Occupations and Risk of Head and Neck Cancers: A Case–Control Study in Tanzania
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Setting
2.2. Study Population
2.3. Data Collection
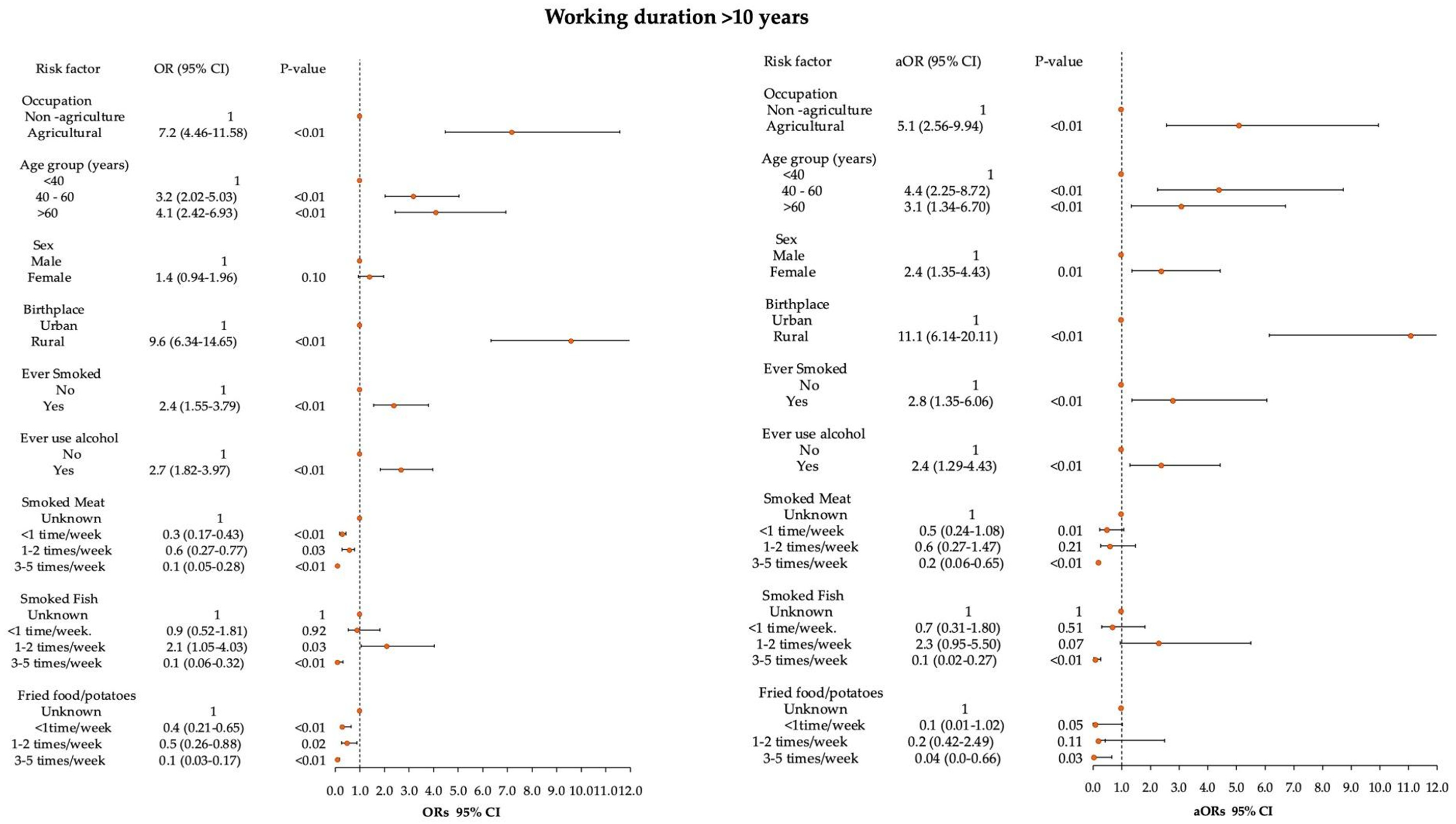
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Melariri, H.; Els, T.; Oyedele, O.; Suttle, T.K.; Bermosky, K.T.; De Freitas, A.; Murtaza, A.; Moosajee, M.; Melariri, P.E. Prevalence of locoregional recurrence and survival post-treatment of head and neck cancers in Africa: A systematic review and meta-analysis. eClinicalMedicine 2023, 59, 101964. [Google Scholar] [CrossRef] [PubMed]
- Mwelange, L.P.; Mamuya, S.H.D.; Mwaiselage, J.; Bråtveit, M.; Moen, B.E. Esophageal and Head and Neck Cancer Patients Attending Ocean Road Cancer Institute in Tanzania from 2019 to 2021: An Observational Study. Int. J. Environ. Res. Public Health 2023, 20, 3305. [Google Scholar] [CrossRef] [PubMed]
- Leonel, A.; Bonan, R.; Pinto, M.; Kowalski, L.; Perez, D. The pesticides use and the risk for head and neck cancer: A review of case-control studies. Med. Oral Patol. Oral Cir. Bucal 2021, 26, e56–e63. [Google Scholar] [CrossRef] [PubMed]
- European Environment Agency. European Environment Agency. Beating Cancer—The Role of Europe’s Environment—Web Report. 28 June 2022. Available online: https://www.eea.europa.eu/publications/environmental-burden-of-cancer (accessed on 3 September 2025).
- Maasland, D.H.; Brandt, P.A.v.D.; Kremer, B.; Goldbohm, R.A.S.; Schouten, L.J. Alcohol consumption, cigarette smoking and the risk of subtypes of head-neck cancer: Results from the Netherlands Cohort Study. BMC Cancer 2014, 14, 187. [Google Scholar] [CrossRef] [PubMed]
- Di Credico, G.; Polesel, J.; Maso, L.D.; Pauli, F.; Torelli, N.; Luce, D.; Radoï, L.; Matsuo, K.; Serraino, D.; Brennan, P.; et al. Alcohol drinking and head and neck cancer risk: The joint effect of intensity and duration. Br. J. Cancer 2020, 123, 1456–1463. [Google Scholar] [CrossRef] [PubMed]
- Hashim, D.; Boffetta, P. Occupational and Environmental Exposures and Cancers in Developing Countries. Ann. Glob. Health 2014, 80, 393–411. [Google Scholar] [CrossRef] [PubMed]
- Naghibzadeh-Tahami, A.; Khosravi, Y.; Es’haghi, M.; Haghdoost, A.-A. Scoping review of 5 common occupational cancers and their related exposures. Med. J. Islam. Repub. Iran 2022, 6, 84. [Google Scholar] [CrossRef] [PubMed]
- Purdue, M.P.; Hutchings, S.J.; Rushton, L.; Silverman, D.T. The proportion of cancer attributable to occupational exposures. Ann. Epidemiol. 2015, 25, 188–192. [Google Scholar] [CrossRef] [PubMed]
- Amizadeh, M.; Safari-Kamalabadi, M.; Askari-Saryazdi, G.; Amizadeh, M.; Reihani-Kermani, H. Pesticide exposure and head and neck cancers: A case-control study in an agricultural region. Iran. J. Otorhinolaryngol. 2017, 29, 275. [Google Scholar] [PubMed]
- Mwelange, L.; Mamuya, S.; Nyarubeli, I.; Bråtveit, M.; Moen, B. Carcinogenic chemicals used in selected industries and agriculture sectors in Tanzania. A descriptive study. Int. J. Occup. Saf. Health 2025, 15, 202–215. [Google Scholar] [CrossRef]
- Bigman, G.; Otieno, L.; Adebamowo, S.N.; Adebamowo, C. Dietary Intake and Cancer in Sub-Saharan Africa: A Critical Review of Epidemiological Studies. Nutr. Cancer 2022, 74, 2803–2814. [Google Scholar] [CrossRef] [PubMed]
- Lucenteforte, E.; Garavello, W.; Bosetti, C.; La Vecchia, C. Dietary factors and oral and pharyngeal cancer risk. Oral Oncol. 2009, 45, 461–467. [Google Scholar] [CrossRef] [PubMed]
- Joury, E.; Naja, F.; Nour, A.; Itani, L.; Rafii, B.; Nakhleh, K.; Manadili, A. Dietary patterns and the risk of oral, pharyngeal and laryngeal cancer in Syria: A case control study. BMC Nutr. 2016, 2, 8. [Google Scholar] [CrossRef]
- Tanzania National Bureau of Statistics. 2018 Tanzania Global Adult Tobacco Survey. Key Findings. Report. 2018. Available online: https://www.nbs.go.tz/uploads/statistics/documents/en-1705492591-2018TanzaniaGATS_Key%20Findings.pdf (accessed on 23 October 2025).
- Louis, L.M.; Lerro, C.C.; Friesen, M.C.; Andreotti, G.; Koutros, S.; Sandler, D.P.; Blair, A.; Robson, M.G.; Freeman, L.E.B. A prospective study of cancer risk among Agricultural Health Study farm spouses associated with personal use of organochlorine insecticides. Environ. Health 2017, 16, 95. [Google Scholar] [CrossRef] [PubMed]
- Uzcudun, A.E.; Retolaza, I.R.; Grande, A.G.; Olivar, L.M.; García, A.G.; Barón, M.G.; Bouzas, J.G. Pharyngeal cancer prevention: Evidence from a case–control study involving 232 consecutive patients. J. Laryngol. Otol. 2002, 116, 523–531. [Google Scholar] [CrossRef] [PubMed]
- Boras, V.V.; Fučić, A.; Baranović, S.; Blivajs, I.; Milenović, M.; Bišof, V.; Rakušić, Z.; Ceppi, M.; Bruzzone, M. Environmental and behavioural head and neck cancer risk factors. Cent. Eur. J. Public Health 2019, 27, 106–109. [Google Scholar] [CrossRef] [PubMed]
- Carton, M.; Barul, C.; Menvielle, G.; Cyr, D.; Sanchez, M.; Pilorget, C.; Trétarre, B.; Stücker, I.; Luce, D. Occupational exposure to solvents and risk of head and neck cancer in women: A population-based case–control study in France. BMJ Open 2017, 7, e012833. [Google Scholar] [CrossRef] [PubMed]
- Seedat, J.; Coutts, K.; Vlok, E. Epidemiology and demographics of head and neck cancer in Africa: A scoping review. Afr. J. Prim. Health Care Fam. Med. 2023, 15, e1–e13. [Google Scholar] [CrossRef] [PubMed]
- Barul, C.; Matrat, M.; Auguste, A.; Dugas, J.; Radoï, L.; Menvielle, G.; Févotte, J.; Guizard, A.-V.; Stücker, I.; Luce, D. Welding and the risk of head and neck cancer: The ICARE study. Occup. Environ. Med. 2020, 77, 293–300. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | Cases (n = 298) | Controls (n = 305) | p-Value 1 | ||
|---|---|---|---|---|---|
| Age in Years Mean (SD) | 51 (15.4) | 43 (15.9) | |||
| n | % | n | % | ||
| Age group (years) | |||||
| <40 | 72 | 24.2 | 152 | 49.8 | <0.001 |
| 40–60 | 142 | 47.7 | 106 | 34.7 | |
| >60 | 84 | 28.2 | 47 | 15.4 | |
| Sex | |||||
| Female | 130 | 43.6 | 109 | 35.7 | 0.05 |
| Male | 168 | 56.4 | 196 | 64.3 | |
| Educational level | |||||
| Informal | 51 | 17.1 | 42 | 13.8 | 0.50 |
| Primary School | 174 | 58.4 | 182 | 59.6 | |
| Secondary and above | 73 | 24.5 | 81 | 26.6 | |
| Marital Status | |||||
| Unmarried | 97 | 32.5 | 130 | 42.6 | <0.001 |
| Married | 201 | 67.5 | 175 | 57.4 | |
| Birthplace | |||||
| Urban | 65 | 21.8 | 206 | 67.5 | <0.001 |
| Rural | 233 | 78.2 | 99 | 32.5 | |
| Residence place | |||||
| Urban | 127 | 42.6 | 217 | 71.2 | <0.001 |
| Rural | 171 | 57.4 | 88 | 28.9 | |
| Family history of cancer | |||||
| No | 267 | 89.6 | 267 | 87.5 | 0.42 |
| Yes | 31 | 10.4 | 38 | 12.5 | |
| Ever smoked tobacco | |||||
| No | 214 | 71.8 | 265 | 86.9 | <0.001 |
| Yes | 84 | 28.2 | 40 | 13.1 | |
| Current tobacco smoker | |||||
| No | 80 | 94.2 | 19 | 47.5 | <0.001 |
| Yes | 4 | 4.8 | 21 | 52.5 | |
| Ever used alcohol | |||||
| No | 168 | 56.4 | 231 | 75.7 | <0.001 |
| Yes | 130 | 43.6 | 74 | 24.3 | |
| Currently using alcohol | |||||
| No | 123 | 94.6 | 31 | 41.9 | <0.001 |
| Yes | 7 | 5.4 | 43 | 58.1 | |
| Cooking | |||||
| Firewood and/or charcoal | 276 | 92.6 | 263 | 86.2 | <0.001 |
| Gas | 22 | 7.4 | 42 | 13.8 | |
| Occupations * | |||||
| Service and Sales Workers | 99 | 33.2 | 152 | 49.8 | <0.001 |
| Cooks | 23 | 7.7 | 49 | 16.1 | |
| Building and Related Trades | 22 | 7.4 | 36 | 11.8 | |
| Sum non-agriculture | 144 | 48.3 | 237 | 77.7 | |
| Agriculture | 154 | 51.7 | 68 | 22.3 | |
| Working experience (years) | |||||
| <10 | 34 | 11.4 | 71 | 23.3 | <0.001 |
| 10–20 | 31 | 10.4 | 69 | 22.6 | |
| 21–30 | 61 | 20.5 | 50 | 16.4 | |
| 31–40 | 75 | 25.2 | 60 | 19.7 | |
| >40 | 97 | 32.6 | 55 | 18.0 | |
| Smoked Meat | |||||
| Unknown | 99 | 33.3 | 39 | 14.7 | <0.001 |
| <1 time/week | 110 | 36.9 | 130 | 49.1 | |
| 1–2 times/week | 80 | 26.8 | 60 | 22.6 | |
| 3–5 times/week | 9 | 3.0 | 36 | 13.6 | |
| Smoked Fish | |||||
| Unknown | 32 | 10.7 | 30 | 9.8 | <0.001 |
| <1 time/week | 132 | 44.3 | 126 | 41.4 | |
| 1–2 times/week | 121 | 40.6 | 52 | 17.0 | |
| 3–5 times/week | 13 | 4.4 | 97 | 31.8 | |
| Chips/fried potatoes | |||||
| Unknown | 78 | 26.2 | 32 | 10.4 | <0.001 |
| <1 time/week | 125 | 41.9 | 131 | 43.0 | |
| 1–2 times/week | 82 | 27.5 | 70 | 23.0 | |
| 3–5 times/week | 13 | 4.4 | 72 | 23.6 | |
| Head and neck cancer types | |||||
| Pharynx | 82 | 27.5 | - | ||
| Larynx | 35 | 11.7 | - | - | |
| Oral cavity | 92 | 30.9 | - | - | |
| Nasal Cavity and Paranasal Sinuses | 89 | 29.9 | - | - | |
| Risk Factors | Cases (n = 298) | Control (n = 305) | OR | p-Value | aOR | p-Value |
|---|---|---|---|---|---|---|
| n (%) | n (%) | (95% CI) | (95% CI) | |||
| Occupation | ||||||
| Non-agriculture | 144 (48.3) | 237 (77.7) | 1 | 1 | ||
| Agricultural | 154 (51.7) | 68 (22.3) | 3.7 (2.62–5.30) | <0.01 | 2.6 (1.60–4.37) | <0.01 |
| Age group (years) | ||||||
| <40 | 72 (24.2) | 152 (49.8) | 1 | 1 | ||
| 40–60 | 142 (47.7) | 106 (34.7) | 2.8 (1.94–4.12) | <0.01 | 3.2 (1.86–5.39) | <0.01 |
| >60 | 84 (28.2) | 47 (15.4) | 3.8 (2.40–5.94) | <0.01 | 2.2 (1.15–4.17) | <0.02 |
| Sex | ||||||
| Male | 168 (56.4) | 196 (64.3) | 1 | |||
| Female | 130 (43.6) | 109 (35.7) | 1.4 (1.00–1.93) | 0.05 | 2.1 (1.27–3.43) | <0.01 |
| Birthplace | ||||||
| Urban | 65 (21.8) | 206 (67.5) | 1 | |||
| Rural | 233 (78.2) | 99 (32.5) | 7.5 (5.18–10.74) | <0.01 | 8.6 (5.23–14.06) | <0.01 |
| Ever smoked | ||||||
| No | 214 (71.8) | 265 (86.9) | 1 | 1 | ||
| Yes | 84 (28.2) | 40 (13.1) | 2.6 (1.71–3.94) | <0.01 | 3.0 (1.56–5.94) | <0.01 |
| Ever used alcohol | ||||||
| No | 168 (56.4) | 231 (75.7) | 1 | 1 | ||
| Yes | 130 (43.6) | 74 (24.3) | 2.4 (1.701–3.42) | <0.01 | 1.72 (1.94–2.94) | 0.05 |
| Smoked Meat | ||||||
| Unknown | 99 (33.2) | 48 (15.7) | 1 | |||
| <1 time/week | 110 (36.9) | 148 (48.5) | 0.4 (0.23–0.55) | <0.01 | 0.4 (0.22–0.80) | 0.01 |
| 1–2 times/week | 80 (26.9) | 68 (22.3) | 0.6 (0.36–0.92) | 0.02 | 0.6 (0.31–1.30) | 0.21 |
| 3–5 times/week | 9 (3.0) | 41 (13.4) | 0.1 (0.05–0.24) | <0.01 | 0.2 (0.06–0.50) | <0.01 |
| Smoked Fish | ||||||
| Unknown | 32 (10.7) | 30 (9.8) | 1 | 1 | ||
| <1 time/week | 132 (44.3) | 126 (41.3) | 1.0 (0.56–1.71) | 0.95 | 1.1 (0.48–2.32) | 0.9 |
| 1–2 times/week | 121 (40.6) | 52 (17.1) | 2.2 (1.20–3.95) | 0.01 | 2.7 (1.24–6.02) | 0.01 |
| 3–5 times/week | 13 (4.6) | 97 (31.8) | 0.1 (0.10–0.27) | 0.00 | 0.1 (0.04–0.31) | <0.01 |
| Chips/fried potatoes | ||||||
| Unknown | 78 (26.2) | 32 (10.5) | 1 | 1 | ||
| <1 time/week | 125 (42.0) | 131 (43.0) | 0.3 (0.24–0.63) | <0.01 | 0.5 (0.26–103) | 0.06 |
| 1–2 times/week | 82 (27.5) | 70 (23.0) | 0.5 (0.3–0.81) | <0.01 | 0.8 (0.40–1.78) | 0.66 |
| 3–5 times/week | 13 (4.4) | 72 (23.6) | 0.1 (0.04–0.15) | <0.01 | 0.2 (0.07–0.48) | 0.001 |
| Non-Smoking Group | |||||||
|---|---|---|---|---|---|---|---|
| Risk Factor | Cases (n = 214) | Control (n = 265) | OR (95% CI) | p-Value | aOR (95% CI) | p-Value | |
| n (%) | n (%) | ||||||
| Occupation | |||||||
| Non–agriculture | 106 (49.5) | 205 (77.4) | 1 | 1 | 1 | ||
| Agriculture | 108 (50.5) | 60 (22.6) | 3.3 (2.12–4.98) | <0.01 | 2.6 (1.48–4.53) | <0.01 | |
| Age group (years) | |||||||
| <40 | 67 (31.3) | 136 (51.3) | 1 | 1 | |||
| 40–60 | 97 (45.3) | 88 (33.2) | 2.2 (1.48–3.37) | <0.01 | 3.2 (1.80–5.90) | <0.01 | |
| >60 | 50 (23.4) | 41 (15.5) | 2.4 (1.49–4.11) | <0.01 | 1.9 (0.92–3.90) | 0.08 | |
| Sex | |||||||
| Male | 121 (56.5) | 108 (40.8) | 1 | 1 | |||
| Female | 93 (43.5) | 157 (59.3) | 1.9 (1.31–2.72) | <0.01 | 1.7 (1.12–2.92) | 0.04 | |
| Birthplace | |||||||
| Urban | 43 (20.0) | 180 (67.9) | 1 | 1 | |||
| Rural | 171 (80.0) | 85 (32.1) | 8.4 (5.52–12.84) | <0.01 | 11.0 (6.2115.56) | <0.01 | |
| Smoked Meat | |||||||
| Unknown | 81 (37.9) | 39 (14.7) | |||||
| <1 time/week | 72 (33.6) | 130 (49.1) | 0.3 (0.17–0.43) | <0.01 | 0.2 (0.11–0.52) | <0.01 | |
| 1–2 times/week | 57 (26.6) | 60 (22.6) | 0.5 (0.27–0.77) | 0.004 | 0.5 (0.20–1.05) | 0.07 | |
| 3–5 times/week | 4 (1.9) | 36 (13.6) | 0.1 (0.02-0.17) | <0.001 | 0.1 (0.02–0.32) | <0.01 | |
| Smoked Fish | |||||||
| Unknown | 25 (11.7) | 25 (9.4) | 1 | 1 | |||
| <1 time/week | 90 (42.1) | 110 (41.5) | 0.8 (0.44–1.52) | 0.53 | 1.4 (0.56–3.48) | 0.45 | |
| 1–2 times/week | 92 (43.0) | 46 (17.4) | 2.0 (1.04–3.86) | 0.04 | 4.0 (1.58–10.13) | <0.01 | |
| 3–5 times/week | 7 (3.3) | 84 (31.7) | 0.1 (0.03–0.22) | 0.00 | 0.1 (0.04–0.42) | <0.01 | |
| Chips/fried potatoes | |||||||
| Unknown | 54 (25.2) | 29 (10.9) | 1 | 1 | |||
| <1 time/week | 87 (40.7) | 110 (41.5) | 0.42 (0.24–0.72) | 0.002 | 0.8 (0.35–1.78) | 0.60 | |
| 1–2 times/week | 62 (29.0) | 64 (24.2) | 0.5 (0.29–0.92) | 0.03 | 1.2 (0.49–1.05) | 0.78 | |
| 3–5 times/week | 11 (5.1) | 62 (23.4) | 0.1 (0.04–0.20) | <0.01 | 0.4 (0.12–1.02) | 0.05 | |
| Non-Smokers and Non-Alcohol Users | ||||||
|---|---|---|---|---|---|---|
| Risk Factor | Cases (n = 148) | Control (n = 215) | OR (95% CI) | p-Value | aOR (95% CI) | p-Value |
| n (%) | n (%) | |||||
| Occupation | ||||||
| Non-agriculture | 80 (54.1) | 167 (77.7) | 1 | 1 | ||
| Agriculture | 68 (46.0) | 48 (22.3) | 3.0 (1.88–4.66) | <0.01 | 3.0 (1.52–5.97) | <0.01 |
| Age group (years) | ||||||
| <40 | 54 (36.5) | 112 (52.1) | 1 | 1 | ||
| 40–60 | 66 (44.6) | 71 (33.0) | 1.9 (1.21–3.07) | 0.01 | 2.5 (1.25–4. 98) | <0.01 |
| >60 | 28 (18.9) | 32 (14.9) | 1.8 (0.99–3.31) | 0.05 | 1.2 (0.48–2.83) | 0.74 |
| Sex | ||||||
| Male | 60 (40.5) | 85 (39.5) | 1 | 1 | ||
| Female | 88 (59.5) | 130 (60.5) | 2.2 (1.46–3.44) | <0.01 | 2.0 (1.063. 84) | 0.03 |
| Birthplace | ||||||
| Urban | 31 (21.0) | 148 (68.8) | 1 | 1 | ||
| Rural | 117 (79.0) | 67 (31.2) | 8.3 (5.11–13.60) | <0.01 | 14 (7.24–28.19) | <0.01 |
| Smoked Meat | ||||||
| Unknown | 58 (39.2) | 34 (15.8) | ||||
| <1 time/week | 52 (35.1) | 106 (49.3) | 0.3 (0.17–0.49) | <0.01 | 0.4 (0.15–0.98) | 0.03 |
| 1–2 times/week | 37 (25.0) | 49 (22.8) | 0.4 (0.24–0.80) | <0.01 | 0.6 (0.22–1.53) | 0.27 |
| 3–5 times/week | 1 (0.7) | 26 (12.1) | 0.02 (0.01–0.17) | <0.01 | 0.01 (0.00–0.31) | 0.003 |
| Smoked Fish | ||||||
| Unknown | 21 (14.2) | 22 (10.2) | ||||
| <1 time/week | 64 (43.2) | 96 (44.7) | 0.7 (0.36–1.37) | 0.30 | 1.2 (0.42–3.56) | 0.7 |
| 1–2 times/week | 60 (40.6) | 34 (15.8) | 1.8 (0.89–3.84) | 0.10 | 4.4 (1.51–13.13) | 0.007 |
| 3–5 times/week | 3 (2.0) | 63 (29.3) | 0.04 (0.01–0.18) | 0.00 | 0.1 (0.01–0.26) | <0.001 |
| Chips/fried potatoes | ||||||
| Unknown | 39 (26.4) | 26 (12.1) | ||||
| <1 time/week | 59 (39.9) | 88 (40.9) | 0.4 (0.24–0.81) | 0.01 | 0.7 (0.42–3.56) | 0.40 |
| 1–2 times/week | 43 (29.1) | 52 (24.2) | 0.6 (0.29–1.05) | 0.07 | 0.9 (0.31–2.39) | 0.27 |
| 3–5 times/week | 7 (4.7) | 49 (22.8) | 0.1 (0.03–0.24) | <0.01 | 0.3 (0.08–1.04) | 0.06 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mwelange, L.P.; Nyarubeli, I.P.; Sakwari, G.; Mamuya, S.H.; Moen, B.E. Occupations and Risk of Head and Neck Cancers: A Case–Control Study in Tanzania. Int. J. Environ. Res. Public Health 2025, 22, 1643. https://doi.org/10.3390/ijerph22111643
Mwelange LP, Nyarubeli IP, Sakwari G, Mamuya SH, Moen BE. Occupations and Risk of Head and Neck Cancers: A Case–Control Study in Tanzania. International Journal of Environmental Research and Public Health. 2025; 22(11):1643. https://doi.org/10.3390/ijerph22111643
Chicago/Turabian StyleMwelange, Luco Patson, Israel Paul Nyarubeli, Gloria Sakwari, Simon Henry Mamuya, and Bente Elisabeth Moen. 2025. "Occupations and Risk of Head and Neck Cancers: A Case–Control Study in Tanzania" International Journal of Environmental Research and Public Health 22, no. 11: 1643. https://doi.org/10.3390/ijerph22111643
APA StyleMwelange, L. P., Nyarubeli, I. P., Sakwari, G., Mamuya, S. H., & Moen, B. E. (2025). Occupations and Risk of Head and Neck Cancers: A Case–Control Study in Tanzania. International Journal of Environmental Research and Public Health, 22(11), 1643. https://doi.org/10.3390/ijerph22111643







