottom-up emotional regulation | Psycho-education about interoceptive awareness (heart rate, breath, and arousal), association among emotions and body posture, and how sadness manifests in our body. |
| Module 7 | Strategies for bottom-up emotional regulation | Practicing bottom-up emotion regulation: practicing attention and posture correction, Jacobson’s relaxation technique and other emotion regulation skills (music, PA, smile, etc.). | |
| 5 | Module 8 | Top-down emotional regulation | Psycho-education about how our thoughts can activate or intensify negative emotions. |
| Module 9 | Strategies for top-down emotional regulation | Practicing top-down emotion regulation: Learn to identify and modify negative thoughts and use reappraisal. | |
| 6 | Module 10 | Emotional regulation through behavior | Psycho-education about how depression or negative mood modulates body posture, facial expression, and arousal, and how exercise can improve posture, increase energy levels, and positively impact mood. |
| Module 11 | Cognitive flexibility | Cognitive flexibility and factors and behaviors that contribute to intensifying our emotional discomfort. | |
| 7 | Module 12 | Coping skills | Psycho-education about different coping skills focused on solving problems and focused on emotion and avoidance coping. |
| Module 13 | Applying coping skills | Practical examples of the application of different coping strategies to real problems. | |
| 8 | Module 14 | The role of serotonin as a neurotransmitter | What is serotonin, what are its functions, and some non-pharmacological methods that can help regulate serotonin. |
| 9 | Module 15 | The role of dopamine as a neurotransmitter | What is dopamine, what are its functions, and some non-pharmacological methods that can help regulate dopamine. |
| 10 | Module 16 | The role of endorphins as a neurotransmitter | What are endorphins, what are their functions, and some non-pharmacological methods that can help regulate endorphins. |
| 11 | Module 17 | The role of oxytocin as a neurotransmitter | What is oxytocin, what are its functions, and some non-pharmacological methods that can help regulate oxytocin. |
| 12 | Module 18 | Farewell and Conclusion | Summary, main conclusions, and closure. |
References
- Kupcova, I.; Danisovic, L.; Klein, M.; Harsanyi, S. Effects of the COVID-19 Pandemic on Mental Health, Anxiety, and Depression. BMC Psychol. 2023, 11, 108. [Google Scholar] [CrossRef]
- BOE-A-2020-3692 Real Decreto 463/2020, de 14 de Marzo, por El Que Se Declara El Estado de Alarma para La Gestión de La Situación de Crisis Sanitaria Ocasionada por El COVID-19. Available online: https://www.boe.es/buscar/act.php?id=BOE-A-2020-3692 (accessed on 22 May 2024).
- Coronavirus Disease (COVID-19) Pandemic. Available online: https://www.who.int/europe/emergencies/situations/covid-19 (accessed on 22 May 2024).
- Kong, H.; Government, C.; Ministry, T. Mitigate the Effects of Home Confinement on Children during the COVID-19 Outbreak. Lancet 2020, 395, 945–947. [Google Scholar]
- Cao, W.; Fang, Z.; Hou, G.; Han, M.; Xu, X.; Dong, J.; Zheng, J. The Psychological Impact of the COVID-19 Epidemic on College Students in China. Psychiatry Res. 2020, 287, 112934. [Google Scholar] [CrossRef]
- Santomauro, D.F.; Mantilla Herrera, A.M.; Shadid, J.; Zheng, P.; Ashbaugh, C.; Pigott, D.M.; Abbafati, C.; Adolph, C.; Amlag, J.O.; Aravkin, A.Y.; et al. Global Prevalence and Burden of Depressive and Anxiety Disorders in 204 Countries and Territories in 2020 Due to the COVID-19 Pandemic. Lancet 2021, 398, 1700–1712. [Google Scholar] [CrossRef]
- Norouzi, E.; Naseri, A.; Rezaie, L.; Bender, A.M.; Salari, N.; Khazaie, H. Combined Mindfulness-Based Stress Reduction and Physical Activity Improved Psychological Factors and Sleep Quality in Patients with MDD: A Randomized Controlled Trial Study. Arch. Psychiatr. Nurs. 2024, 53, 215–223. [Google Scholar] [CrossRef] [PubMed]
- WHO. OMS | La depresión. OMS. 2016; 369. Available online: https://www.who.int/es/news-room/fact-sheets/detail/depression (accessed on 20 February 2023).
- Malhi, G.S.; Mann, J.J. Depression. Lancet 2018, 392, 2299–2312. [Google Scholar] [CrossRef] [PubMed]
- Khazaie, H.; Norouzi, E.; Rezaie, L.; Safari-Faramani, R. Effect of Physical Activity on Sleep Quality in Patients with Major Depression Disorder: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Curr. Psychol. 2023, 42, 28846–28856. [Google Scholar] [CrossRef]
- Rezaie, L.; Norouzi, E.; Bratty, A.J.; Khazaie, H. Better Sleep Quality and Higher Physical Activity Levels Predict Lower Emotion Dysregulation among Persons with Major Depression Disorder. BMC Psychol. 2023, 11, 171. [Google Scholar] [CrossRef]
- Gorzalka, B.B.; Hill, M.N. Putative Role of Endocannabinoid Signaling in the Etiology of Depression and Actions of Antidepressants. Prog. Neuropsychopharmacol. Biol. Psychiatry 2011, 35, 1575–1585. [Google Scholar] [CrossRef]
- Huang, W.J.; Chen, W.W.; Zhang, X. Endocannabinoid System: Role in Depression, Reward and Pain Control (Review). Mol. Med. Rep. 2016, 14, 2899–2903. [Google Scholar] [CrossRef]
- Zou, S.; Somvanshi, R.K.; Paik, S.; Kumar, U. Colocalization of Cannabinoid Receptor 1 with Somatostatin and Neuronal Nitric Oxide Synthase in Rat Brain Hypothalamus. J. Mol. Neurosci. 2015, 55, 480–491. [Google Scholar] [CrossRef]
- Brellenthin, A.G.; Crombie, K.M.; Hillard, C.J.; Koltyn, K.F. Endocannabinoid and Mood Responses to Exercise in Adults with Varying Activity Levels. Med. Sci. Sports Exerc. 2017, 49, 1688–1696. [Google Scholar] [CrossRef] [PubMed]
- Szuhany, K.L.; Bugatti, M.; Otto, M.W. A Meta-Analytic Review of the Effects of Exercise on Brain-Derived Neurotrophic Factor. J. Psychiatr. Res. 2015, 60, 56. [Google Scholar] [CrossRef] [PubMed]
- Hill, M.N.; McEwen, B.S. Involvement of the Endocannabinoid System in the Neurobehavioural Effects of Stress and Glucocorticoids. Prog. Neuropsychopharmacol. Biol. Psychiatry 2010, 34, 791–797. [Google Scholar] [CrossRef] [PubMed]
- Hillard, C.J. Circulating Endocannabinoids: From Whence Do They Come and Where Are They Going? Neuropsychopharmacology 2018, 43, 155. [Google Scholar] [CrossRef]
- Zarazúa-Guzmán, S.; Vicente-Martínez, J.G.; Pinos-Rodríguez, J.M.; Arevalo-Villalobos, J.I. An Overview of Major Depression Disorder: The Endocannabinoid System as a Potential Target for Therapy. Basic. Clin. Pharmacol. Toxicol. 2024, 135, 669–684. [Google Scholar] [CrossRef]
- Piomelli, D. The Molecular Logic of Endocannabinoid Signalling. Nat. Rev. Neurosci. 2003, 4, 873–884. [Google Scholar] [CrossRef]
- Matei, D.; Trofin, D.; Iordan, D.A.; Onu, I.; Condurache, I.; Ionite, C.; Buculei, I. The Endocannabinoid System and Physical Exercise. Int. J. Mol. Sci. 2023, 24, 1989. [Google Scholar] [CrossRef]
- Desai, S.; Borg, B.; Cuttler, C.; Crombie, K.M.; Rabinak, C.A.; Hill, M.N.; Marusak, H.A. A Systematic Review and Meta-Analysis on the Effects of Exercise on the Endocannabinoid System. Cannabis Cannabinoid Res. 2022, 7, 388–408. [Google Scholar] [CrossRef]
- Navarro, D.; Gasparyan, A.; Navarrete, F.; Torregrosa, A.B.; Rubio, G.; Marín-Mayor, M.; Acosta, G.B.; Garcia-Gutiérrez, M.S.; Manzanares, J. Molecular Alterations of the Endocannabinoid System in Psychiatric Disorders. Int. J. Mol. Sci. 2022, 23, 4764. [Google Scholar] [CrossRef]
- Bright, U.; Akirav, I. Modulation of Endocannabinoid System Components in Depression: Pre-Clinical and Clinical Evidence. Int. J. Mol. Sci. 2022, 23, 5526. [Google Scholar] [CrossRef] [PubMed]
- Zou, S.; Kumar, U. Cannabinoid Receptors and the Endocannabinoid System: Signaling and Function in the Central Nervous System. Int. J. Mol. Sci. 2018, 19, 833. [Google Scholar] [CrossRef]
- Bashshur, R.; Shannon, G.; Krupinski, E.; Grigsby, J. The Taxonomy of Telemedicine. Telemed. J. e-Health 2011, 17, 484–494. [Google Scholar] [CrossRef]
- Shih, Y.H.; Wang, J.Y.; Chou, P.H.; Lin, K.H. The Effects of Treatment via Telemedicine Interventions for Patients with Depression on Depressive Symptoms and Quality of Life: A Systematic Review and Meta-Ranalysis. Ann. Med. 2023, 55, 1092–1101. [Google Scholar] [CrossRef] [PubMed]
- Andrews, G.; Basu, A.; Cuijpers, P.; Craske, M.G.; McEvoy, P.; English, C.L.; Newby, J.M. Computer Therapy for the Anxiety and Depression Disorders Is Effective, Acceptable and Practical Health Care: An Updated Meta-Analysis. J. Anxiety Disord. 2018, 55, 70–78. [Google Scholar] [CrossRef]
- Carlbring, P.; Andersson, G.; Cuijpers, P.; Riper, H.; Hedman-Lagerlöf, E. Internet-Based vs. Face-to-Face Cognitive Behavior Therapy for Psychiatric and Somatic Disorders: An Updated Systematic Review and Meta-Analysis. Cogn. Behav. Ther. 2018, 47, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Cuijpers, P.; Cristea, I.A.; Karyotaki, E.; Reijnders, M.; Huibers, M.J.H. How Effective Are Cognitive Behavior Therapies for Major Depression and Anxiety Disorders? A Meta-Analytic Update of the Evidence. World Psychiatry 2016, 15, 245–258. [Google Scholar] [CrossRef] [PubMed]
- Biesheuvel-Leliefeld, K.E.M.; Kok, G.D.; Bockting, C.L.H.; Cuijpers, P.; Hollon, S.D.; Van Marwijk, H.W.J.; Smit, F. Effectiveness of Psychological Interventions in Preventing Recurrence of Depressive Disorder: Meta-Analysis and Meta-Regression. J. Affect. Disord. 2015, 174, 400–410. [Google Scholar] [CrossRef]
- Kandola, A.; Ashdown-Franks, G.; Hendrikse, J.; Sabiston, C.M.; Stubbs, B. Physical Activity and Depression: Towards Understanding the Antidepressant Mechanisms of Physical Activity. Neurosci. Biobehav. Rev. 2019, 107, 525–539. [Google Scholar] [CrossRef]
- Pereira-Miranda, E.; Costa, P.R.F.; Queiroz, V.A.O.; Pereira-Santos, M.; Santana, M.L.P. Overweight and Obesity Associated with Higher Depression Prevalence in Adults: A Systematic Review and Meta-Analysis. J. Am. Coll. Nutr. 2017, 36, 223–233. [Google Scholar] [CrossRef]
- Marques, A.; Gomez-Baya, D.; Peralta, M.; Frasquilho, D.; Santos, T.; Martins, J.; Ferrari, G.; de Matos, M.G. The Effect of Muscular Strength on Depression Symptoms in Adults: A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2020, 17, 5674. [Google Scholar] [CrossRef]
- Pedersen, B.K.; Saltin, B. Exercise as Medicine—Evidence for Prescribing Exercise as Therapy in 26 Different Chronic Diseases. Scand. J. Med. Sci. Sports 2015, 25 (Suppl. S3), 1–72. [Google Scholar] [CrossRef] [PubMed]
- World Medical Association. World Medical Association Declaration of Helsinki: Ethical Principles for Medical Research Involving Human Subjects. JAMA 2013, 310, 2191–2194. [Google Scholar] [CrossRef] [PubMed]
- The ICD-10 Classification of Mental and Behavioural Disorders: Clinical Descriptions and Diagnostic Guidelines. Available online: https://www.who.int/publications/i/item/9241544228 (accessed on 30 May 2024).
- Stelzer, E.M.; Book, S.; Graessel, E.; Hofner, B.; Kornhuber, J.; Luttenberger, K. Bouldering Psychotherapy Reduces Depressive Symptoms Even When General Physical Activity Is Controlled for: A Randomized Controlled Trial. Heliyon 2018, 4, e00580. [Google Scholar] [CrossRef]
- Eysenbach, G. The Law of Attrition. J. Med. Internet Res. 2005, 7, e11. [Google Scholar] [CrossRef]
- Christensen, H.; Griffiths, K.M.; Farrer, L. Adherence in Internet Interventions for Anxiety and Depression. J. Med. Internet Res. 2009, 11, e13. [Google Scholar] [CrossRef] [PubMed]
- Beck, A.T.; Ward, C.H.; Mendelson, M.; Mock, J.; Erbaugh, J. An Inventory for Measuring Depression. Arch. Gen. Psychiatry 1961, 4, 561–571. [Google Scholar] [CrossRef]
- Richter, P.; Werner, J.; Heerlein, A.; Kraus, A.; Sauer, H. On the Validity of the Beck Depression Inventory. A Review. Psychopathology 1998, 31, 160–168. [Google Scholar] [CrossRef]
- Scalco, A.Z.; Scalco, M.Z.; Azul, J.B.S.; Lotufo Neto, F. Hypertension and Depression. Clinics 2005, 60, 241–250. [Google Scholar] [CrossRef]
- Pietrobelli, A.; Rubiano, F.; St-Onge, M.P.; Heymsfield, S.B. New Bioimpedance Analysis System: Improved Phenotyping with Whole-Body Analysis. Eur. J. Clin. Nutr. 2004, 58, 1479–1484. [Google Scholar] [CrossRef]
- Norton, K.; Eston, R.G. Kinanthropometry and Exercise Physiology; Routledge Taylor and Francis Group: New York, NY, USA, 2019; ISBN 9781138230521. [Google Scholar]
- Sasaki, J.E.; John, D.; Freedson, P.S. Validation and Comparison of ActiGraph Activity Monitors. J. Sci. Med. Sport. 2011, 14, 411–416. [Google Scholar] [CrossRef]
- Troiano, R.P.; Berrigan, D.; Dodd, K.W.; Mâsse, L.C.; Tilert, T.; Mcdowell, M. Physical Activity in the United States Measured by Accelerometer. Med. Sci. Sports Exerc. 2008, 40, 181–188. [Google Scholar] [CrossRef]
- Freedson, P.S.; Melanson, E.; Sirard, J. Calibration of the Computer Science and Applications, Inc. Accelerometer. Med. Sci. Sports Exerc. 1998, 30, 777–781. [Google Scholar] [CrossRef]
- Cleland, C.L.; Hunter, R.F.; Kee, F.; Cupples, M.E.; Sallis, J.F.; Tully, M.A. Validity of the Global Physical Activity Questionnaire (GPAQ) in Assessing Levels and Change in Moderate-Vigorous Physical Activity and Sedentary Behaviour. BMC Public Health 2014, 14, 1255. [Google Scholar] [CrossRef] [PubMed]
- Arango Vélez, E.F.; Echavarría Rodríguez, A.M.; Aguilar González, F.A.; Patiño Villada, F.A. Validación de Dos Cuestionarios Para Evaluar El Nivel de Actividad Física y El Tiempo Sedentario En Una Comunidad Universitaria de Colombia. Rev. Fac. Nac. Salud Pública 2020, 38, 1–11. [Google Scholar] [CrossRef][Green Version]
- Guyatt, G.H.; Sullivan, M.J.; Thompson, P.J.; Fallen, E.L.; Pugsley, S.O.; Taylor, D.W.; Berman, L.B. The 6-Minute Walk: A New Measure of Exercise Capacity in Patients with Chronic Heart Failure. Can. Med. Assoc. J. 1985, 132, 919. [Google Scholar] [PubMed]
- Rikli, R.E.; Jones, C.J. Development and Validation of a Functional Fitness Test for Community-Residing Older Adults. J. Aging Phys. Act. 1999, 7, 129–161. [Google Scholar] [CrossRef]
- Jones, C.J.; Rikli, R.E.; Beam, W.C. A 30-s Chair-Stand Test as a Measure of Lower Body Strength in Community-Residing Older Adults. Res. Q. Exerc. Sport. 1999, 70, 113–119. [Google Scholar] [CrossRef]
- Guralnik, J.M.; Ferrucci, L.; Pieper, C.F.; Leveille, S.G.; Markides, K.S.; Ostir, G.V.; Studenski, S.; Berkman, L.F.; Wallace, R.B. Lower Extremity Function and Subsequent Disability: Consistency across Studies, Predictive Models, and Value of Gait Speed Alone Compared with the Short Physical Performance Battery. J. Gerontol. A Biol. Sci. Med. Sci. 2000, 55, M221–M231. [Google Scholar] [CrossRef]
- Marin-Jimenez, N.; Perez-Bey, A.; Cruz-Leon, C.; Conde-Caveda, J.; Segura-Jimenez, V.; Castro-Piñero, J.; Cuenca-Garcia, M. Criterion-Related Validity and Reliability of the Standing Long Jump Test in Adults: The Adult-Fit Project. Eur. J. Sport Sci. 2024, 24, 1379–1392. [Google Scholar] [CrossRef]
- Castro-Piñero, J.; González-Montesinos, J.L.; Mora, J.; Keating, X.D.; Girela-Rejón, M.J.; Sjöström, M.; Ruiz, J.R. Percentile Values for Muscular Strength Field Tests in Children Aged 6 to 17 Years: Influence of Weight Status. J. Strength. Cond. Res. 2009, 23, 2295–2310. [Google Scholar] [CrossRef] [PubMed]
- Osness, W.H. Functional Fitness Assessment for Adults Over 60 Years [Internet]. Reston: American Alliance for Health, Physical Education, Recreation and Dance. 1990, 37p. Available online: https://www.scienceopen.com/book?vid=24e042cb-a996-4b68-a239-64b397f9dd53 (accessed on 11 March 2025).
- Cadenas-Sanchez, C.; Sanchez-Delgado, G.; Martinez-Tellez, B.; Mora-Gonzalez, J.; Löf, M.; España-Romero, V.; Ruiz, J.R.; Ortega, F.B. Reliability and Validity of Different Models of TKK Hand Dynamometers. Am. J. Occup. Ther. 2016, 70, 7004300010p1–7004300010p9. [Google Scholar] [CrossRef]
- Ruiz-Ruiz, J.; Mesa, J.L.M.; Gutiérrez, A.; Castillo, M.J. Hand Size Influences Optimal Grip Span in Women but Not in Men. J. Hand Surg. 2002, 27, 897–901. [Google Scholar] [CrossRef]
- Springer, B.A.; Marin, R.; Cyhan, T.; Roberts, H.; Gill, N.W. Normative Values for the Unipedal Stance Test with Eyes Open and Closed. J. Geriatr. Phys. Ther. 2007, 30, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Gómez Montes, J.F.; Curcio, C.L.; Alvarado, B.; Zunzunegui, M.V.; Guralnik, J. Validity and Reliability of the Short Physical Performance Battery (SPPB): A Pilot Study on Mobility in the Colombian Andes. Colomb. Médica 2013, 44, 165. [Google Scholar] [CrossRef]
- Merellano-Navarro, E.; Collado-Mateo, D.; García-Rubio, J.; Gusi, N.; Olivares, P.R. Validity of the International Fitness Scale “IFIS” in Older Adults. Exp. Gerontol. 2017, 95, 77–81. [Google Scholar] [CrossRef] [PubMed]
- Pastor, A.; Farré, M.; Fitó, M.; Fernandez-Aranda, F.; De La Torre, R. Analysis of ECs and Related Compounds in Plasma: Artifactual Isomerization and Ex Vivo Enzymatic Generation of 2-MGs. J. Lipid Res. 2014, 55, 966–977. [Google Scholar] [CrossRef]
- Di Marzo, V.; Matias, I. Endocannabinoid Control of Food Intake and Energy Balance. Nat. Neurosci. 2005, 8, 585–589. [Google Scholar] [CrossRef]
- Mechoulam, R.; Fride, E.; Di Marzo, V. Endocannabinoids. Eur. J. Pharmacol. 1998, 359, 1–18. [Google Scholar] [CrossRef]
- Chakrapani, S.; Eskander, N.; Santos, L.A.D.L.; Omisore, B.A.; Mostafa, J.A.; Chakrapani, S.; Eskander, N.; Santos, L.A.D.L.; Omisore, B.A.; Mostafa, J.A. Neuroplasticity and the Biological Role of Brain Derived Neurotrophic Factor in the Pathophysiology and Management of Depression. Cureus 2020, 12, e11396. [Google Scholar] [CrossRef]
- Spitzer, R.L.; Kroenke, K.; Williams, J.B.W.; Löwe, B. A Brief Measure for Assessing Generalized Anxiety Disorder: The GAD-7. Arch. Intern. Med. 2006, 166, 1092–1097. [Google Scholar] [CrossRef] [PubMed]
- Crawford, J.R.; Henry, J.D. The Positive and Negative Affect Schedule (PANAS): Construct Validity, Measurement Properties and Normative Data in a Large Non-Clinical Sample. Br. J. Clin. Psychol. 2004, 43, 245–265. [Google Scholar] [CrossRef] [PubMed]
- Watson, D.; Clark, L.A.; Tellegen, A. Development and Validation of Brief Measures of Positive and Negative Affect: The PANAS Scales. J. Pers. Soc. Psychol. 1988, 54, 1063–1070. [Google Scholar] [CrossRef]
- Robles García, R.; Páez, F. El Estudio Sobre La Traducción El Español y Las Propiedades Psicométricas de Las Escalas de Afecto Positivo y Negativo (PANAS). Salud Ment. 2003, 26, 69–75. [Google Scholar]
- Mehling, W.E.; Price, C.; Daubenmier, J.J.; Acree, M.; Bartmess, E.; Stewart, A. The Multidimensional Assessment of Interoceptive Awareness (MAIA). PLoS ONE 2012, 7, e48230. [Google Scholar] [CrossRef]
- Ryff, C.D. Happiness Is Everything, or Is It? Explorations on the Meaning of Psychological Well-Being. J. Pers. Soc. Psychol. 1989, 57, 1069–1081. [Google Scholar] [CrossRef]
- Díaz, D.; Rodríguez-Carvajal, R.; Blanco, A.; Moreno-Jiménez, B.; Gallardo, I.; Valle, C.; van Dierendonck, D. Spanish Adaptation of the Psychological Well-Being Scales (PWBS). Psicothema 2006, 18, 572–577. [Google Scholar]
- Vilagut, G.; Ferrer, M.; Rajmil, L.; Rebollo, P.; Permanyer-Miralda, G.; Quintana, J.M.; Santed, R.; Valderas, J.M.; Ribera, A.; Domingo-Salvany, A.; et al. The Spanish Version of the Short Form 36 Health Survey: A Decade of Experience and New Developments. Gac. Sanit. 2005, 19, 135–150. [Google Scholar] [CrossRef]
- Martínez-González, M.A.; García-Arellano, A.; Toledo, E.; Salas-Salvadó, J.; Buil-Cosiales, P.; Corella, D.; Covas, M.I.; Schröder, H.; Arós, F.; Gómez-Gracia, E.; et al. A 14-Item Mediterranean Diet Assessment Tool and Obesity Indexes among High-Risk Subjects: The PREDIMED Trial. PLoS ONE 2012, 7, e43134. [Google Scholar] [CrossRef]
- Schröder, H.; Fitó, M.; Estruch, R.; Martínez-González, M.A.; Corella, D.; Salas-Salvadó, J.; Lamuela-Raventós, R.; Ros, E.; Salaverría, I.; Fiol, M.; et al. A Short Screener Is Valid for Assessing Mediterranean Diet Adherence among Older Spanish Men and Women. J. Nutr. 2011, 141, 1140–1145. [Google Scholar] [CrossRef]
- Fernández-Ballart, J.D.; Piñol, J.L.; Zazpe, I.; Corella, D.; Carrasco, P.; Toledo, E.; Perez-Bauer, M.; Martínez-González, M.Á.; Salas-Salvadó, J.; Martn-Moreno, J.M. Relative Validity of a Semi-Quantitative Food-Frequency Questionnaire in an Elderly Mediterranean Population of Spain. Br. J. Nutr. 2010, 103, 1808–1816. [Google Scholar] [CrossRef] [PubMed]
- Kroes, R.; Müller, D.; Lambe, J.; Löwik, M.R.H.; Van Klaveren, J.; Kleiner, J.; Massey, R.; Mayer, S.; Urieta, I.; Verger, P.; et al. Assessment of Intake from the Diet. Food Chem. Toxicol. 2002, 40, 327–385. [Google Scholar] [CrossRef]
- Slade, S.C.; Dionne, C.E.; Underwood, M.; Buchbinder, R. Consensus on Exercise Reporting Template (CERT): Explanation and Elaboration Statement. Br. J. Sports Med. 2016, 50, 1428–1437. [Google Scholar] [CrossRef] [PubMed]
- WHO Guidelines on Physical Activity and Sedentary Behaviour. Available online: https://www.who.int/publications/i/item/9789240015128 (accessed on 28 May 2024).
- ACSM’s Guidelines for Exercise Testing and Prescription. Available online: https://www.acsm.org/education-resources/books/guidelines-exercise-testing-prescription (accessed on 27 May 2024).
- Toigo, M.; Boutellier, U. New Fundamental Resistance Exercise Determinants of Molecular and Cellular Muscle Adaptations. Eur. J. Appl. Physiol. 2006, 97, 643–663. [Google Scholar] [CrossRef]
- Barlow, D.H.; Harris, B.A.; Eustis, E.H.; Farchione, T.J. The Unified Protocol for Transdiagnostic Treatment of Emotional Disorders. World Psychiatry 2020, 19, 245. [Google Scholar] [CrossRef]
- Fritz, C.O.; Morris, P.E.; Richler, J.J. Effect Size Estimates: Current Use, Calculations, and Interpretation. J. Exp. Psychol. Gen. 2012, 141, 2–18. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J. A Power Primer. Psychol. Bull. 1992, 112, 155–159. [Google Scholar] [CrossRef] [PubMed]
- Bland, J.M.; Altman, D.G. Multiple Significance Tests: The Bonferroni Method. BMJ 1995, 310, 170. [Google Scholar] [CrossRef]
- Althubaiti, A. Information Bias in Health Research: Definition, Pitfalls, and Adjustment Methods. J. Multidiscip. Healthc. 2016, 9, 211. [Google Scholar] [CrossRef]
- Vindegaard, N.; Benros, M.E. COVID-19 Pandemic and Mental Health Consequences: Systematic Review of the Current Evidence. Brain Behav. Immun. 2020, 89, 531–542. [Google Scholar] [CrossRef]
- Fucarino, A.; Fabbrizio, A.; Garrido, N.D.; Iuliano, E.; Reis, V.M.; Sausa, M.; Vilaça-Alves, J.; Zimatore, G.; Baldari, C.; Macaluso, F.; et al. Emerging Technologies and Open-Source Platforms for Remote Physical Exercise: Innovations and Opportunities for Healthy Population-A Narrative Review. Healthcare 2024, 12, 1466. [Google Scholar] [CrossRef] [PubMed]
- Scully, D.; Kremer, J.; Meade, M.M.; Graham, R.; Dudgeon, K. Physical Exercise and Psychological Well Being: A Critical Review. Br. J. Sports Med. 1998, 32, 111. [Google Scholar] [CrossRef] [PubMed]
- Schuch, F.B.; Vancampfort, D.; Richards, J.; Rosenbaum, S.; Ward, P.B.; Stubbs, B. Exercise as a Treatment for Depression: A Meta-Analysis Adjusting for Publication Bias. J. Psychiatr. Res. 2016, 77, 42–51. [Google Scholar] [CrossRef] [PubMed]
| Outcome | Assessment Tool |
|---|---|
| Primary outcome | |
| Depressive Symptoms |
|
| Secondary outcome | |
| Physical Domain | |
| Body Composition |
|
| Blood Pressure and Heart Rate |
|
| Physical Activity and Sedentary Time |
|
| Health-Related Physical Fitness |
|
| Blood Biomarker Analyses |
|
| Psychological Domain | |
| Anxiety and Stress |
|
| Emotional States |
|
| Interoceptive Awareness |
|
| Well-being and Quality of Life |
|
| Control Variables | |
| Sociodemographic and Medical History Information |
|
| Sleep Time and Quality |
|
| Dietary Habits |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Escudier-Vázquez, J.M.; Ruiz-Muñoz, M.; Garrido-Palomino, I.; Ortega-Gómez, S.; Valmisa Gómez de Lara, E.J.; Espinosa Nogales, M.d.M.; Viglerio Montero, A.; Rosety-Rodríguez, M.Á.; Jiménez-Pavón, D.; Carbonell-Baeza, A.; et al. Internet-Based Psycho-Physical Exercise Intervention Program in Mild-to-Moderate Depression: The Study Protocol of the SONRIE Randomized Controlled Trial. Int. J. Environ. Res. Public Health 2025, 22, 540. https://doi.org/10.3390/ijerph22040540
Escudier-Vázquez JM, Ruiz-Muñoz M, Garrido-Palomino I, Ortega-Gómez S, Valmisa Gómez de Lara EJ, Espinosa Nogales MdM, Viglerio Montero A, Rosety-Rodríguez MÁ, Jiménez-Pavón D, Carbonell-Baeza A, et al. Internet-Based Psycho-Physical Exercise Intervention Program in Mild-to-Moderate Depression: The Study Protocol of the SONRIE Randomized Controlled Trial. International Journal of Environmental Research and Public Health. 2025; 22(4):540. https://doi.org/10.3390/ijerph22040540
Chicago/Turabian StyleEscudier-Vázquez, Juan Manuel, Manuel Ruiz-Muñoz, Inmaculada Garrido-Palomino, Sonia Ortega-Gómez, Eulalio Juan Valmisa Gómez de Lara, María del Mar Espinosa Nogales, Alicia Viglerio Montero, Miguel Ángel Rosety-Rodríguez, David Jiménez-Pavón, Ana Carbonell-Baeza, and et al. 2025. "Internet-Based Psycho-Physical Exercise Intervention Program in Mild-to-Moderate Depression: The Study Protocol of the SONRIE Randomized Controlled Trial" International Journal of Environmental Research and Public Health 22, no. 4: 540. https://doi.org/10.3390/ijerph22040540
APA StyleEscudier-Vázquez, J. M., Ruiz-Muñoz, M., Garrido-Palomino, I., Ortega-Gómez, S., Valmisa Gómez de Lara, E. J., Espinosa Nogales, M. d. M., Viglerio Montero, A., Rosety-Rodríguez, M. Á., Jiménez-Pavón, D., Carbonell-Baeza, A., & España-Romero, V. (2025). Internet-Based Psycho-Physical Exercise Intervention Program in Mild-to-Moderate Depression: The Study Protocol of the SONRIE Randomized Controlled Trial. International Journal of Environmental Research and Public Health, 22(4), 540. https://doi.org/10.3390/ijerph22040540









