Distribution of Hematologic Parameters of Complete Blood Count in Anemic and Nonanemic Children in a Mining-Exposed Highland Peruvian Community
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Settings
2.2. Sampling, Inclusion Criteria, and Variables
2.3. Instruments and Serological Markers
2.4. Data Gathering and Analysis
2.5. Ethical Aspects
3. Results
3.1. Hematological Parameters Distribution by Sex
3.2. Hematological Parameter Distribution According Age Groups
3.3. Hematological Distribution According Anemia Outcomes
4. Discussion
4.1. Strengths
4.2. Main Findings
4.3. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- von der Goltz, J.; Barnwal, P. Mines: The local wealth and health effects of mineral mining in developing countries. J. Dev. Econ. 2019, 139, 1–16. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, X.; Sun, D.; Cao, J.; Zhang, Y.; Huo, X. Alteration of the number and percentage of innate immune cells in preschool children from an e-waste recycling area. Ecotoxicol. Environ. Saf. 2017, 145, 615–622. [Google Scholar] [CrossRef]
- Udom, G.J.; Turyahabwe, B.; Aturamu, A.; Aziakpono, O.M.; Agbana, R.D.; Joseph, O.G.; Udom, N.-W.G.; Mugide, N.; Odey, O.P.; Olot, H.; et al. Heavy metal and metalloid pollution: A systematic review of health implications for pregnant women, children, and geriatrics in the East African region. Environ. Adv. 2025, 19, 100620. [Google Scholar] [CrossRef]
- Islam, M.d.H.; Nowar, A.; Islam, S.; Nayan, M.d.M.; Jubayer, A. A Systematic Review on Heavy Metals Contamination in Bangladeshi Fruits and Their Associated Health Risks. Environ. Health Insights 2024, 18, 11786302241309280. [Google Scholar] [CrossRef]
- Lee, C.R.; Yoo, C.I.; Lee, J.H.; Kim, S.R.; Kim, Y. Hematological changes of children exposed to volatile organic compounds containing low levels of benzene. Sci. Total Environ. 2002, 299, 237–245. [Google Scholar] [CrossRef] [PubMed]
- Oulhote, Y.; Shamim, Z.; Kielsen, K.; Weihe, P.; Grandjean, P.; Ryder, L.; Heilmann, C. Children’s white blood cell counts in relation to developmental exposures to methylmercury and persistent organic pollutants. Reprod. Toxicol. 2017, 68, 207–214. [Google Scholar] [CrossRef]
- Fu, Y.; He, M.; Liu, Y.; Li, M.; Zhu, M.; Wang, Y.; Lin, W.; Yu, L.; Yang, L.; Zhang, Y.; et al. Reduction of haemoglobin is related to metal mixtures exposure in Chinese preschoolers: Joint effect models. J. Trace Elem. Med. Biol. 2024, 84, 127427. [Google Scholar] [CrossRef] [PubMed]
- Pumallanqui-Ramirez, B.; Li, J.; Lenin Rueda-Torres Rosales-Rimache, J. Relationship between Blood Lead Levels and Anemia: A Cross-Sectional Study on Mining Workers from Peru. Adv. Public Health 2024, 2024, 5517405. [Google Scholar] [CrossRef]
- Costalat, H.C.M.; Rodrigues, N.P.; Ribeiro, A.F.; Rocha, R.M.R.; Rosane, P.L.R.; Corvelo, T.C.O. Exposure to Aluminum and the Prevalence of Anemia in Communities in Barcarena Pará, Brazil. Int. J. Adv. Eng. Res. Sci. 2022, 9, 491–497. [Google Scholar] [CrossRef]
- Wigle, D.T.; Arbuckle, T.E.; Walker, M.; Wade, M.G.; Liu, S.; Krewski, D. Environmental Hazards: Evidence for Effects on Child Health. J. Toxicol. Environ. Health Part B 2007, 10, 3–39. [Google Scholar] [CrossRef]
- Landrigan, P.J.; Schechter, C.B.; Lipton, J.M.; Fahs, M.C.; Schwartz, J. Environmental pollutants and disease in American children: Estimates of morbidity, mortality, and costs for lead poisoning, asthma, cancer, and developmental disabilities. Environ. Health Perspect. 2002, 110, 721–728. [Google Scholar] [CrossRef] [PubMed]
- Landrigan, P.; Garg, A. Chronic Effects of Toxic Environmental Exposures on Children’s Health. J. Toxicol. Clin. Toxicol. 2002, 40, 449–456. [Google Scholar] [CrossRef] [PubMed]
- Goldman, L. Children—unique and vulnerable. Environmental risks facing children and recommendations for response. Environ. Health Perspect. 1995, 103, 13–18. [Google Scholar] [CrossRef]
- Teixeira, R.A.; Pereira, W.V.D.S.; Souza, E.S.; Ramos, S.J.; Dias, Y.N.; Lima, M.W.; de Souza Neto, H.F.; Oliveira, E.S.; Fernandes, A.R. Artisanal gold mining in the eastern Amazon: Environmental and human health risks of mercury from different mining methods. Chemosphere 2021, 284, 131220. [Google Scholar] [CrossRef]
- Gillings, M.M.; Ton, R.; Harris, T.; Swaddle, J.P.; Taylor, M.P.; Griffith, S.C. House Sparrows as Sentinels of Childhood Lead Exposure. Environ. Sci. Technol. 2024, 58, 10028–10040. [Google Scholar] [CrossRef]
- Wong, C.; Roberts, S.M.; Saab, I.N. Review of regulatory reference values and background levels for heavy metals in the human diet. Regul. Toxicol. Pharmacol. 2022, 130, 105122. [Google Scholar] [CrossRef]
- Lee, H.R.; Shin, S.; Yoon, J.H.; Roh, E.Y.; Chang, J.Y. Reference Intervals of Hematology and Clinical Chemistry Analytes for 1-Year-Old Korean Children. Ann. Lab. Med. 2016, 36, 481–488. [Google Scholar] [CrossRef]
- Lazón Mansilla, D.F.; Lagos Castillo, M.A.; Prado Maggia, C.T.; Llerena Paredes, V.; Mayhua Gutiérrez, B.S. Lead in blood and its relationship with hemoglobin and hematocrit in children exposed to mining pollution, Apurímac 2023. World Health J. 2024, 5, 7–15. [Google Scholar] [CrossRef]
- Instituto Geológico Minero y Metalúrgico. Mapa de Ubicación de Labores Mineras Artesanales—Región Apurímac. Gobierno del Perú. 2016. Available online: https://portal.ingemmet.gob.pe/documents/73138/883057/Ubicaci\%C3\%B3n+Labores+Mineras_regi\%C3\%B3n+Apur\%C3\%ADmac+2015.pdf (accessed on 11 March 2024).
- Custodio, L.M. “¿Cómo Vamos a Vivir?” El Impacto de la Minería en las Comunidades del sur de Perú. Dialogue Earth. 2022. Available online: https://dialogue.earth/es/polucion/361618-como-vamos-a-vivir-el-impacto-de-la-mineria-en-las-comunidades-del-sur-de-peru/ (accessed on 11 March 2024).
- Huarachi, L.A.; Lozano-Zanelly, G.; Acosta, J.; Huarachi, C.A.; Moya-Salazar, J. Inequality in the distribution of resources and health care in the poverty quintiles: Evidence from Peruvian comprehensive health insurance 2018–2019. Electron. J. Gen. Med. 2024, 21, em568. [Google Scholar] [CrossRef]
- Maiza-Larrarte, A.; Claudio-Quiroga, G. The impact of Las Bambas megamine on development in Apurímac, Peru. Miner. Econ. 2025, 38, 569–583. [Google Scholar] [CrossRef]
- Astete, J.; Gastañaga, M.C.; Pérez, D. Levels of heavy metals in the environment and population exposure after five years of mineral exploration in the Las Bambas project, Peru 2010. Rev. Perú. Med. Exp. Salud Publica 2014, 31, 695–701. Available online: http://www.scielo.org.pe/scielo.php?script=sci_arttext&pid=S1726-46342014000400012&lng=es (accessed on 11 March 2024). [PubMed]
- Correa Cuba, O.; Fuentes Bernedo, F.E.; Coral Surco, R.G.; Correa Cuba, O.; Fuentes Bernedo, F.E.; Coral Surco, R.G. Contaminación por metales pesados de la microcuenca agropecuaria del río Huancaray—Perú. Rev. Soc. Quím. Perú 2021, 87, 26–38. [Google Scholar] [CrossRef]
- Observatorio de Conflictos Mineros de América Latina. Perú: Regiones Mineras Presentan Escandalosas Cifras de Desnutrición Crónica en Niños. OCMAL. 2012. Available online: https://www.ocmal.org/peru-regiones-mineras-presentan-escandalosas-cifras-de-desnutricion-cronica-en-ninos/# (accessed on 11 March 2024).
- Clinical and Laboratory Standards Institute. Defining, Establishing, and Verifying Reference Intervals in Clinical Laboratory; Approved Guidelines, 3rd ed.; CLSI document C28-A3; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2008; Available online: https://clsi.org/shop/standards/ep28/ (accessed on 11 March 2024).
- Ministerio de Salud (MINSA). Resolución Ministerial N.° 251-2024-MINSA. Gobierno del Perú. 2024. Available online: https://www.gob.pe/institucion/minsa/normas-legales/5440166-251-2024-minsa (accessed on 11 March 2024).
- Bais, R.; Vassault, A.; Blasutig, I.M.; Dabla, P.K.; Lin, J.; Perret-Liaudet, A.; Thomas, A.; Cendejas, K.A.; Wheeler, S.E.; Giannoli, J.-M.; et al. External quality assessment performance in ten countries: An IFCC global laboratory quality project. Clin. Chem. Lab. Med. 2024, 62, 2435–2443. [Google Scholar] [CrossRef]
- Cornet, E.; Behier, C.; Troussard, X. Guidance for storing blood samples in laboratories performing complete blood count with differential. Int. J. Lab. Hematol. 2012, 34, 655–660. [Google Scholar] [CrossRef]
- Jaya, A.; Kakkar, N.; John, M. Effect of room temperature and refrigerated storage on automated complete blood count: A longitudinal study. CHRISMED J. Health Res. 2022, 9, 57–61. [Google Scholar] [CrossRef]
- Ministerio de Salud (MINSA). Resolución Directoral N.° 173-2023-HNHU-DG. Gobierno del Perú. 2023. Available online: https://www.gob.pe/institucion/hnhu/normas-legales/4305070-173-2023-hnhu-dg (accessed on 11 March 2024).
- Ministerio de Salud (MINSA). Resolución Directoral N.° 086-2022-DG-HVLH. Gobierno del Perú. 2022. Available online: https://larcoherrera.gob.pe/wp-content/uploads/2022/06/RD-086-2022-DG-HVLH-MINSA.pdf (accessed on 11 March 2024).
- Wu, D.; Li, Y.; Wang, F. How Long can we Store Blood Samples: A Systematic Review and Meta-Analysis. eBioMedicine 2017, 24, 277–285. [Google Scholar] [CrossRef]
- Tukey, J.W. Exploratory Data Analysis; Addison Wesley: Reading, MA, USA, 1997. [Google Scholar]
- World Medical Association. World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA 2013, 310, 2191–2194. [Google Scholar] [CrossRef]
- Congreso de la República. Ley N.° 29733—Ley de Protección de Datos Personales; Gobierno del Perú: Lima, Peru, 2011. Available online: https://www.gob.pe/institucion/congreso-de-la-republica/normas-legales/243470-29733 (accessed on 11 March 2024).
- Linares, A.M.; Unrine, J.M.; Wigging, A.T.; Tantalean, J.C.; Radulescu, V.C. Blood’s Concentration of Lead and Arsenic Associated with Anemia in Peruvian Children. J. Environ. Public Health 2021, 2021, 7283514. [Google Scholar] [CrossRef] [PubMed]
- Capitao, C.; Martins, R.; Santos, O.; Bicho, M.; Szigeti, T.; Katsonouri, A.; Bocca, B.; Ruggieri, F.; Wasowicz, W.; Tolonen, H.; et al. Exposure to heavy metals and red blood cell parameters in children: A systematic review of observational studies. Front. Pediatr. 2022, 10, 921239. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Deng, Y.H.; Ke, H.J.; Wu, J.L. Iron Status in Relation to Low-Level Lead Exposure in a Large Population of Children Aged 0–5 Years. Biol. Trace Elem. Res. 2020, 199, 1253–1258. [Google Scholar] [CrossRef]
- Heng, Y.Y.; Asad, I.; Coleman, B.; Menard, L.; Benki-Nugent, S.; Were, F.H.; Karr, C.J.; McHenry, M.S. Heavy metals and neurodevelopment of children in low and middle-income countries: A systematic review. PLoS ONE 2022, 17, e0265536. [Google Scholar] [CrossRef]
- Disalvo, L.; Varea, A.; Matamoros, N.; Sala, M.; Fasano, M.V.; González, H.F. Blood Lead Levels and Their Association with Iron Deficiency and Anemia in Children. Biol. Trace Element Res. 2025, 203, 69–75. [Google Scholar] [CrossRef]
- Kuang, W.; Chen, Z.; Shi, K.; Sun, H.; Li, H.; Huang, L.; Bi, J. Adverse health effects of lead exposure on physical growth, erythrocyte parameters and school performances for school-aged children in eastern China. Environ. Int. 2020, 145, 106130. [Google Scholar] [CrossRef] [PubMed]
- Balali-Mood, M.; Naseri, K.; Tahergorabi, Z.; Khazdair, M.R.; Sadeghi, M. Toxic Mechanisms of Five Heavy Metals: Mercury, Lead, Chromium, Cadmium, and Arsenic. Front. Pharmacol. 2021, 12, 643972. [Google Scholar] [CrossRef] [PubMed]
- Adeli, K.; Raizman, J.; Chen, Y.; Higgins, V.; Nieuwesteeg, M.; Abdelhaleem, M.; Wong, S.; Blais, D. Complex biological profile of hematologic markers across pediatric, adult, and geriatric ages: Establishment of robust pediatric and adult reference intervals on the basis of the Canadian Health Measures Survey. Clin. Chem. 2015, 61, 1075–1086. [Google Scholar] [CrossRef]
- Song, W.; Yan, R.; Peng, M.; Jiang, H.; Li, G.; Cao, S.; Jiang, Y.; Guo, Z.; Chen, D.; Yang, H.; et al. Age and sex specific reference intervals of 13 hematological analytes in Chinese children and adolescents aged from 28 days up to 20 years: The PRINCE study. Clin. Chem. Lab. Med. 2022, 60, 1250–1260. [Google Scholar] [CrossRef]
- Laugesen, K.; Winther-Larsen, A. Paediatric reference intervals for haematology parameters analysed on Sysmex XN-9000: A comparison of methods in the framework of indirect sampling. Clin. Chem. Lab. Med. 2024, 63, 812–820. [Google Scholar] [CrossRef]
- Entwistle, J.A.; Hursthouse, A.S.; Marinho Reis, P.A.; Stewart, A.G. Metalliferous mine dust: Human health impacts and the potential determinants of disease in mining communities. Curr. Pollut. Rep. 2019, 5, 67–83. [Google Scholar] [CrossRef]
- Lafta, M.H.; Afra, A.; Patra, I.; Jalil, A.T.; Mohammadi, M.J.; Al-Dhalimy, A.M.B.; Ziyadullaev, S.; Kiani, F.; Ekrami, H.A.; Asban, P. Toxic effects due to exposure heavy metals and increased health risk assessment (leukemia). Rev. Environ. Health 2022, 39, 351–362. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, H. A mechanistic review of silica-induced inhalation toxicity. Inhal. Toxicol. 2015, 27, 363–377. [Google Scholar] [CrossRef]
- McFadden, J.P.; Basketter, D.A.; Dearman, R.J.; Puangpet, P.; Kimber, I. The hapten–atopy hypothesis III: The potential role of airborne chemicals. Br. J. Dermatol. 2014, 170, 45–51. [Google Scholar] [CrossRef]
- Girard, D. Activation of human polymorphonuclear neutrophils by environmental contaminants. Rev. Environ. Health 2003, 18, 75–90. [Google Scholar] [CrossRef]
- Hashemi, M.; Rajabi, S.; Eghbalian, M.; Suliburska, J.; Nasab, H. Demographic and anthropometric characteristics and their effect on the concentration of heavy metals (arsenic, lead, chromium, zinc) in children and adolescents. Heliyon 2023, 9, e13621. [Google Scholar] [CrossRef] [PubMed]
- Moya-Salazar, J.; Cerda, S.P.; Cañari, B.; Moya-Salazar, M.M.; Contreras-Pulache, H. Reference intervals of the sex hormonal profile in healthy women: A retrospective single-center study in Peru. Heliyon 2022, 8, e10592. [Google Scholar] [CrossRef] [PubMed]
- Moya, S.J.; Pio, D.L. Imprecision of erythrocyte parameters (hematocrit, hemoglobin and recount erythroid) with rule 3 according to guidelines CLSI H26-A2. Infinitum 2015, 5, 2–8. [Google Scholar]
- Fernández, B.C. Correlación Diagnóstica en el Recuento Diferencial de Leucocitos Entre el Sistema Automatizado Genrui kt—40 y Lámina Periférica en el Consultorio Médico Privado Lima, 2023. Bachelor’s Thesis, Facultad de Ciencias de la Salud, Universidad Norbert Wiener, Lima, Peru, 2024. Available online: https://hdl.handle.net/20.500.13053/11601 (accessed on 11 March 2024).
- Moya-Salazar, J. Day-per-day maintenance and six sigmas of the Landwind LW D3600 hematological analyzer: Clinical aspects and quality verification. Arch. Hematol. Blood Dis. 2019, 2, 12–22. [Google Scholar] [CrossRef]
| Variable | Categories | Frequency | % |
|---|---|---|---|
| Sex | Male | 74 | 47.4 |
| Female | 82 | 52.6 | |
| Age (year) | 3 | 40 | 25.6 |
| 4 | 34 | 21.8 | |
| 5 | 30 | 19.2 | |
| 6 | 22 | 14.1 | |
| 7 | 30 | 19.2 | |
| District | Chiara | 34 | 21.8 |
| Huayana | 30 | 19.2 | |
| Huancarama | 27 | 17.3 | |
| Talavera | 24 | 15.4 | |
| Huancaray | 19 | 12.2 | |
| Andarapa | 9 | 5.8 | |
| San Jerónimo | 7 | 4.5 | |
| Otros * | 6 | 3.8 |
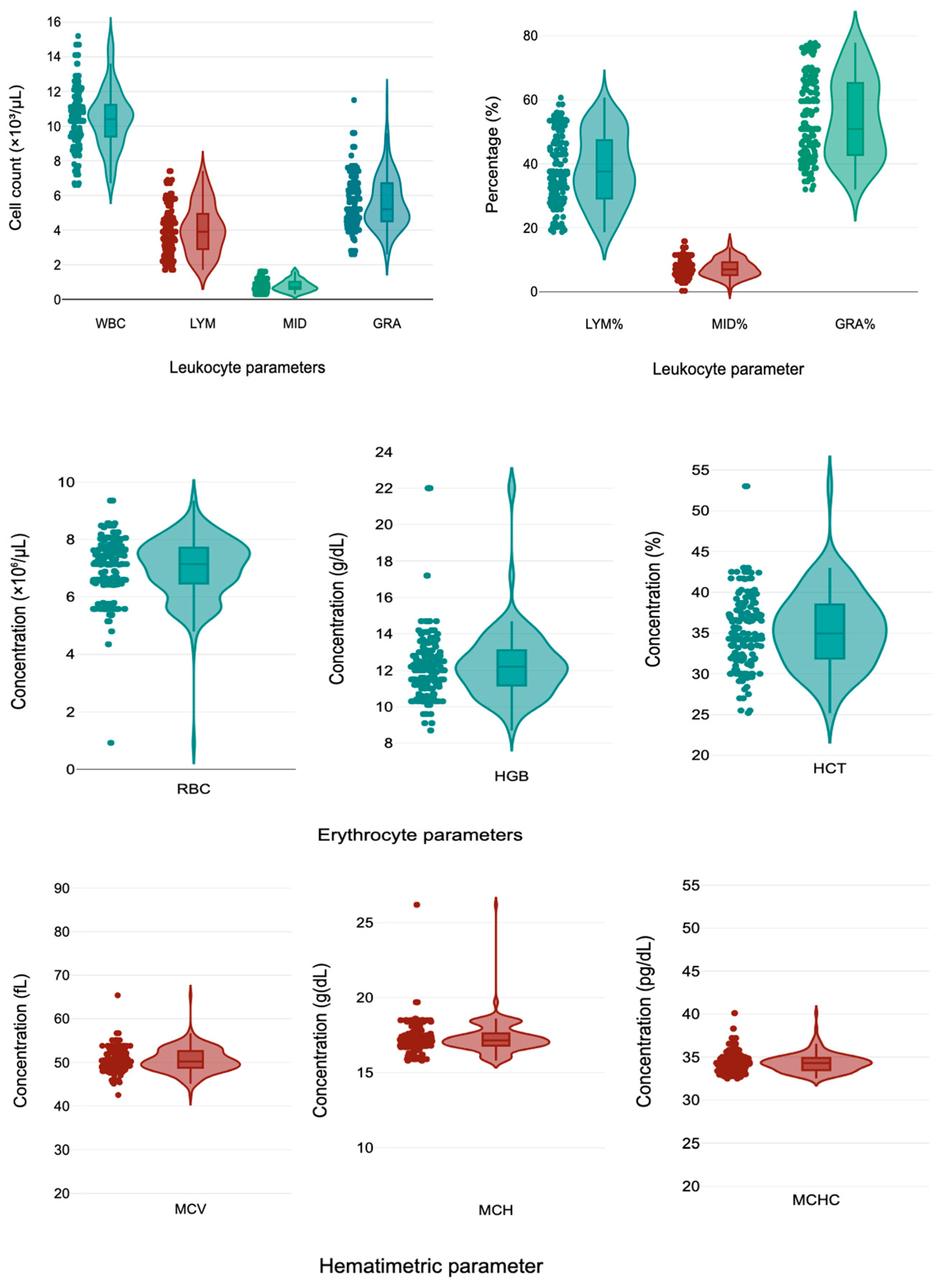
| Hematological Parameters | Total | Male (n = 74) | Female (n = 82) | p-Value |
|---|---|---|---|---|
| WBC (×103/µL) | 10.42 ± 1.76 (6.70 to 14.70) | 10.47 ± 1.58 (6.60 to 14.29) | 10.39 ± 1.87 (6.70 to 14.70) | 0.770 |
| LYM (×103/µL) | 4.05 ± 1.44 (1.70 to 6.83) | 4.06 ± 1.46 (1.74 to 6.90) | 4.04 ± 1.43 (1.70 to 6.85) | 0.934 |
| MID (×103/µL) | 0.78 ± 0.33 (0.30 to 1.60) | 0.75 ± 0.29 (0.30 to 1.23) | 0.81 ± 0.35 (0.30 to 1.60) | 0.276 |
| GRA (×103/µL) | 5.57 ± 1.52 (2.80 to 9.03) | 5.64 ± 1.54 (3.54 to 9.92) | 5.54 ± 1.51 (2.60 to 8.87) | 0.684 |
| LYM% | 38.50 ± 11.02 (19.30 to 56.00) | 38.27 ± 11.49 (19.03 to 56.80) | 38.64 ± 10.77 (19.26 to 56.05) | 0.836 |
| MID% | 7.34 ± 2.87 (2.80 to 12.84) | 7.12 ± 2.48 (2.84 to 11.50) | 7.48 ± 3.10 (0.30 to 14.07) | 0.445 |
| GRA% | 54.06 ± 12.60 (33.38 to 76.90) | 54.61 ± 12.76 (33.64 to 77.05) | 53.71 ± 12.55 (32.00 to 76.90) | 0.664 |
| RBC (×106/µL) | 6.98 ± 1.08 (4.85 to 8.56) | 7.06 ± 0.89 (5.58 to 8.69) | 6.94 ± 1.18 (4.14 to 8.56) | 0.502 |
| HGB (g/dL) | 12.32 ± 2.01 (9.17 to 17.20) | 12.54 ± 2.27 (10.14 to 22) | 12.18 ± 1.82 (9.08 to 14.93) | 0.283 |
| HCT (%) | 35.35 ± 4.71 (25.71 to 43.00) | 35.52 ± 4.63 (29.19 to 44.70) | 35.24 ± 4.79 (25.48 to 43.00) | 0.717 |
| MCV (fL) | 50.50 ± 2.85 (45.40 to 55.10) | 50.44 ± 2.63 (45.17 to 55.37) | 50.54 ± 2.99 (45.51 to 55.24) | 0.818 |
| MCH (g/dL) | 17.33 ± 1.06 (15.90 to 18.60) | 17.30 ± 0.81 (15.90 to 18.79) | 17.34 ± 1.20 (15.80 to 18.70) | 0.817 |
| MCHC (pg/dL) | 34.31 ± 1.11 (32.71 to 36.73) | 34.32 ± 0.99 (32.56 to 37.20) | 34.31 ± 1.18 (32.69 to 36.70) | 0.974 |
| PLT (×103/µL) | 226.47 ± 35.10 (167.00 to 295.06) | 224.50 ± 27.86 (167.00 to 284.36) | 227.70 ± 39.04 (135.94 to 311.93) | 0.581 |
| Hematological Parameters | Age Group | p-Value | |
|---|---|---|---|
| 3–4 (n = 74) | 5–7 (n = 84) | ||
| WBC (×103/µL) | 10.28 ± 1.74 (6.65 to 14.55) | 10.55 ± 1.78 (7 to 14.7) | 0.350 |
| LYM (×103/µL) | 3.94 ± 1.45 (1.79 to 6.8) | 4.15 ± 1.43 (1.83 to 7.16) | 0.362 |
| MID (×103/µL) | 0.73 ± 0.31 (0.30 to 1.6) | 0.83 ± 0.34 (0.30 to 1.6) | 0.053 |
| GRA (×103/µL) | 5.59 ± 1.43 (2.60 to 9.12) | 5.56 ± 1.61 (2.8 to 9.21) | 0.906 |
| LYM% | 37.86 ± 11.14 (18.7 to 55.77) | 39.1 ± 10.94 (19.89 to 57.33) | 0.494 |
| MID% | 6.79 ± 2.72 (0.30 to 11.5) | 7.85 ± 2.93 (2.93 to 14.87) | 0.021 |
| GRA% | 55.14 ± 12.56 (34.2 to 77.58) | 53.08 ± 12.63 (32 to 75.91) | 0.310 |
| RBC (×106/µL) | 7.06 ± 0.96 (4.98 to 8.56) | 6.91 ± 1.18 (3.18 to 8.52) | 0.369 |
| HGB (g/dL) | 12.61 ± 2.48 (8.9 to 22) | 12.06 ± 1.42 (10.23 to 14.7) | 0.085 |
| HCT (%) | 35.64 ± 5 (25.35 to 43) | 35.09 ± 4.46 (28.09 to 42.75) | 0.474 |
| MCV (fL) | 50.51 ± 2.43 (45.4 to 55.1) | 50.5 ± 3.19 (44.41 to 55.92) | 0.975 |
| MCH (g/dL) | 17.26 ± 0.70 (15.9 to 18.5) | 17.38 ± 1.31 (15.8 to 19.7) | 0.467 |
| MCHC (pg/dL) | 34.19 ± 0.92 (32.63 to 36.31) | 34.42 ± 1.25 (32.63 to 37.76) | 0.198 |
| PLT (×103/µL) | 226.61 ± 31 (151 to 301.5) | 226.34 ± 38.6 (156.46 to 333.49) | 0.962 |
| Hematological Parameters | 3–4 Years (n = 74) | 5–7 Years (n = 82) | ||||
|---|---|---|---|---|---|---|
| Anemia (n = 14) | No Anemia (n = 60) | p-Value | Anemia (n = 34) | No Anemia (n = 48) | p-Value | |
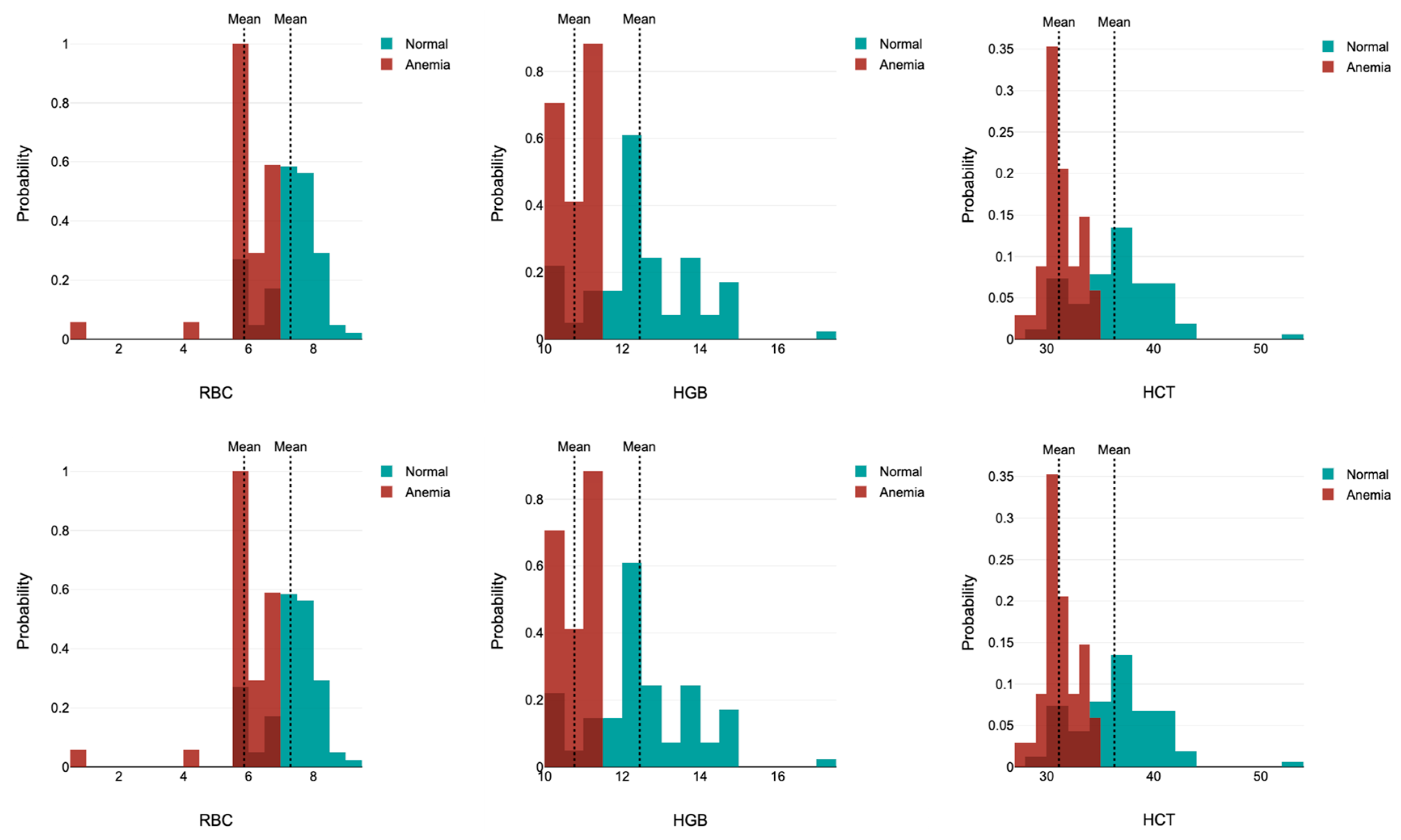
| RBC (×106/µL) | 5.56 ± 0.47 (4.91 to 6.52) | 7.06 ± 0.96 (4.98 to 8.56) | <0.00001 | 5.87 ± 1.02 (4.46 to 6.62) | 7.36 ± 0.79 (5.58 to 8.54) | <0.00001 |
| HCT (%) | 33.97 ± 6.89 (25.3 to 39.8) | 35.64 ± 5 (25.43 to 43) | <0.00001 | 31.13 ± 1.73 (27.86 to 34.45) | 36.54 ± 4 (29.74 to 42.89) | <0.00001 |
| MCV (fL) | 49.95 ± 6.43 (46.35 to 53.8) | 50.51 ± 2.43 (45.4 to 55.1) | 0.223 | 51.83 ± 3.86 (42.91 to 55.1) | 49.75 ± 2.49 (45.54 to 55.1) | 0.0018 |
| MCH (g/dL) | 17.12 ± 2.15 (15.93 to 18.5) | 17.26 ± 0.70 (15.9 to 18.5) | 0.001 | 17.98 ± 1.75 (16.06 to 19.88) | 17.08 ± 0.77 (15.8 to 18.58) | 0.0004 |
| MCHC (pg/dL) | 33.84 ± 4.11 (33.59 to 35.6) | 34.19 ± 0.92 (32.6 to 36.31) | 0.020 | 34.64 ± 1.54 (32.94 to 37.79) | 34.35 ± 0.99 (32.6 to 36.54) | 0.165 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cruz-Gonzales, G.; Hurtado-Concha, A.; Bejarano-Benites, H.; Bedoya-Vílchez, H.; Sarabia-Tarrillo, M.; Goicochea-Palomino, E.A.; Moya-Salazar, J. Distribution of Hematologic Parameters of Complete Blood Count in Anemic and Nonanemic Children in a Mining-Exposed Highland Peruvian Community. Int. J. Environ. Res. Public Health 2025, 22, 1637. https://doi.org/10.3390/ijerph22111637
Cruz-Gonzales G, Hurtado-Concha A, Bejarano-Benites H, Bedoya-Vílchez H, Sarabia-Tarrillo M, Goicochea-Palomino EA, Moya-Salazar J. Distribution of Hematologic Parameters of Complete Blood Count in Anemic and Nonanemic Children in a Mining-Exposed Highland Peruvian Community. International Journal of Environmental Research and Public Health. 2025; 22(11):1637. https://doi.org/10.3390/ijerph22111637
Chicago/Turabian StyleCruz-Gonzales, Gloria, Arístides Hurtado-Concha, Héctor Bejarano-Benites, Hernán Bedoya-Vílchez, Merly Sarabia-Tarrillo, Eliane A. Goicochea-Palomino, and Jeel Moya-Salazar. 2025. "Distribution of Hematologic Parameters of Complete Blood Count in Anemic and Nonanemic Children in a Mining-Exposed Highland Peruvian Community" International Journal of Environmental Research and Public Health 22, no. 11: 1637. https://doi.org/10.3390/ijerph22111637
APA StyleCruz-Gonzales, G., Hurtado-Concha, A., Bejarano-Benites, H., Bedoya-Vílchez, H., Sarabia-Tarrillo, M., Goicochea-Palomino, E. A., & Moya-Salazar, J. (2025). Distribution of Hematologic Parameters of Complete Blood Count in Anemic and Nonanemic Children in a Mining-Exposed Highland Peruvian Community. International Journal of Environmental Research and Public Health, 22(11), 1637. https://doi.org/10.3390/ijerph22111637






