Evaluating the Cost-Effectiveness of Chlorhexidine-Coated vs. Standard Peripheral Insertion Central Catheters in Patients with Hematologic Disease: A Health Economic Analysis
Abstract
1. Background
2. Methods
2.1. Study Design and Compliance
2.2. Target Population and Economic Perspective
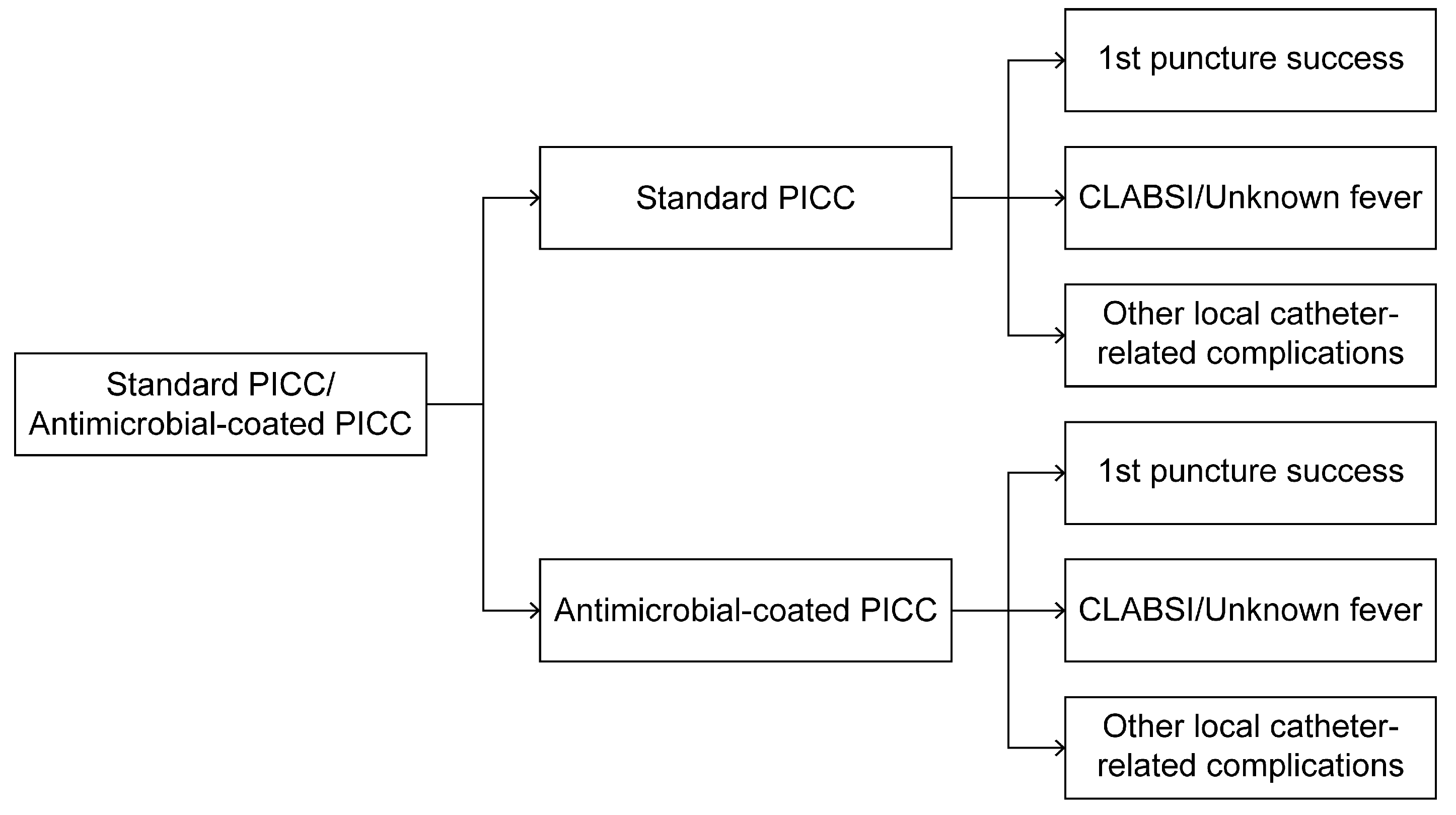
2.3. Conceptual Model: Decision Tree of Two PICCs Selected at >30 Days for Maintenance
2.4. Data Collection: Probabilities, Costs, and Quality-Adjusted Life Years (QALYs)
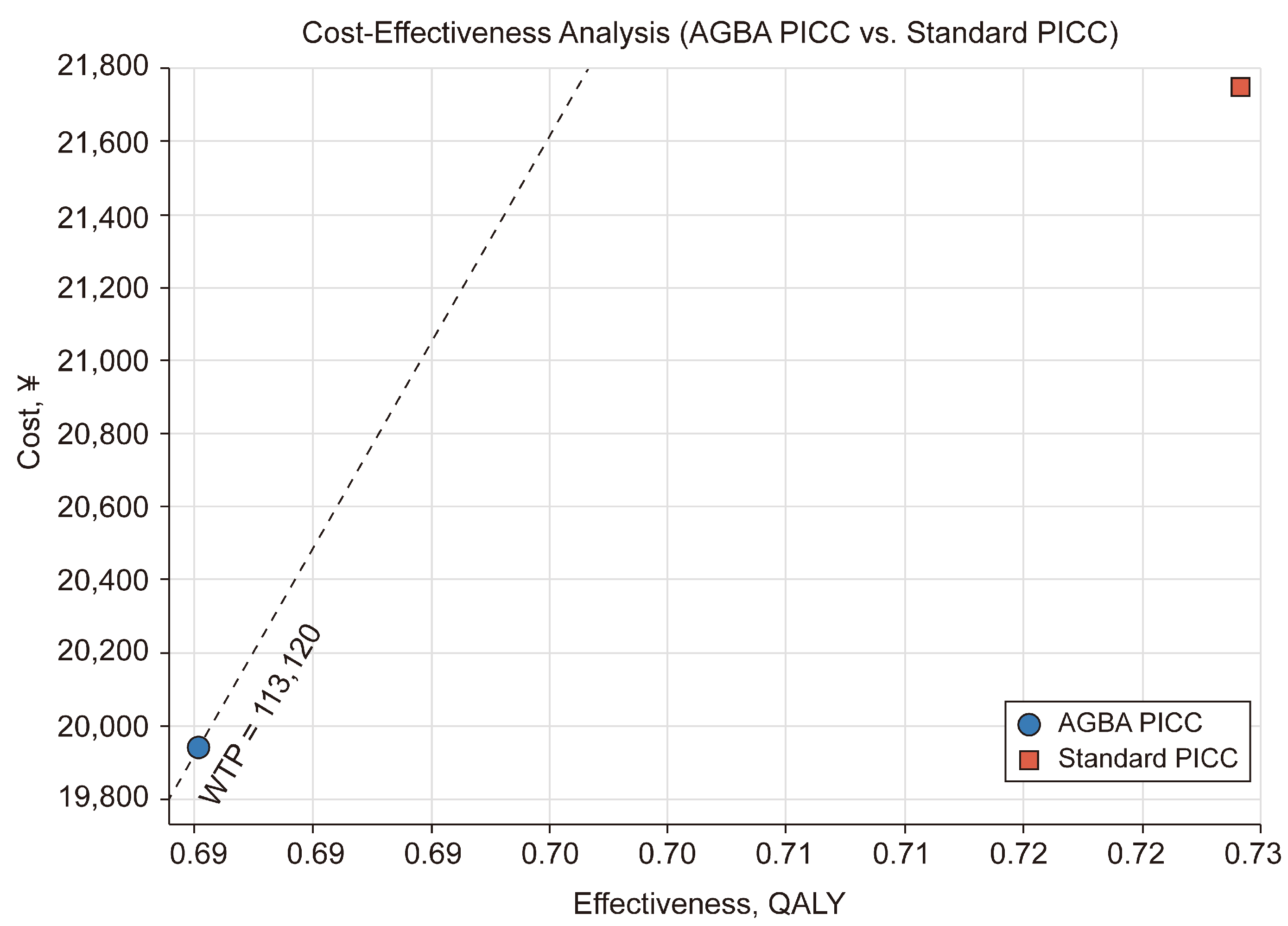
2.5. ICER and Threshold
2.6. Sensitivity Analyses
3. Results
3.1. Sensitivity Analysis
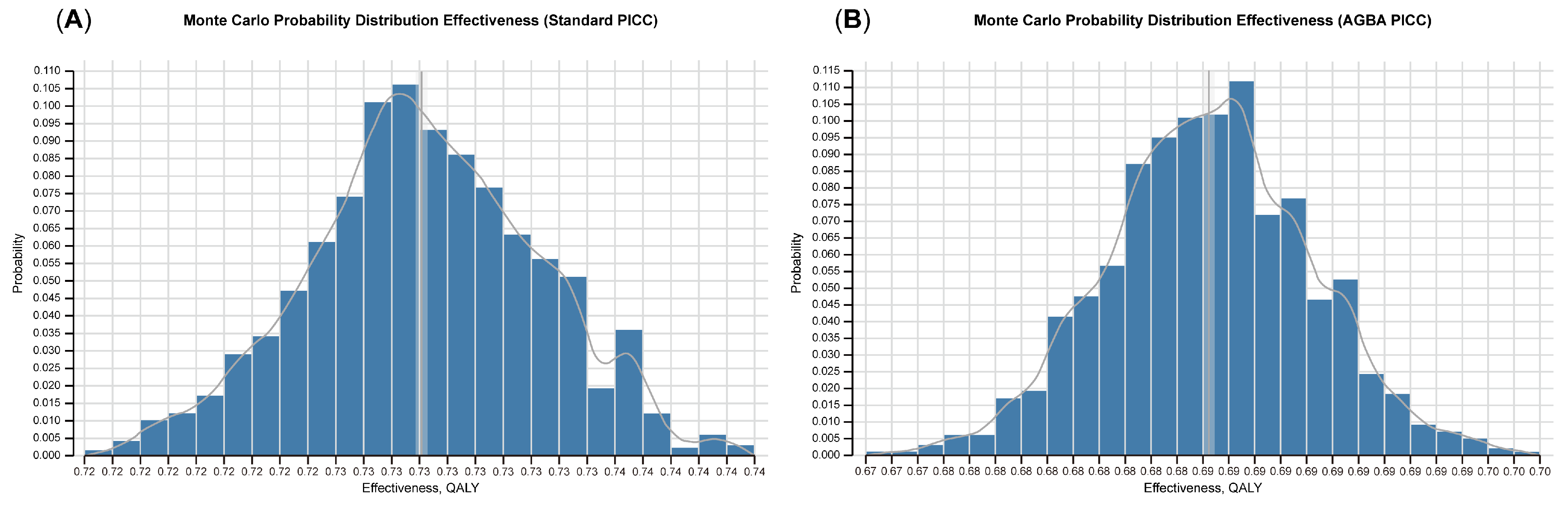
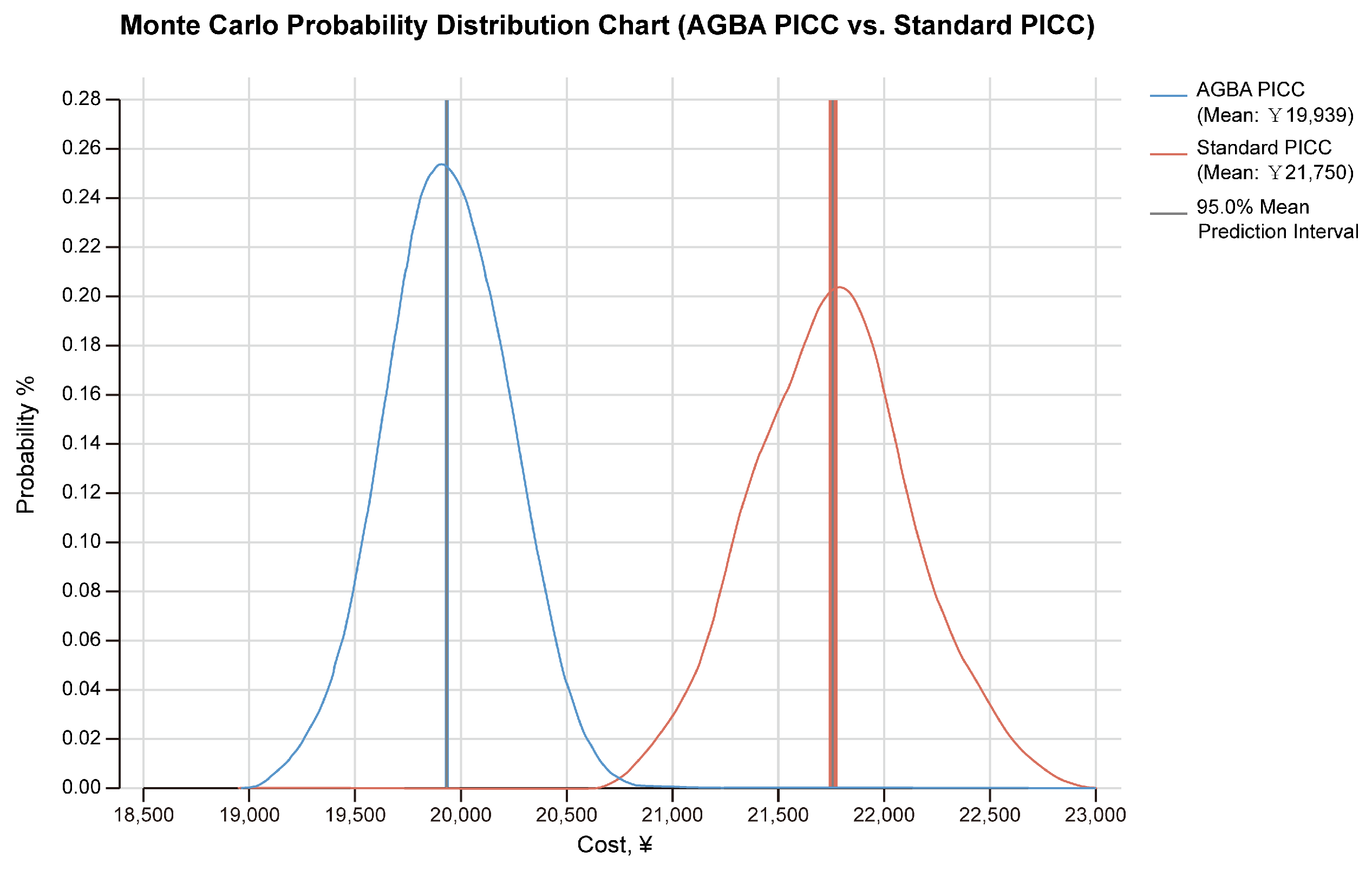
3.2. Monte Carlo Probabilistic Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AGBA | Chlorhexidine-coated PICC |
| CLABSI | Central-line-associated bloodstream infection |
| ICER | Incremental cost-effectiveness ratio |
| PICC | Peripherally inserted central catheter |
| QALY | Quality-adjusted life years |
| WTP | Willingness to pay |
References
- Marschall, J.; Mermel, L.A.; Fakih, M.; Hadaway, L.; Kallen, A.; O’grady, N.P.; Pettis, A.M.; Rupp, M.E.; Sandora, T.; Maragakis, L.L.; et al. Strategies to Prevent Central Line-Associated Bloodstream Infections in Acute Care Hospitals: 2014 Update. Hosp. Epidemiol. 2014, 35, 753–771. [Google Scholar] [CrossRef]
- Böll, B.; Schalk, E.; Buchheidt, D.; Hasenkamp, J.; Kiehl, M.; Kiderlen, T.R.; Kochanek, M.; Koldehoff, M.; Kostrewa, P.; Claßen, A.Y.; et al. Central venous catheter–related infections in hematology and oncology: 2020 updated guidelines on diagnosis, management, and prevention by the Infectious Diseases Working Party (AGIHO) of the German Society of Hematology and Medical Oncology (DGHO). Ann. Hematol. 2021, 100, 239–259. [Google Scholar] [CrossRef]
- Baskin, K.M.; Hunnicutt, C.; Beck, M.E.; Cohen, E.D.; Crowley, J.J.; Fitz, C.R. Long-term central venous access in pediatric patients at high risk: Conventional versus antibiotic-impregnated catheters. J. Vasc. Interv. Radiol. 2014, 25, 411–418. [Google Scholar] [CrossRef]
- Caris, M.G.; de Jonge, N.A.; Punt, H.J.; Salet, D.M.; de Jong, V.M.T.; Lissenberg-Witte, B.I.; Zweegman, S.; Vandenbroucke-Grauls, C.M.J.E.; van Agtmael, M.A.; Janssen, J.J.W.M. Indwelling time of peripherally inserted central catheters and incidence of bloodstream infections in haematology patients: A cohort study. Antimicrob. Resist. Infect. Control. 2022, 11, 37. [Google Scholar] [CrossRef]
- Chang, Y.-J.; Wang, Y.; Liu, Y.-R.; Xu, L.-P.; Zhang, X.-H.; Chen, H.; Chen, Y.-H.; Wang, F.-R.; Han, W.; Sun, Y.-Q.; et al. Haploidentical allograft is superior to matched sibling donor allograft in eradicating pre-transplantation minimal residual disease of AML patients as determined by multiparameter flow cytometry: A retrospective and prospective analysis. J. Hematol. Oncol. 2017, 10, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Li, S.-Q.; Fan, Q.-Z.; Xu, L.-P.; Wang, Y.; Zhang, X.-H.; Chen, H.; Chen, Y.-H.; Wang, F.-R.; Han, W.; Sun, Y.-Q.; et al. Different Effects of Pre-transplantation Measurable Residual Disease on Outcomes According to Transplant Modality in Patients with Philadelphia Chromosome Positive ALL. Front. Oncol. 2020, 10, 320. [Google Scholar] [CrossRef]
- Buetti, N.; Marschall, J.; Drees, M.; Fakih, M.G.; Hadaway, L.; Maragakis, L.L.; Monsees, E.; Novosad, S.; O’grady, N.P.; Rupp, M.E.; et al. Strategies to prevent central line-associated bloodstream infections in acute-care hospitals: 2022 Update. Infect. Control. Hosp. Epidemiol. 2022, 43, 553–569. [Google Scholar] [CrossRef] [PubMed]
- Bradford, N.K.; Edwards, R.M.; Chan, R.J. Normal saline (0.9% sodium chloride) versus heparin intermittent flushing for the prevention of occlusion in long-term central venous catheters in infants and children. Cochrane Database Syst. Rev. 2020, 2020, CD010996. [Google Scholar] [CrossRef]
- Kitamura, H.; Kubota, Y.; Komukai, S.; Yoshida, H.; Kaneko, Y.; Mihara, Y.; Nagasawa, Z.; Kawaguchi, A.; Aoki, Y.; Kimura, S. Venue of catheter insertion does not significantly impact the event of central line-associated bloodstream infection in patients with haematological diseases Infection Prevention in Practice. Infect. Prev. Pract. 2020, 2, 100050. [Google Scholar] [CrossRef] [PubMed]
- Husereau, D.; Drummond, M.; Petrou, S.; Carswell, C.; Moher, D.; Greenberg, D.; Augustovski, F.; Briggs, A.H.; Mauskopf, J.; Loder, E.; et al. Consolidated Health Economic Evaluation Reporting Standards (CHEERS) statement. BMJ 2013, 346, f1049. [Google Scholar] [CrossRef]
- Earl, D.J.; Deem, M.W. Monte Carlo simulations. Methods Mol. Biol. 2008, 443, 25–36. [Google Scholar] [CrossRef]
- Briggs, A.H. Statistical Issues in Economic Evaluations. Encycl. Health Econ. 2014, 3, 352–361. [Google Scholar] [CrossRef]
- Kuntz, K.; Sainfort, F.; Butler, M.; Taylor, B.; Kulasingam, S.; Gregory, S.; Mann, E.; Anderson, J.M.; Kane, R.L. Overview of Decision Models Used in Research. 2013. Available online: https://www.ncbi.nlm.nih.gov/books/NBK127474/ (accessed on 18 June 2023).
- CDC; Ncezid; DHQP. Bloodstream Infection Event (Central Line-Associated Bloodstream Infection and Non-central Line Associated Bloodstream Infection). Available online: https://www.cdc.gov/nhsn/pdfs/pscmanual/4psc_clabscurrent.pdf (accessed on 18 July 2024).
- Novikov, A.; Lam, M.Y.; Mermel, L.A.; Casey, A.L.; Elliott, T.S.; Nightingale, P. Impact of catheter antimicrobial coating on species-specific risk of catheter colonization: A meta-analysis. Antimicrob. Resist. Infect. Control 2012, 1, 40. [Google Scholar] [CrossRef] [PubMed]
- Hanna, H.; Benjamin, R.; Chatzinikolaou, I.; Alakech, B.; Richardson, D.; Mansfield, P.; Dvorak, T.; Munsell, M.F.; Darouiche, R.; Kantarjian, H.; et al. Long-Term Silicone Central Venous Catheters Impregnated With Minocycline and Rifampin Decrease Rates of Catheter-Related Bloodstream Infection in Cancer Patients: A Prospective Randomized Clinical Trial. J. Clin. Oncol. 2016, 22, 3163–3171. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, R.S.; Noritomi, D.T.; Degaspare, N.V.; Muñoz, G.O.C.; Porto, A.P.M.; Costa, S.F.; Ranzani, O.T. Peripherally inserted central catheters are associated with lower risk of bloodstream infection compared with central venous catheters in paediatric intensive care patients: A propensity-adjusted analysis. Intensive Care Med. 2017, 43, 1097–1104. [Google Scholar] [CrossRef]
- Liang, Y.; Wang, H.; Niu, M.; Zhu, X.; Cai, J.; Wang, X. Health-related quality of life before and after hematopoietic stem cell transplant: Evidence from a survey in Suzhou, China. Hematology 2018, 23, 626–632. [Google Scholar] [CrossRef] [PubMed]
- Xie, F.; Zhou, T.; Humphries, B.; Neumann, P.J. Do Quality-Adjusted Life-Years Discriminate Against the Elderly? An Empirical Analysis of Published Cost-Effectiveness Analyses. Value Health 2024, 27, 706–712. [Google Scholar] [CrossRef]
- Leech, A.A.; Kim, D.D.; Cohen, J.T.; Neumann, P.J. Are low and middle-income countries prioritising high-value healthcare interventions? BMJ Glob. Health 2020, 5, 1850. [Google Scholar] [CrossRef]
- Liu, X.; Xu, J.; Yuan, B.; Ma, X.; Fang, H.; Meng, Q. Containing medical expenditure: Lessons from reform of Beijing public hospitals. BMJ 2019, 365, l2369. [Google Scholar] [CrossRef]
- Butt, T.; Liu, G.G.; Kim, D.D.; Neumann, P.J.; Gordon, D.; Liu, G. Taking stock of cost-effectiveness analysis of healthcare in China What do the new findings imply? BMJ Glob. Health 2019, 4, 1418. [Google Scholar] [CrossRef]
- Wu, J.T.; Jit, M.; Zheng, Y.; Leung, K.; Xing, W.; Yang, J.; Liao, Q.; Cowling, B.J.; Yang, B.; Lau, E.H.Y.; et al. Routine Pediatric Enterovirus 71 Vaccination in China: A Cost-Effectiveness Analysis. PLoS Med. 2016, 13, e1001975. [Google Scholar] [CrossRef]
- Lan, T.; Guan, L.; Pang, X.; Li, X.; Yu, Q. Impact of the National Centralized Drug Procurement Policy (4 + 7 policy) on the drug expenditures of patients treated in outpatient and emergency departments in a large tertiary level-A hospital in China: A single centre, interrupted time series. J. Clin. Pharm. Ther. 2022, 47, 104–111. [Google Scholar] [CrossRef]
- Pan, L.; Xiao, K.; Zhu, H.; Luo, L. The impacts of public hospital comprehensive reform policies on hospital medicine cost, revenues and healthcare expenditures 2014–2019: An analysis of 103 tertiary public hospitals in China. Front. Health Serv. 2023, 3, 1079370. [Google Scholar] [CrossRef]
- Ye, Z.; Abduhilil, R.; Huang, J.; Sun, L. Willingness to Pay for One Additional Quality Adjusted Life Year: A Population Based Survey from China Key Points for Decision Makers. Appl. Health Econ. Health Policy 2022, 20, 893–904. [Google Scholar] [CrossRef] [PubMed]
- Choong, S.H.C.; Poon, M.M.; Soh, T.G.; Lieow, J.; Tan, L.K.; Koh, L.P.; Chng, W.-J.; Lee, Y.M.; Lee, J.S.X.; Ramos, D.G.; et al. Use of Peripherally Inserted Central Catheter (PICC) for the Infusion of Peripheral Blood Stem Cell Products Is Safe and Effective. Blood 2020, 136 (Suppl. S1), 41–42. [Google Scholar] [CrossRef]
- Comas, M.; Domingo, L.; Jansana, A.M.; Lafuente, E.M.; Civit, A.M.; García-Pérez, L.; de la Vega, C.M.L.; Cots, F.; Sala, M.; Castells, X. Cost-effectiveness Analysis of Peripherally Inserted Central Catheters Versus Central Venous Catheters for in-Hospital Parenteral Nutrition. J. Patient Saf. 2022, 18, E1109–E1115. [Google Scholar] [CrossRef]
- Huang, Y.; Yan, S.; Xie, H.; Wang, B.; Zhao, Z.; Huang, Y.; Li, J. Health Related Quality of Life of Rosacea Patients in China Assessed by Dermatology Life Quality Index and Willingness to Pay. Patient Prefer. Adherence 2022, 16, 659–670. [Google Scholar] [CrossRef]
- Health Economic Evaluation: Important Principles and Methodology. Available online: https://onlinelibrary.wiley.com/doi/epdf/10.1002/lary.23943?saml_referrer (accessed on 8 January 2023).

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, J.; Zare, H.; Chiu, H.-C.; Castillo, R.C. Evaluating the Cost-Effectiveness of Chlorhexidine-Coated vs. Standard Peripheral Insertion Central Catheters in Patients with Hematologic Disease: A Health Economic Analysis. Int. J. Environ. Res. Public Health 2025, 22, 373. https://doi.org/10.3390/ijerph22030373
Xu J, Zare H, Chiu H-C, Castillo RC. Evaluating the Cost-Effectiveness of Chlorhexidine-Coated vs. Standard Peripheral Insertion Central Catheters in Patients with Hematologic Disease: A Health Economic Analysis. International Journal of Environmental Research and Public Health. 2025; 22(3):373. https://doi.org/10.3390/ijerph22030373
Chicago/Turabian StyleXu, Jia, Hossein Zare, Herng-Chia Chiu, and Renan C. Castillo. 2025. "Evaluating the Cost-Effectiveness of Chlorhexidine-Coated vs. Standard Peripheral Insertion Central Catheters in Patients with Hematologic Disease: A Health Economic Analysis" International Journal of Environmental Research and Public Health 22, no. 3: 373. https://doi.org/10.3390/ijerph22030373
APA StyleXu, J., Zare, H., Chiu, H.-C., & Castillo, R. C. (2025). Evaluating the Cost-Effectiveness of Chlorhexidine-Coated vs. Standard Peripheral Insertion Central Catheters in Patients with Hematologic Disease: A Health Economic Analysis. International Journal of Environmental Research and Public Health, 22(3), 373. https://doi.org/10.3390/ijerph22030373






