Incidence and Risk Factors for the Development of Stress Fractures in Military Recruits and Qualified Personnel: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Protocol and Registration
2.2. Eligibility Criteria, Information Sources, and Search Terms
2.3. Inclusion and Exclusion Criteria
2.4. Study Selection, Data Collection Process, and Data Items
2.5. Levels of Evidence and Methodological Quality, Summary of Measures, and Synthesis of Results
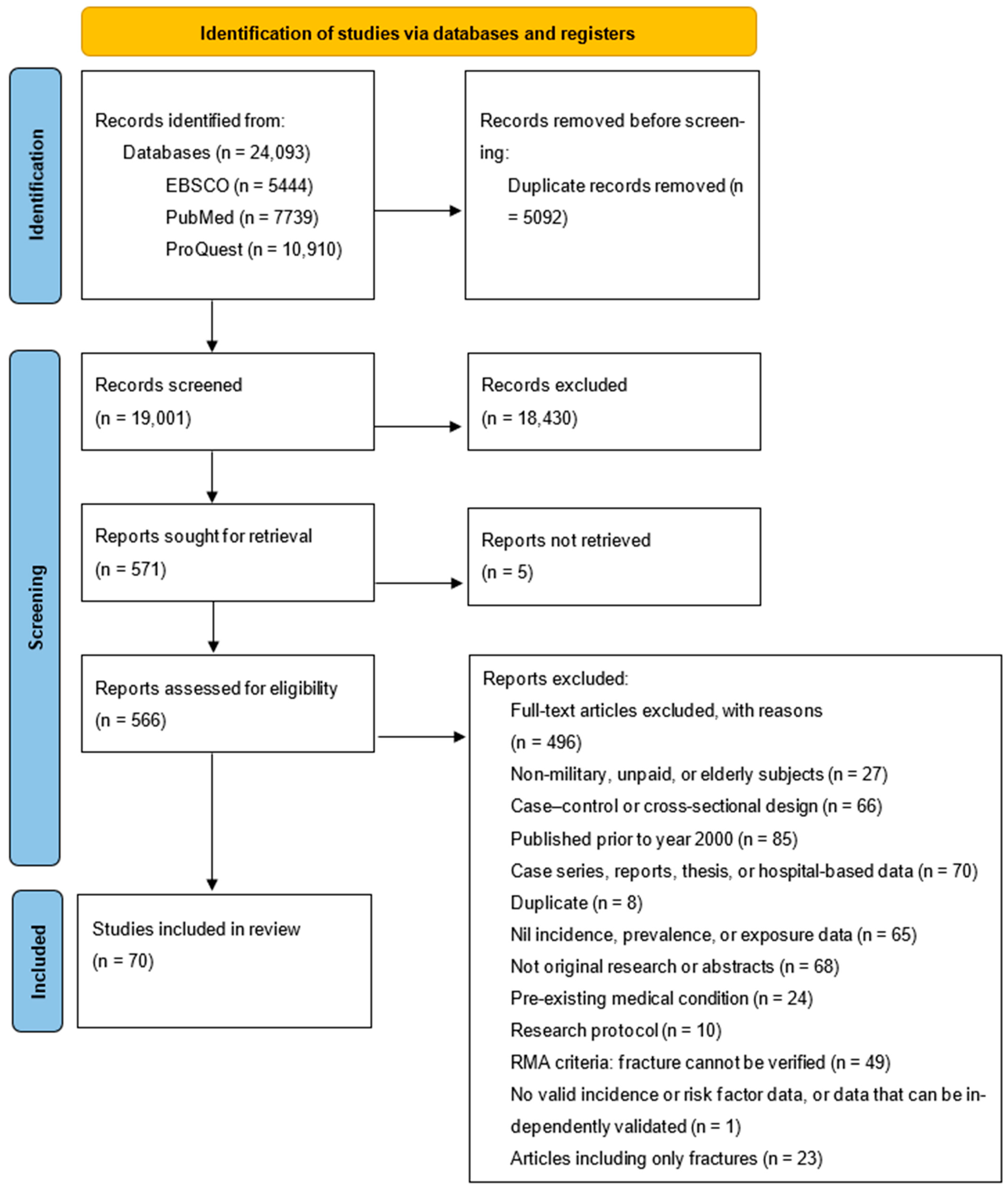
3. Results
3.1. Occupational Incidence of Stress Fractures in Military Recruits/Trainees
3.2. Occupational Incidence of Stress Fractures in Qualified Military Personnel
3.3. Occupational Tasks and Injury Mechanisms Associated with Stress Fractures in Military Recruit or Trainee Populations
3.4. Occupational Tasks and Injury Mechanisms Associated with Stress Fractures in Qualified Military Personnel
3.5. Other Factors Associated with Stress Fractures in Military Recruit or Trainee Populations
3.5.1. Health or Medical History
3.5.2. Sex
3.5.3. Age
3.5.4. Body Composition
3.5.5. Race and Ethnicity
3.5.6. Prior Physical Activity Levels
3.5.7. Aerobic or Strength Test Performance
3.5.8. Pharmacological Factors and Blood-Markers
3.5.9. Biomechanical Factors
3.5.10. Genetic Markers
3.5.11. Other Factors
3.6. Other Factors Associated with Stress Fractures in Qualified Military Personnel
3.6.1. Sex
3.6.2. Race/Ethnicity
3.6.3. Age
4. Discussion
4.1. Stress Fracture Incidence
4.2. Stress Fracture Risk Factors
4.3. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wentz, L.; Liu, P.Y.; Haymes, E.; Ilich, J.Z. Females have a greater incidence of stress fractures than males in both military and athletic populations: A systemic review. Mil. Med. 2011, 176, 420–430. [Google Scholar] [CrossRef] [PubMed]
- Reis, J.P.; Trone, D.W.; Macera, C.A.; Rauh, M.J. Factors associated with discharge during marine corps basic training. Mil. Med. 2007, 172, 936–941. [Google Scholar] [CrossRef] [PubMed]
- Knapik, J.J.; Sharp, M.A.; Canham-Chervak, M.; Hauret, K.; Patton, J.F.; Jones, B.H. Risk factors for training-related injuries among men and women in basic combat training. Med. Sci. Sports Exerc. 2001, 33, 946–954. [Google Scholar] [CrossRef]
- Donaldson, L.J.; Reckless, I.P.; Scholes, S.; Mindell, J.S.; Shelton, N.J. The epidemiology of fractures in England. J. Epidemiol. Community Health 2008, 62, 174–180. [Google Scholar] [CrossRef] [PubMed]
- Hamstra-Wright, K.L.; Huxel Bliven, K.C.; Napier, C. Training Load Capacity, Cumulative Risk, and Bone Stress Injuries: A Narrative Review of a Holistic Approach. Front. Sports Act. Living 2021, 3, 665683. [Google Scholar] [CrossRef]
- Knapik, J.J.; Reynolds, K.; Hoedebecke, K.L. Stress Fractures: Etiology, Epidemiology, Diagnosis, Treatment, and Prevention. J. Spec. Oper. Med. 2017, 17, 120–130. [Google Scholar] [CrossRef]
- Bennell, K.; Matheson, G.; Meeuwisse, W.; Brukner, P. Risk Factors for Stress Fractures. Sports Med. 1999, 28, 91–122. [Google Scholar] [CrossRef]
- Barrack, M.T.; Gibbs, J.C.; De Souza, M.J.; Williams, N.I.; Nichols, J.F.; Rauh, M.J.; Nattiv, A. Higher incidence of bone stress injuries with increasing female athlete triad-related risk factors: A prospective multisite study of exercising girls and women. Am. J. Sports Med. 2014, 42, 949–958. [Google Scholar] [CrossRef]
- Kalkhoven, J.T.; Watsford, M.L.; Coutts, A.J.; Edwards, W.B.; Impellizzeri, F.M. Training load and injury: Causal pathways and future directions. Sports Med. 2021, 51, 1137–1150. [Google Scholar] [CrossRef]
- Claassen, J.; Hu, Z.; Rohrbeck, P. Fractures among active component, recruit trainees, and deployed service members, U.S. Armed Forces, 2003–2012. Med. Surveill. Mon. Rep. 2014, 21, 2–7. [Google Scholar]
- Schram, B.; Pope, R.; Norman, A.; Orr, R. A Detailed Analysis of Serious Personal Injuries Suffered by Full Time and Part Time Soldiers of the Australian Army. Mil. Med. 2020, 185, e364–e369. [Google Scholar] [CrossRef] [PubMed]
- Sharma, J.; Greeves, J.P.; Byers, M.; Bennett, A.N.; Spears, I.R. Musculoskeletal injuries in British Army recruits: A prospective study of diagnosis-specific incidence and rehabilitation times. BMC Musculoskelet. Disord. 2015, 16, 106. [Google Scholar] [CrossRef] [PubMed]
- Rauh, M.J.; Macera, C.A.; Trone, D.W.; Shaffer, R.A.; Brodine, S.K. Epidemiology of stress fracture and lower-extremity overuse injury in female recruits. Med. Sci. Sports Exerc. 2006, 38, 1571–1577. [Google Scholar] [CrossRef]
- Jones, B.H.; Thacker, S.B.; Gilchrist, J.; Kimsey, C.D., Jr.; Sosin, D.M. Prevention of lower extremity stress fractures in athletes and soldiers: A systematic review. Epidemiol. Rev. 2002, 24, 228–247. [Google Scholar] [CrossRef]
- Jacobs, J.M.; Cameron, K.L.; Bojescul, J.A. Lower extremity stress fractures in the military. Clin. Sports Med. 2014, 33, 591–613. [Google Scholar] [CrossRef]
- Milgrom, C.; Simkin, A.; Arieh, E.; Nyska, M.; Finestone, A. Using bone’s adaptation ability to lower the incidence of stress fractures. Am. J. Sports Med. 2000, 28, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Finestone, A.; Milgrom, C. How stress fracture incidence was lowered in the Israeli army: A 25-yr struggle. Med. Sci. Sports Exerc. 2008, 40, S623–S629. [Google Scholar] [CrossRef]
- Finestone, A.; Giladi, M.; Elad, H.; Salmon, A.; Mendelson, S.; Eldad, A.; Milgrom, C. Prevention of stress fractures using custom biomechanical shoe orthoses. Clin. Orthop. Relat. Res. 1999, 360, 182–190. [Google Scholar] [CrossRef]
- Dembowski, S.C.; Tragord, B.S.; Hand, A.F.; Rohena-Quinquilla, I.R.; Lee, I.E.; Thoma, D.C.; Molloy, J.M. Injury Surveillance and Reporting for Trainees with Bone Stress Injury: Current Practices and Recommendations. Mil. Med. 2018, 183, e455–e461. [Google Scholar] [CrossRef]
- Zhao, L.; Chang, Q.; Huang, T.; Huang, C. Prospective cohort study of the risk factors for stress fractures in Chinese male infantry recruits. J. Int. Med. Res. 2016, 44, 787–795. [Google Scholar] [CrossRef]
- Korvala, J.; Hartikka, H.; Pihlajamäki, H.; Solovieva, S.; Ruohola, J.P.; Sahi, T.; Barral, S.; Ott, J.; Ala-Kokko, L.; Männikkö, M. Genetic predisposition for femoral neck stress fractures in military conscripts. BMC Genet. 2010, 11, 95. [Google Scholar] [CrossRef]
- Fedgo, A.A.; Stahlman, S. Increased risk for stress fractures and delayed healing with NSAID receipt, U.S. Armed Forces, 2014–2018. Med. Surveill. Mon. Rep. 2020, 27, 18–25. [Google Scholar]
- Ben-Ami, I.S.; Ankory, R.; Kadar, A.; Rotman, D.; Snir, N.; Schermann, H. The Effect of Previous Methylphenidate Use on Incidence of Stress Fractures in Military Recruits: A Retrospective Cohort. J. Bone Jt. Surg. Am. 2018, 100, 930–935. [Google Scholar] [CrossRef]
- Ruohola, J.P.; Laaksi, I.; Ylikomi, T.; Haataja, R.; Mattila, V.M.; Sahi, T.; Tuohimaa, P.; Pihlajamäki, H. Association between serum 25(OH)D concentrations and bone stress fractures in Finnish young men. J. Bone Miner. Res. 2006, 21, 1483–1488. [Google Scholar] [CrossRef]
- Merkel, D.; Moran, D.S.; Yanovich, R.; Evans, R.K.; Finestone, A.S.; Constantini, N.; Israeli, E. The association between hematological and inflammatory factors and stress fractures among female military recruits. Med. Sci. Sports Exerc. 2008, 40, S691–S697. [Google Scholar] [CrossRef]
- Yanovich, R.; Merkel, D.; Israeli, E.; Evans, R.K.; Erlich, T.; Moran, D.S. Anemia, iron deficiency, and stress fractures in female combatants during 16 months. J. Strength Cond. Res. 2011, 25, 3412–3421. [Google Scholar] [CrossRef] [PubMed]
- Lappe, J.; Cullen, D.; Haynatzki, G.; Recker, R.; Ahlf, R.; Thompson, K. Calcium and vitamin d supplementation decreases incidence of stress fractures in female navy recruits. J. Bone Miner. Res. 2008, 23, 741–749. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Campbell, P.; Canetti, E.F.; Simas, V.; Schram, B.; Pope, R.; Orr, R.M. Factors That Increase the Risk of Fractures in Occupational Settings: A Systematic Review Protocol. 2020. Available online: https://osf.io/w6e7x/ (accessed on 9 October 2025).
- Rohatgi, A. WebPlotDigitizer User Manual Version 3.4. 2014, pp. 1–18. Available online: https://dl1.icdst.org/pdfs/files3/c9490eacaa7707e65e02ad7eba1e4e14.pdf (accessed on 10 November 2020).
- Robson, K.; Pope, R.; Orr, R. Incidence and risk factors for acute articular cartilage tears in military and other occupational settings: A systematic review. Healthcare 2024, 12, 595. [Google Scholar] [CrossRef] [PubMed]
- Aromataris, E.; Lockwood, C.; Porritt, K.; Pilla, B.; Jordan, Z. (Eds.) JBI Manual for Evidence Synthesis; JBI: Adelaide, Australia, 2024. [Google Scholar] [CrossRef]
- Munn, Z.; Moola, S.; Lisy, K.; Riitano, D.; Tufanaru, C. Methodological guidance for systematic reviews of observational epidemiological studies reporting prevalence and cumulative incidence data. Int. J. Evid.-Based Healthc. 2015, 13, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Orr, R.; Simas, V.; Canetti, E.; Schram, B. A profile of injuries sustained by firefighters: A critical review. Int. J. Environ. Res. Public Health 2019, 16, 3931. [Google Scholar] [CrossRef]
- Bulathsinhala, L.; Hughes, J.M.; McKinnon, C.J.; Kardouni, J.R.; Guerriere, K.I.; Popp, K.L.; Matheny, R.W., Jr.; Bouxsein, M.L. Risk of Stress Fracture Varies by Race/Ethnic Origin in a Cohort Study of 1.3 Million US Army Soldiers. J. Bone Miner. Res. 2017, 32, 1546–1553. [Google Scholar] [CrossRef]
- Cowan, D.N.; Bedno, S.A.; Urban, N.; Lee, D.S.; Niebuhr, D.W. Step test performance and risk of stress fractures among female army trainees. Am. J. Prev. Med. 2012, 42, 620–624. [Google Scholar] [CrossRef]
- Cowan, D.N.; Bedno, S.A.; Urban, N.; Yi, B.; Niebuhr, D.W. Musculoskeletal injuries among overweight army trainees: Incidence and health care utilization. Occup. Med. 2011, 61, 247–252. [Google Scholar] [CrossRef]
- Evans, J.T.; Guyver, P.M.; Kassam, A.M.; Hubble, M.J. Displaced femoral neck stress fractures in Royal Marine recruits--management and results of operative treatment. J. R. Nav. Med. Serv. 2012, 98, 3–5. [Google Scholar] [PubMed]
- Hauret, K.G. The Physical Training and Rehabilitation Program: Duration of rehabilitation and final outcome of injuries in basic combat training. Mil. Med. 2001, 166, 820–826. [Google Scholar] [CrossRef] [PubMed]
- Heagerty, R.; Sharma, J.; Cayton, J.; Goodwin, N. Retrospective analysis of four-year injury data from the Infantry Training Centre, Catterick. J. R. Army Med. Corps 2018, 164, 35–40. [Google Scholar] [CrossRef]
- House, C.; Reece, A.; Roiz de Sa, D. Shock-absorbing insoles reduce the incidence of lower limb overuse injuries sustained during Royal Marine training. Mil. Med. 2013, 178, 683–689. [Google Scholar] [CrossRef] [PubMed]
- Hughes, J.M.; McKinnon, C.J.; Taylor, K.M.; Kardouni, J.R.; Bulathsinhala, L.; Guerriere, K.I.; Popp, K.L.; Bouxsein, M.L.; Proctor, S.P.; Matheny, R.W., Jr. Nonsteroidal anti-inflammatory drug prescriptions are associated with increased stress fracture diagnosis in the US Army population. J. Bone Miner. Res. 2019, 34, 429–436. [Google Scholar] [CrossRef]
- Itskoviz, D.; Marom, T.; Ostfeld, I. Trends of stress fracture prevalence among Israel Defense Forces basic trainees. Mil. Med. 2011, 176, 56–59. [Google Scholar] [CrossRef]
- Knapik, J.; Montain, S.J.; McGraw, S.; Grier, T.; Ely, M.; Jones, B.H. Stress fracture risk factors in basic combat training. Int. J. Sports Med. 2012, 33, 940–946. [Google Scholar] [CrossRef]
- Knapik, J.J.; Sharp, M.A.; Montain, S.J. Association between stress fracture incidence and predicted body fat in United States Army Basic Combat Training recruits. BMC Musculoskelet. Disord. 2018, 19, 161. [Google Scholar] [CrossRef]
- Milgrom, C.; Finestone, A.S. The effect of stress fracture interventions in a single elite infantry training unit (1983–2015). Bone 2017, 103, 125–130. [Google Scholar] [CrossRef]
- Montain, S.J.; McGraw, S.M.; Ely, M.R.; Grier, T.L.; Knapik, J.J. A retrospective cohort study on the influence of UV index and race/ethnicity on risk of stress and lower limb fractures. BMC Musculoskelet. Disord. 2013, 14, 135. [Google Scholar] [CrossRef]
- Oetting, A.A.; Garvin, N.U.; Boivin, M.R.; Cowan, D.N. Non-cognitive personality assessment and risk of injuries among army trainees. Am. J. Prev. Med. 2017, 52, 324–330. [Google Scholar] [CrossRef]
- Orr, R.M.; Cohen, B.S.; Allison, S.C.; Bulathsinhala, L.; Zambraski, E.J.; Jaffrey, M. Models to predict injury, physical fitness failure and attrition in recruit training: A retrospective cohort study. Mil. Med. Res. 2020, 7, 26. [Google Scholar] [CrossRef]
- Piantanida, N.A.; Knapik, J.J.; Brannen, S.; O’Connor, F. Injuries during Marine Corps officer basic training. Mil. Med. 2000, 165, 515–520. [Google Scholar] [CrossRef] [PubMed]
- Pihlajamäki, H.; Parviainen, M.; Kyröläinen, H.; Kautiainen, H.; Kiviranta, I. Regular physical exercise before entering military service may protect young adult men from fatigue fractures. BMC Musculoskelet. Disord. 2019, 20, 126. [Google Scholar] [CrossRef]
- Pihlajamäki, H.K.; Ruohola, J.; Weckström, M.; Kiuru, M.J.; Visuri, T.I. Long-term outcome of undisplaced fatigue fractures of the femoral neck in young male adults. J. Bone Jt. Surg. Br. 2006, 88B, 1574–1579. [Google Scholar] [CrossRef] [PubMed]
- Pihlajamäki, H.K.; Ruohola, J.P.; Kiuru, M.J.; Visuri, T.I. Displaced femoral neck fatigue fractures in military recruits. J. Bone Jt. Surg. Am. 2006, 88, 1989–1997. [Google Scholar] [CrossRef]
- Salminen, S.T.; Pihlajamäki, H.K.; Visuri, T.I.; Böstman, O.M. Displaced fatigue fractures of the femoral shaft. Clin. Orthop. Relat. Res. 2003, 409, 250–259. [Google Scholar] [CrossRef]
- Sormaala, M.J.; Niva, M.H.; Kiuru, M.J.; Mattila, V.M.; Pihlajamäki, H.K. Bone stress injuries of the talus in military recruits. Bone 2006, 39, 199–204. [Google Scholar] [CrossRef]
- Waterman, B.R.; Gun, B.; Bader, J.O.; Orr, J.D.; Belmont, P.J., Jr. Epidemiology of lower extremity stress fractures in the United States military. Mil. Med. 2016, 181, 1308–1313. [Google Scholar] [CrossRef]
- Wood, A.M.; Hales, R.; Keenan, A.; Moss, A.; Chapman, M.; Davey, T.; Nelstrop, A. Incidence and time to return to training for stress fractures during military basic training. J. Sports Med. 2014, 2014, 282980. [Google Scholar] [CrossRef] [PubMed]
- MacGregor, A.J.; Fogleman, S.A.; Dougherty, A.L.; Ryans, C.P.; Janney, C.F.; Fraser, J.J. Sex differences in the incidence and risk of ankle-foot complex stress fractures among U.S. military personnel. J. Womens Health 2022, 31, 586–592. [Google Scholar] [CrossRef]
- Shapiro, M.; Zubkov, K.; Landau, R. Diagnosis of Stress fractures in military trainees: A large-scale cohort. BMJ Mil. Health 2022, 168, 382–385. [Google Scholar] [CrossRef] [PubMed]
- Griffis, C.E.; Pletta, A.M.; Mutschler, C.; Ahmed, A.E.; Lorimer, S.D. Proportion of navy recruits diagnosed with symptomatic stress fractures during training and monetary impact of these injuries. Clin. Orthop. Relat. Res. 2022, 480, 2111–2119. [Google Scholar] [CrossRef]
- Johnson, A.S.; Brismée, J.M.; Hooper, T.L.; Hintz, C.N.; Hando, B.R. Incidence and risk factors for bone stress injuries in United States Air Force special warfare trainees. Mil. Med. 2024, 189, e1790–e1796. [Google Scholar] [CrossRef]
- Kelly, K.; Niederberger, B.; Givens, A.; Bernards, J.; Orr, R. Profiling injuries sustained following implementation of a progressive load carriage program in United States Marine Corps recruit training. Work 2024, 77, 1391–1399. [Google Scholar] [CrossRef]
- Koltun, K.J.; Sekel, N.M.; Bird, M.B.; Lovalekar, M.; Mi, Q.; Martin, B.J.; Nindl, B.C. Tibial bone geometry is associated with bone stress injury during military training in men and women. Front. Physiol. 2022, 13, 803219. [Google Scholar] [CrossRef]
- Kardouni, J.R.; McKinnon, C.J.; Taylor, K.M.; Hughes, J.M. Timing of stress fracture in soldiers during the first 6 career months: A retrospective cohort study. J. Athl. Train. 2021, 56, 1278–1284. [Google Scholar] [CrossRef] [PubMed]
- Ross, R.A.; Allsopp, A. Stress fractures in Royal Marines recruits. Mil. Med. 2002, 167, 560–565. [Google Scholar] [CrossRef] [PubMed]
- Bar-Dayan, Y.; Gam, A.; Goldstein, L.; Karmon, Y.; Mintser, I.; Grotto, I.; Guri, A.; Goldberg, A.; Ohana, N.; Onn, E.; et al. Comparison of stress fractures of male and female recruits during basic training in the Israeli anti-aircraft forces. Mil. Med. 2005, 170, 710–712. [Google Scholar] [CrossRef]
- Constantini, N.; Finestone, A.S.; Hod, N.; Shub, I.; Heinemann, S.; Foldes, A.J.; Mann, G. Equipment modification is associated with fewer stress fractures in female Israel Border Police recruits. Mil. Med. 2010, 175, 799–804. [Google Scholar] [CrossRef]
- Cosman, F.; Ruffing, J.; Zion, M.; Uhorchak, J.; Ralston, S.; Tendy, S.; McGuigan, F.E.; Lindsay, R.; Nieves, J. Determinants of stress fracture risk in United States Military Academy cadets. Bone 2013, 55, 359–366. [Google Scholar] [CrossRef] [PubMed]
- Dash, N.; Kushwaha, A.S. Stress fractures—A prospective study amongst recruits. Med. J. Armed Forces India 2012, 68, 118–122. [Google Scholar] [CrossRef]
- Davey, T.; Lanham-New, S.; Shaw, A.; Hale, B.; Cobley, R.; Berry, J.; Roch, M.; Allsopp, A.; Fallowfield, J. Low serum 25-hydroxyvitamin D is associated with increased risk of stress fracture during Royal Marine recruit training. Osteoporos. Int. 2016, 27, 171–179. [Google Scholar] [CrossRef]
- Dixon, S.; Nunns, M.; House, C.; Rice, H.; Mostazir, M.; Stiles, V.; Davey, T.; Fallowfield, J.; Allsopp, A. Prospective study of biomechanical risk factors for second and third metatarsal stress fractures in military recruits. J. Sci. Med. Sport 2019, 22, 135–139. [Google Scholar] [CrossRef]
- Finestone, A.S.; Milgrom, C.; Yanovich, R.; Evans, R.; Constantini, N.; Moran, D.S. Evaluation of the performance of females as light infantry soldiers. Biomed. Res. Int. 2014, 2014, 572953. [Google Scholar] [CrossRef]
- Hetsroni, I.; Finestone, A.; Milgrom, C.; Ben-Sira, D.; Nyska, M.; Mann, G.; Almosnino, S.; Ayalon, M. The role of foot pronation in the development of femoral and tibial stress fractures: A prospective biomechanical study. Clin. J. Sport Med. 2008, 18, 18–23. [Google Scholar] [CrossRef]
- Krauss, M.R.; Garvin, N.U.; Boivin, M.R.; Cowan, D.N. Excess stress fractures, musculoskeletal injuries, and health care utilization among unfit and overweight female Army trainees. Am. J. Sports Med. 2017, 45, 311–316. [Google Scholar] [CrossRef]
- Kucera, K.L.; Marshall, S.W.; Wolf, S.H.; Padua, D.A.; Cameron, K.L.; Beutler, A.I. Association of injury history and incident injury in cadet basic military training. Med. Sci. Sports Exerc. 2016, 48, 1053–1061. [Google Scholar] [CrossRef] [PubMed]
- Lappe, J.; Davies, K.; Recker, R.; Heaney, R. Quantitative ultrasound: Use in screening for susceptibility to stress fractures in female Army recruits. J. Bone Miner. Res. 2005, 20, 571–578. [Google Scholar] [CrossRef] [PubMed]
- Lappe, J.M.; Stegman, M.R.; Recker, R.R. The impact of lifestyle factors on stress fractures in female Army recruits. Osteoporos. Int. 2001, 12, 35–42. [Google Scholar] [CrossRef]
- Mattila, V.M.; Niva, M.; Kiuru, M.; Pihlajamäki, H. Risk factors for bone stress injuries: A follow-up study of 102,515 person-years. Med. Sci. Sports Exerc. 2007, 39, 1061–1066. [Google Scholar] [CrossRef] [PubMed]
- Moran, D.S.; Evans, R.; Arbel, Y.; Luria, O.; Hadid, A.; Yanovich, R.; Milgrom, C.; Finestone, A.S. Physical and psychological stressors linked with stress fractures in recruit training. Scand. J. Med. Sci. Sports 2013, 23, 443–450. [Google Scholar] [CrossRef]
- Moran, D.S.; Finestone, A.S.; Arbel, Y.; Shabshin, N.; Laor, A. A simplified model to predict stress fracture in young elite combat recruits. J. Strength Cond. Res. 2012, 26, 2585–2592. [Google Scholar] [CrossRef] [PubMed]
- Moran, D.S.; Israeli, E.; Evans, R.K.; Yanovich, R.; Constantini, N.; Shabshin, N.; Merkel, D.; Luria, O.; Erlich, T.; Laor, A.; et al. Prediction model for stress fracture in young female recruits during basic training. Med. Sci. Sports Exerc. 2008, 40 (Suppl. S11), S636–S644. [Google Scholar] [CrossRef]
- Potter, R.N.; Gardner, J.W.; Deuster, P.A.; Jenkins, P.; McKee, K., Jr.; Jones, B. Musculoskeletal injuries in an Army airborne population. Mil. Med. 2002, 167, 1033–1040. [Google Scholar] [CrossRef]
- Ruohola, J.P.; Mulari, M.; Haataja, R.I.; Väänänen, H.K.; Pihlajamäki, H.K. Can elevated serum TRACP-5b levels predict stress fractures? A cohort study. Scand. J. Surg. 2009, 98, 239–243. [Google Scholar] [CrossRef]
- Schaffer, R.A.; Rauh, M.J.; Brodine, S.K.; Trone, D.W.; Macera, C.A. Predictors of stress fracture susceptibility in young female recruits. Am. J. Sports Med. 2006, 34, 108–115. [Google Scholar] [CrossRef]
- Välimäki, V.V.; Alfthan, H.; Lehmuskallio, E.; Löyttyniemi, E.; Sahi, T.; Suominen, H.; Välimäki, M.J. Risk factors for clinical stress fractures in male military recruits: A prospective cohort study. Bone 2005, 37, 267–273. [Google Scholar] [CrossRef]
- Carswell, A.T.; O’Leary, T.J.; Swinton, P.; Jackson, S.; Tang, J.C.; Oliver, S.J.; Izard, R.M.; Walsh, N.P.; Fraser, W.D.; Greeves, J.P. Vitamin D metabolites are associated with musculoskeletal injury in young adults: A prospective cohort study. J. Bone Miner. Res. 2023, 38, 1453–1464. [Google Scholar] [CrossRef] [PubMed]
- Eastman, K.; O’Leary, T.J.; Carswell, A.; Walsh, N.; Izard, R.; Fraser, W.; Greeves, J. Distal tibial bone properties and bone stress injury risk in young men undergoing arduous physical training. Calcif. Tissue Int. 2023, 113, 317–328. [Google Scholar] [CrossRef]
- Merlin, T.; Weston, A.; Tooher, R.; Middleton, P.; Salisbury, J.; Coleman, K. NHMRC Levels of Evidence and Grades for Recommendations for Developers of Guidelines; National Health and Medical Research Council (NHRMC): Canberra, Australia, 2009.
- Fisher, R.; Esparza, S.; Nye, N.S.; Gottfredson, R.; Pawlak, M.T.; Cropper, T.L.; Casey, T.; Tchandja, J.; de la Motte, S.J.; Webber, B.J. Outcomes of embedded athletic training services within United States air force basic military training. J. Athl. Train. 2021, 56, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Palmanovich, E.; Frankl, M.; Nyska, M.; Hetsroni, I.; Constantini, N.; Trejo, L.; Bechar, R.; Novak, G.; Lankovsky, Z.; Mann, G. The effect of army vest design on the occurrence of stress fractures and overuse injuries in female military recruits. J. R. Army Med. Corps 2017, 163, 251. [Google Scholar] [CrossRef]
- Finestone, A.; Novack, V.; Farfel, A.; Berg, A.; Amir, H.; Milgrom, C. A prospective study of the effect of foot orthoses composition and fabrication on comfort and the incidence of overuse injuries. Foot Ankle Int. 2004, 25, 462–466. [Google Scholar] [CrossRef]
- Popovich, R.M.; Gardner, J.W.; Potter, R.; Knapik, J.J.; Jones, B.H. Effect of rest from running on overuse injuries in Army basic training. Am. J. Prev. Med. 2000, 18, 147–155. [Google Scholar] [CrossRef]
- Sheehan, K.M.; Murphy, M.M.; Reynolds, K.; Creedon, J.F.; White, J.; Kazel, M. The response of a bone resorption marker to Marine recruit training. Mil. Med. 2003, 168, 797–801. [Google Scholar] [CrossRef]
- Wood, P.S.; Krüger, P.E. Flexibility as risk factor for stress-fracture development in South African male soldiers. S. Afr. Fam. Pract. 2015, 57, 235–240. [Google Scholar] [CrossRef]
- Devlin, J.D.; Knapik, J.J.; Solomon, Z.; Hauret, K.G.; Morris, K.; Carter, R.; McGill, R.; Paoli, L. Incidence of admission to the physical training and rehabilitation programs in initial entry training during fiscal year 2011. Mil. Med. 2014, 179, 547–552. [Google Scholar] [CrossRef]
- Sormaala, M.J.; Niva, M.H.; Kiuru, M.J.; Mattila, V.M.; Pihlajamäki, H.K. Stress injuries of the calcaneus detected with magnetic resonance imaging in military recruits. J. Bone Jt. Surg. Am. 2006, 88, 2237–2242. [Google Scholar] [CrossRef]
- Moran, D.S.; Evans, R.K.; Hadad, E. Imaging of lower extremity stress fracture injuries. Sports Med. 2008, 38, 345–356. [Google Scholar] [CrossRef]
- Arendt, E.; Agel, J.; Heikes, C.; Griffiths, H. Stress injuries to bone in college athletes: A retrospective review of experience at a single institution. Am. J. Sports Med. 2003, 31, 959–968. [Google Scholar] [CrossRef] [PubMed]
- Hame, S.L.; LaFemina, J.M.; McAllister, D.R.; Schaadt, G.W.; Dorey, F.J. Fractures in the collegiate athlete. Am. J. Sports Med. 2004, 32, 446–451. [Google Scholar] [CrossRef]
- Robinson, P.G.; Campbell, V.B.; Murray, A.D.; Nicol, A.; Robson, J. Stress fractures: Diagnosis and management in the primary care setting. Br. J. Gen. Pract. 2019, 69, 209–300. [Google Scholar] [CrossRef] [PubMed]
- Toohey, L.A.; Drew, M.K.; Cook, J.L.; Finch, C.F.; Gaida, J.E. Is subsequent lower limb injury associated with previous injury? A systematic review and meta-analysis. Br. J. Sports Med. 2017, 51, 1670–1678. [Google Scholar] [CrossRef]
- Hägglund, M.; Waldén, M.; Ekstrand, J. Previous injury as a risk factor for injury in elite football: A prospective study over two consecutive seasons. Br. J. Sports Med. 2006, 40, 767–772. [Google Scholar] [CrossRef]
- Mountjoy, M.; Sundgot-Borgen, J.; Burke, L.; Carter, S.; Constantini, N.; Lebrun, C.; Meyer, N.; Sherman, R.; Steffen, K.; Budgett, R. The IOC consensus statement: Beyond the female athlete triad—Relative energy deficiency in sport (RED-S). Br. J. Sports Med. 2014, 48, 491–497. [Google Scholar] [CrossRef] [PubMed]
- Mountjoy, M.; Sundgot-Borgen, J.; Burke, L.; Ackerman, K.E.; Blauwet, C.; Constantini, N.; Lebrun, C.; Lundy, B.; Melin, A.; Meyer, N. International Olympic Committee (IOC) consensus statement on relative energy deficiency in sport (RED-S): 2018 update. Int. J. Sport Nutr. Exerc. Metab. 2018, 28, 316–331. [Google Scholar] [CrossRef] [PubMed]
- Constantini, N.W.; Alves, E.; Mountjoy, M.; Ackerman, K. Relative energy deficiency in military (RED-M). BMJ Mil. Health 2024, 170, 191–192. [Google Scholar] [CrossRef]
- Cline, A.D.; Jansen, G.R.; Melby, C.L. Stress fractures in female army recruits: Implications of bone density, calcium intake, and exercise. J. Am. Coll. Nutr. 1998, 17, 128–135. [Google Scholar] [CrossRef]
- Jones, B.H.; Bovee, M.W.; Harris III, J.M.; Cowan, D.N. Intrinsic risk factors for exercise-related injuries among male and female army trainees. Am. J. Sports Med. 1993, 21, 705–710. [Google Scholar] [CrossRef]
- Varley-Campbell, J.; Cooper, C.; Wilkerson, D.; Wardle, S.; Greeves, J.; Lorenc, T. Sex-specific changes in physical performance following military training: A systematic review. Sports Med. 2018, 48, 2623–2640. [Google Scholar] [CrossRef] [PubMed]
- Wallace, L.; Slattery, K.; Coutts, A.J. A comparison of methods for quantifying training load: Relationships between modelled and actual training responses. Eur. J. Appl. Physiol. 2014, 114, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Lambert, M.I.; Borresen, J. Measuring training load in sports. Int. J. Sports Physiol. Perform. 2010, 5, 406–411. [Google Scholar] [CrossRef]
- Schram, B.; Canetti, E.; Orr, R.; Pope, R. Risk factors for injuries in female soldiers: A systematic review. BMC Sports Sci. Med. Rehabil. 2022, 14, 54. [Google Scholar] [CrossRef]
- Pope, R.P.; Herbert, R.D.; Kirwan, J.D.; Graham, B.J. A randomized trial of preexercise stretching for prevention of lower-limb injury. Med. Sci. Sports Exerc. 2000, 32, 271. [Google Scholar] [CrossRef] [PubMed]
- Pope, R.P. Prevention of pelvic stress fractures in female army recruits. Mil. Med. 1999, 164, 370–373. [Google Scholar] [CrossRef]
- Dao, D.; Sodhi, S.; Tabasinejad, R.; Peterson, D.; Ayeni, O.R.; Bhandari, M.; Farrokhyar, F. Serum 25-Hydroxyvitamin D levels and stress fractures in military personnel: A systematic review and meta-analysis. Am. J. Sports Med. 2015, 43, 2064–2072. [Google Scholar] [CrossRef]
- Paradise, S.L.; Beer, J.R.; Cruz, C.A.; Fechner, K.M.; MacGregor, A.J.; Fraser, J.J. Prescribed footwear and orthoses are not prophylactic in preventing lower extremity injuries in military tactical athletes: A systematic review with meta-analysis. BMJ Mil. Health 2021, 170, e001955. [Google Scholar] [CrossRef] [PubMed]
- Barnes, K.R.; Tchandja, J.N.; Webber, B.J.; Federinko, S.P.; Cropper, T.L. The effects of prenatal vitamin supplementation on operationally significant health outcomes in female air force trainees. Mil. Med. 2015, 180, 554–558. [Google Scholar] [CrossRef]
- Chowdhury, M.Z.I.; Turin, T.C. Variable selection strategies and its importance in clinical prediction modelling. Fam. Med. Community Health 2020, 8, e000262. [Google Scholar] [CrossRef] [PubMed]
- Altman, D.G.; Royston, P. The cost of dichotomising continuous variables. BMJ 2006, 332, 1080. [Google Scholar] [CrossRef]
| Database | Search Terms | Filters (PubMed) |
|---|---|---|
| PubMed | (risk [Title/Abstract] OR predict * [Title/Abstract] OR prevalence [Title/Abstract] OR incidence [Title/Abstract] OR caus * [Title/Abstract] OR etiol * [Title/Abstract] OR frequenc * [Title/Abstract] OR rate * [Title/Abstract] OR mediat * [Title/Abstract] OR exposure * [Title/Abstract] OR likelihood [Title/Abstract] OR probability [Title/Abstract] OR factor [Title/Abstract] OR factors [Title/Abstract] OR hazard [Title/Abstract] OR hazards [Title/Abstract] OR predisposing [Title/Abstract]) AND (work * [Title/Abstract] OR occupation * [Title/Abstract] OR profession * [Title/Abstract] OR trade [Title/Abstract] OR employ * [Title/Abstract] OR military [Title/Abstract] OR Defence [Title/Abstract] OR Defense [Title/Abstract] OR airforce [Title/Abstract] OR “air force” [Title/Abstract] OR army [Title/Abstract] OR navy [Title/Abstract] OR recruit [Title/Abstract] OR soldier * [Title/Abstract] OR marines [Title/Abstract] OR “Military Personnel” [Title/Abstract]) AND (Fracture * [Title/Abstract] OR stress fracture * [Title/Abstract] OR overuse fracture * [Title/Abstract] OR bone stress * [Title/Abstract] OR bone strain * [Title/Abstract]) | English, Portuguese, Italian, Spanish Languages, Humans |
| Inclusion | Exclusion |
|---|---|
| Studies reporting original quantitative research conducted in humans aged 16 years or older, in which cohorts of participants were followed over time in a longitudinal study design (for example, cohort studies, randomised controlled trials, quasi-experimental studies); | Literature reviews of any type, cross sectional studies, case–control studies (except any nested within cohort studies); |
| Studies published in English, or translatable to English from Portuguese, Spanish, Italian, or French, by members of the research team; | Published abstracts; |
| Studies investigating factors, or exposures, or hazards, or causes, or mediators associated with development or prevention of stress fractures in personnel engaged in military occupations, or the incidence, or prevalence, or likelihood of the condition occurring in military occupational groups; and | Non-peer-reviewed articles and reports; Articles that are not reports of original research; |
| Studies using diagnostic criteria consistent with the criteria proposed by the Repatriation Medical Authority’s Statements of Principles for stress fractures, as follows: | Studies of pharmacologic interventions or ergogenic aids; or |
| Means an acquired break or rupture of bone resulting from fatigue of the bone; and Excludes spondylolysis, pathological fractures, periostitis, stress fractures due to insufficiency of the bone, and bone stress injuries/bone marrow oedema not being a stress fracture. | Studies of unpaid elite athletes, volunteer occupations, or non-military occupations. |
| Military | Branch | Male Incidence Rate * (Stress Fracture; Case-Based) | Female Incidence Rate * (Stress Fracture; Case-Based) | Combined Incidence Rate * | Incidence Rate Ratio (Female: Male) |
|---|---|---|---|---|---|
| United States | Army | 27.42–311.4 [39,64,92]; 25.6–46.5 [39,48] | 106.66–871.7 [39,64,76,77]; 54.3–551.8 [27,36,39,48,74,76,77] | 40.38–158.6 [39,44,64]; 29.2–158.6 [39,44,47,48] | 2.14–4.6 [35,39,44,48,64,82] |
| Marine Corps | N/A; 81.3–270.1 [2,63,93] | 244.4–365 [13,63,84]; 182.9 [93] | 126.4 [50,63]; N/A | 2.25 (95% CI, 0.28–18.3) [93] | |
| Air Force | N/A; 87.6–105.9 [89] | ||||
| Navy | N/A; 383.5–540 [27,74] | N/A; 34.9 [60] | |||
| Cadets | 19.9 [68]; 14.2 [68] | 69.9 [68]; 47.8 [68] | 27.5 [68]; 19.3 [68] | ||
| Military * | 29.6 [10]; N/A | 94.7 [10]; 552 [77] | 44.2 [10]; N/A | ||
| Israel | Infantry and Army | N/A; 300–810.5 [16,73,91] | N/A; 245.1–369.9 [26,81] | 8.93 (95% CI, 2.22–35.90) [91] | |
| Basic and Advanced Training | N/A; 727.2 [79] | N/A; 309 [25] | N/A; 142.2 [43] | ||
| Anti-aircraft training | 1248 [66]; 582 [66] | 2976 [66]; 1243 [66] | 1713 [66]; 760 [66] | 2.83 (95% CI, 1.89–3.00) [66] | |
| Elite combat unit | 517.2 [80]; 387.9 [80] | ||||
| Australia | Basic training | 13.7 [49]; N/A | 59.3 [49]; N/A | 17 [49]; N/A | 4.41 (95% CI, 2.33–8.35) [49] |
| Finland | Basic training | 15.2 [51]; 83.8–116 [24,85] | N/A; 99 [83] | ||
| United Kingdom | Army | 114.6 [40]; 62.6–194 [12,86,87] | N/A; 163.9 [86] | N/A; 242.8 [86] | |
| Royal Marines | 63.4–202.3 [41,57,65,70]; 117.1 [70] | ||||
| India | Basic training | N/A; 87.3 [69] | |||
| China | Basic infantry | N/A; 878.8 [20] | |||
| South Africa | Basic training | 0 [94]; N/A |
| ANATOMICAL LOCATION | POPULATION | STRESS FRACTURE INCIDENCE RATE (PER 1000 PERSON-YEARS) |
|---|---|---|
| TIBIA/FIBULA | U.S. Military ♂♀ | 15.7 stress fractures [10] |
| TIBIA | U.S. Marine Corps ♀ | 200.8 stress fractures [13] |
| British Army ♂ | 27.8 cases [12] | |
| British Royal Marines ♂ | 20.2 and 26.3 cases [57] | |
| Finnish Conscripts ♂ | 22.3 cases [85] | |
| Chinese Infantry ♂ | 293 cases [20] | |
| METATARSALS | U.S. Military ♂♀ | 5.6 cases [10] |
| British Army ♂ | 24.8 cases [12] | |
| British Royal Marines ♂ | 11.4–68.9 stress fractures [41,57,71] | |
| Finnish Conscripts ♂ | 55.9 cases [85] | |
| Chinese Infantry ♂ | 447 cases [20] | |
| HIP/PELVIS | U.S. Marine Corps ♀ | 54.8 stress fractures [13] |
| PELVIS | U.S. Marine Corps ♀ U.S. Military ♂♀ | 52.8 stress fractures [84] 1.4 stress fractures [10] |
| FEMUR | U.S. Marine Corps ♀ | 48.6 stress fractures [84] |
| British Army ♂ | 5.2 cases [12] | |
| British Royal Marines ♂ | 8.1 stress fractures [57] | |
| Chinese Infantry ♂ | 41.6 cases [20] | |
| PELVIS/FEMUR | Finnish Conscripts ♂ | 3.1 stress fractures [78] |
| Chinese Infantry ♂ | 60.5 cases [20] | |
| TALUS | Finnish Conscripts ♂♀ | 0.09 stress fractures [55] |
| CALCANEUS | Finnish Conscripts ♂ | 5.6 cases [85] |
| British Army ♂ | 4.8 cases [12] | |
| FIBULA | British Royal Marines ♂ | 2.3 stress fractures [57] |
| FEMORAL NECK | Chinese Infantry ♂ | 37.1 cases [20] |
| U.S. Military ♂♀ | 1.1 stress fractures [10] | |
| DISPLACED FEMORAL NECK | British Royal Marines ♂ | 1.51 stress fractures [38] |
| Finnish Conscripts ♂ | 0.023–0.053 stress fractures [53] | |
| UNDISPLACED FEMORAL NECK | Finnish Conscripts ♂ | 0.132–0.532 stress fractures [52] |
| DISPLACED FEMORAL SHAFT | Finnish Conscripts ♂♀ | 0.015 cases [54] |
| FEMORAL SHAFT | U.S. Military ♂♀ | 0.7 stress fractures [10] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Campbell, P.G.; Pope, R.; Simas, V.; Canetti, E.F.D.; Schram, B.; Orr, R.M. Incidence and Risk Factors for the Development of Stress Fractures in Military Recruits and Qualified Personnel: A Systematic Review. Int. J. Environ. Res. Public Health 2025, 22, 1760. https://doi.org/10.3390/ijerph22111760
Campbell PG, Pope R, Simas V, Canetti EFD, Schram B, Orr RM. Incidence and Risk Factors for the Development of Stress Fractures in Military Recruits and Qualified Personnel: A Systematic Review. International Journal of Environmental Research and Public Health. 2025; 22(11):1760. https://doi.org/10.3390/ijerph22111760
Chicago/Turabian StyleCampbell, Patrick G., Rodney Pope, Vinicius Simas, Elisa F. D. Canetti, Benjamin Schram, and Robin M. Orr. 2025. "Incidence and Risk Factors for the Development of Stress Fractures in Military Recruits and Qualified Personnel: A Systematic Review" International Journal of Environmental Research and Public Health 22, no. 11: 1760. https://doi.org/10.3390/ijerph22111760
APA StyleCampbell, P. G., Pope, R., Simas, V., Canetti, E. F. D., Schram, B., & Orr, R. M. (2025). Incidence and Risk Factors for the Development of Stress Fractures in Military Recruits and Qualified Personnel: A Systematic Review. International Journal of Environmental Research and Public Health, 22(11), 1760. https://doi.org/10.3390/ijerph22111760










