INdoor Home Air Level Exploration (INHALE) Study: Protocol to Monitor Indoor Pollution in British Dwellings
Abstract
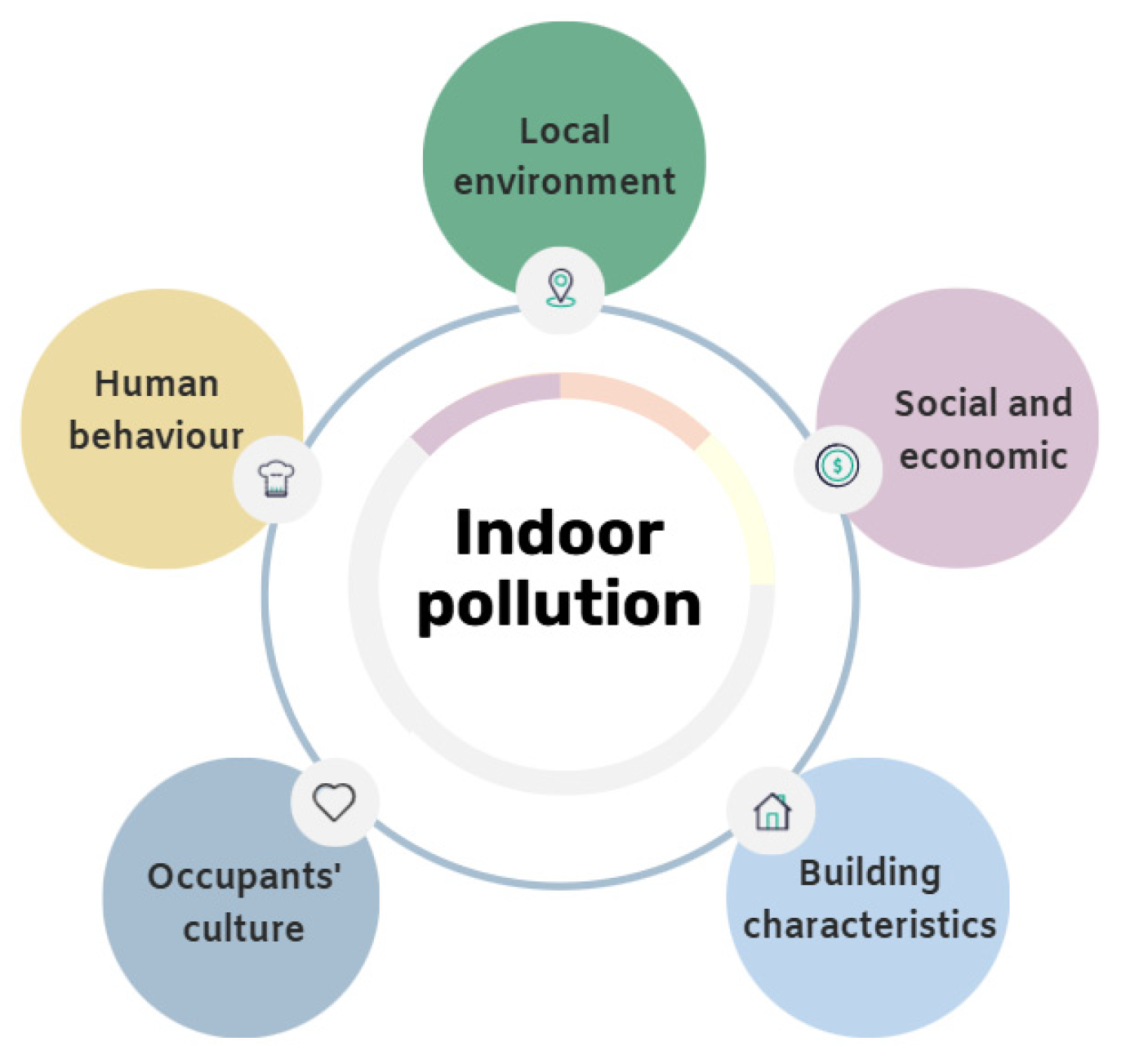
1. Introduction
- Collect high-quality measurements with species-level detail for mould and VOCs, as well as more traditional air pollutants: fine particulate matter (PM2.5), nitrogen oxides (NOx), O3, and CO2, together with relative humidity, temperature and floor dust.
- Maximise acceptability (and minimise drop-out rates) by using non-obtrusive, low- or moderate-cost devices and easy-to-follow protocols.
- Be scalable to a large number of dwellings.
- Minimise staff visits to dwellings (e.g., sample collection devices can be returned by post).
2. Materials and Methods
2.1. Ethics
2.2. The INHALE Study Goals
2.3. Recruitment
2.4. Patient and Public Involvement and Engagement
2.5. Questionnaires
2.6. Instructions
2.7. Study Design
3. Results
3.1. Volatile Organic Compounds (VOCs)
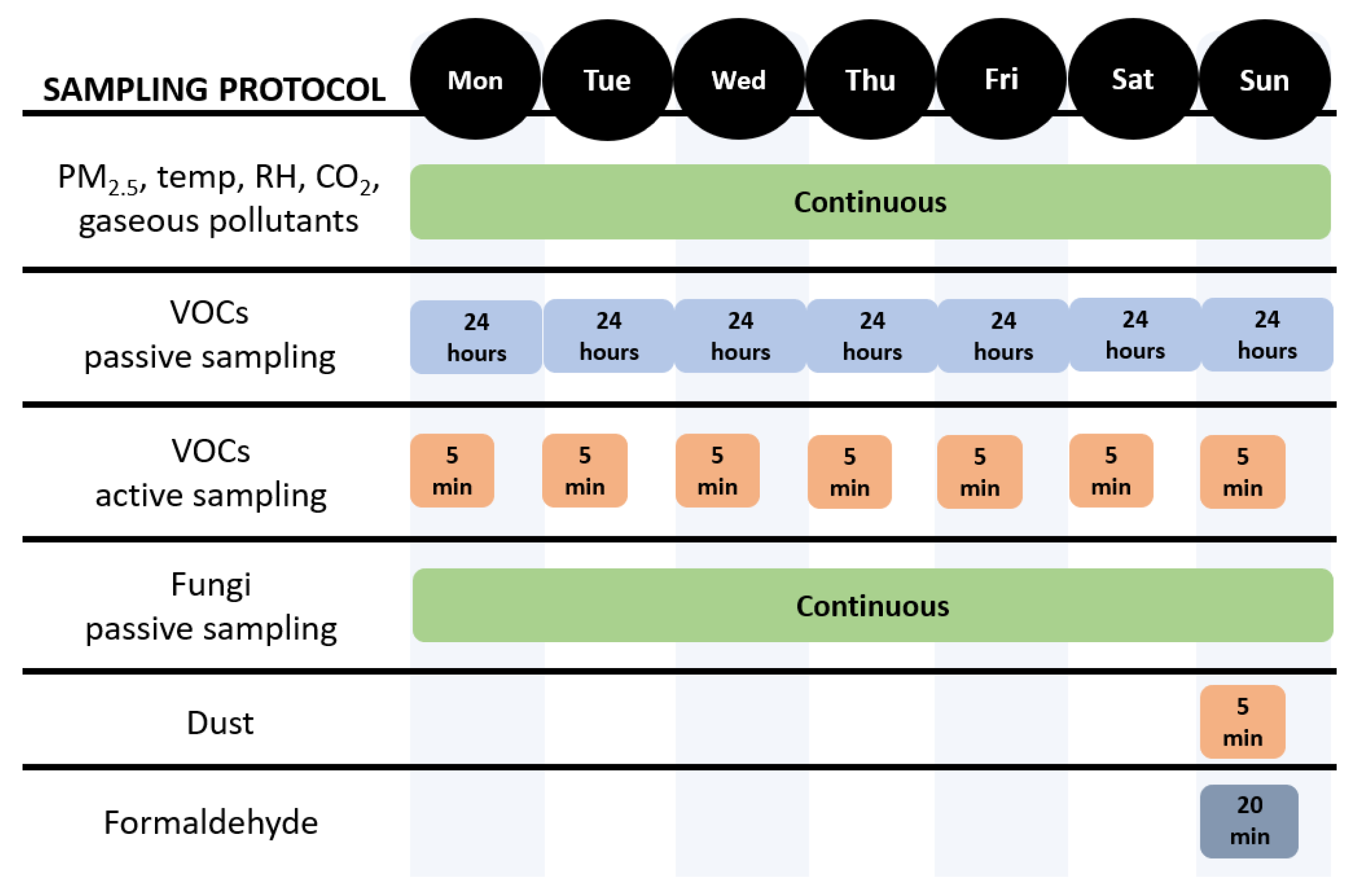
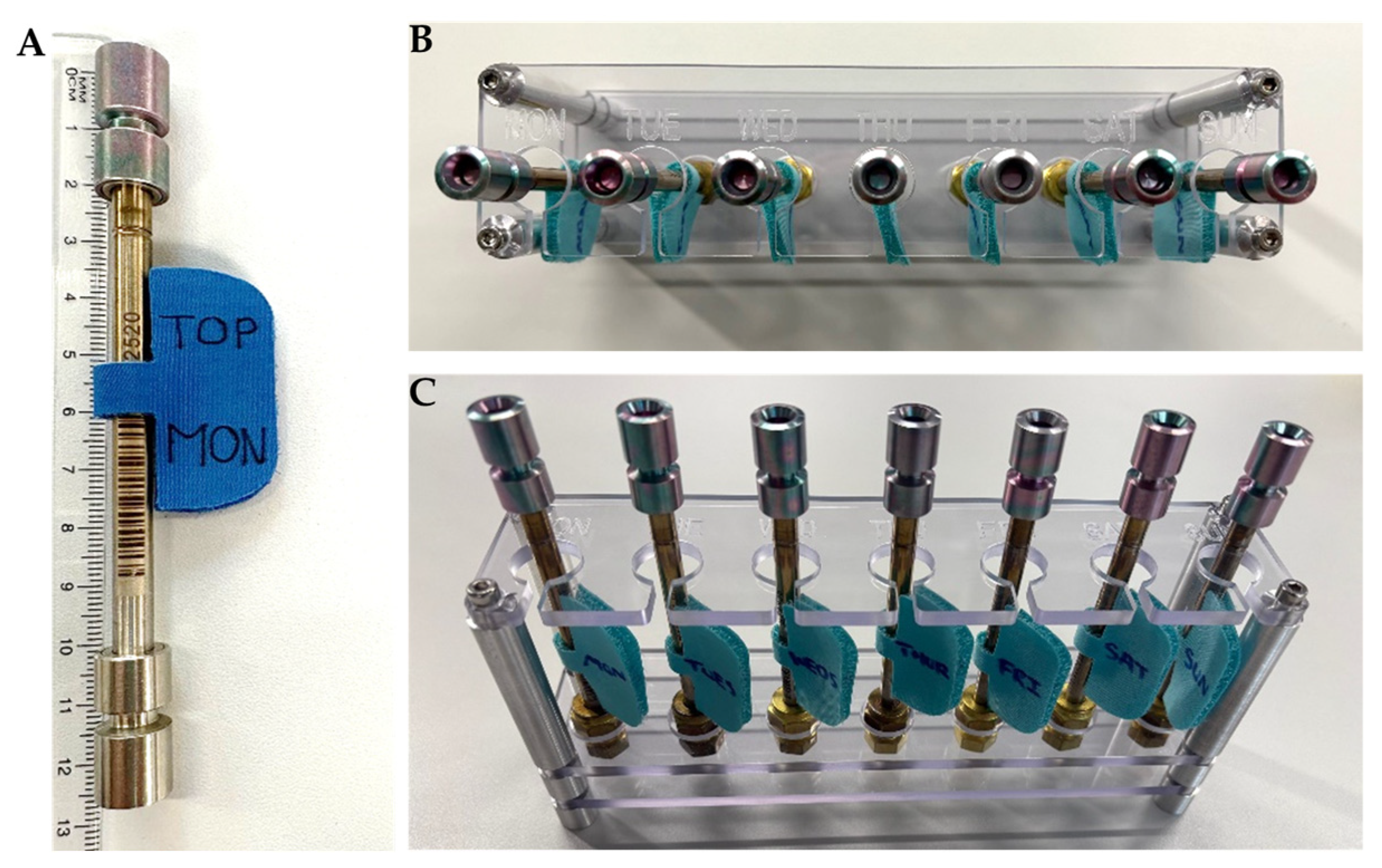
3.1.1. Sampling Protocol
- Option 1: active sampling for untargeted analysis
- Option 2: passive sampling for targeted analysis
3.1.2. Samples and Data Analyses
3.2. Formaldehyde
3.3. Fungal Spores
3.3.1. Sampling Protocol
3.3.2. Samples and Data Analyses
3.4. Dust Samples
3.4.1. Sampling Protocol
3.4.2. Samples and Data Analyses
3.4.3. Future Potential Use of the Dust Samples
3.5. Fine Particulate Matter and Gaseous Pollutants
3.5.1. Sampling Protocol
- Option 1: gravitometry
- Option 2: sensor for PM2.5 and gaseous pollutants
3.5.2. Data Analysis
3.6. Temperature and Relative Humidity (RH)
3.6.1. Sampling Protocol
3.6.2. Data Analysis
3.7. Data Analysis Plan
3.7.1. Data Pre-Processing
3.7.2. Statistical Analysis
3.8. Local Environment
3.9. Social and Economic Status
3.10. Feedback
3.11. Financial Compensation
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AQEG | Air Quality Expert Group |
| CO | Carbon monoxide |
| CO2 | Carbon dioxide |
| DEFRA | Department for Environment, Food and Rural Affairs |
| DNA | Deoxyribonucleic acid |
| GC | Gas chromatography |
| GC-MS | Gas chromatography coupled to Mass Spectrometry |
| HDM | House Dust Mite |
| IAQ | Indoor air quality |
| INHALE | INdoor Home Air Levels Exploration |
| ITS1 | Internal transcribed spacer 1 |
| ITS2 | Internal transcribed spacer 2 |
| LSOA | Lower layer super output area |
| LSU | Large subunit |
| MS | Mass Spectrometry |
| NO2 | Nitrogen Dioxide |
| NOx | Nitrogen oxides |
| O3 | Ozone |
| PCA | Principal Component Analysis |
| PCR | Polymerase Chain Reaction |
| PIS | Participant Information Sheet |
| PM2.5 | Fine particulate matter |
| PM10 | Particulate matter |
| PPIE | Patient and Public Involvement and Engagement |
| q-PCR | Quantitative Polymerase Chain Reaction |
| RH | Relative Humidity |
| rRNA | Ribosomal ribonucleic acid |
| SVOCs | Semi-Volatile Organic Compounds |
| UK | United Kingdom |
| USA | United States of America |
| US EPA | United States Environmental Protection Agency |
| VOCs | Volatile Organic Compounds |
References
- RSC. Indoor Air Quality. 2023. Available online: https://www.rsc.org/globalassets/22-new-perspectives/sustainability/indoor-air-quality/indoor-air-quality-report_v2.pdf (accessed on 22 January 2025).
- DEFRA. Indoor Air Quality in the UK. 2022. Available online: https://uk-air.defra.gov.uk/assets/documents/reports/cat09/2211011000_15062022_Indoor_Air_Quality_Report_Final.pdf (accessed on 22 January 2025).
- Lewis, A.C.; Jenkins, D.; Whitty, C.J.M. Hidden harms of indoor air pollution—Five steps to expose them. Nature 2023, 614, 220–223. [Google Scholar] [CrossRef]
- Klepeis, N.E.; Nelson, W.C.; Ott, W.R.; Robinson, J.P.; Tsang, A.M.; Switzer, P.; Behar, J.V.; Hern, S.C.; Engelmann, W.H. The National Human Activity Pattern Survey (NHAPS): A resource for assessing exposure to environmental pollutants. J. Expo. Anal. Environ. Epidemiol. 2001, 11, 231–252. [Google Scholar] [CrossRef]
- Portnoy, J.M.; Barnes, C.S.; Kennedy, K. Current reviews of allergy and clinical immunology—Sampling for indoor fungi. J. Allergy Clin. Immunol. 2004, 113, 189–198. [Google Scholar] [CrossRef]
- IARC. Outdoor Air Pollution. 2013. Available online: https://publications.iarc.fr/_publications/media/download/6724/271149929ed49b488637baf9f48cd1872aaa514a.pdf (accessed on 22 January 2025).
- Prussin, A.J.; Marr, L.C. Sources of airborne microorganisms in the built environment. Microbiome 2015, 22, 78. [Google Scholar] [CrossRef]
- Viegi, G.; Simoni, M.; Scognamiglio, A.; Baldacci, S.; Pistelli, F.; Carrozzi, L.; Annesi-Maesano, I. Indoor air pollution and airway disease. Int. J. Tuberc. Lung Dis. 2004, 8, 1401–1415. [Google Scholar]
- Andersen, B.; Frisvad, J.C.; Sondergaard, I.; Rasmussen, I.S.; Larsen, L.S. Associations between fungal species and water-damaged building materials. Appl. Environ. Microbiol. 2011, 77, 4180–4188. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Wong, J.W.C. Temperature versus Relative Humidity: Which Is More Important for Indoor Mold Prevention? J. Fungi 2022, 30, 696. [Google Scholar] [CrossRef]
- Stetzenbach, L.D.; Buttner, M.P.; Cruz, P. Detection and enumeration of airborne biocontaminants. Curr. Opin. Biotechnol. 2004, 15, 170–174. [Google Scholar] [CrossRef]
- Jones, A.P. Indoor air quality and health. Atmos. Environ. 1999, 33, 4535–4564. [Google Scholar] [CrossRef]
- Caillaud, D.; Leynaert, B.; Keirsbulck, M.; Nadif, R. Indoor mould exposure, asthma and rhinitis: Findings from systematic reviews and recent longitudinal studies. Eur. Respir. Rev. 2018, 27, 170137. [Google Scholar] [CrossRef] [PubMed]
- Baxi, S.N.; Portnoy, J.M.; Larenas-Linnemann, D.; Phipatanakul, W.; Environmental Allergens Workgroup. Exposure and Health Effects of Fungi on Humans. J. Allergy Clin. Immunol. Pract. 2016, 4, 396–404. [Google Scholar] [CrossRef]
- Vardoulakis, S.; Giagloglou, E.; Steinle, S.; Davis, A.; Sleeuwenhoek, A.; Galea, K.S.; Dixon, K.; Crawford, J.O. Indoor Exposure to Selected Air Pollutants in the Home Environment: A Systematic Review. Int. J. Environ. Res. Public Health 2020, 17, 8972. [Google Scholar] [CrossRef]
- DEFRA. Differentials in Air Pollutant Exposure Across Communities and Regions in the UK; DEFRA: London, UK, 2025.
- English Housing Survey 2022 to 2023: Housing Quality and Condition. 2024. Available online: https://www.gov.uk/government/statistics/english-housing-survey-2022-to-2023-housing-quality-and-condition/english-housing-survey-2022-to-2023-housing-quality-and-condition#:~:text=For%20a%20dwelling%20to%20be,a%20reasonable%20state%20of%20repair (accessed on 13 August 2025).
- Varaden, D.; Barratt, B.; Dallman, M.J.; Skillern, A.; Elmi, M.S.; Green, D.C.; Tremper, A.H.; Hedges, M.; Hicks, W.; Priestman, M.; et al. West London Healthy Home and Environment (WellHome) Study: Protocol for a Community-Based Study Investigating Exposures Across the Indoor-Outdoor Air Pollution Continuum in Urban Communities. Int. J. Environ. Res. Public Health 2025, 22, 249. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, E.; Hamilton, J.; Wood, C.; Chatzidiakou, L.; Warburton, T.; Ruangkanit, A.; Shao, Y.; Genes, D.; Waiblinger, D.; Yang, T.C.; et al. Understanding the patterns and health impact of indoor air pollutant exposures in Bradford, UK: A study protocol. BMJ Open 2023, 13, e081099. [Google Scholar] [CrossRef]
- Raw, G.J.; Coward, S.K.; Brown, V.M.; Crump, D.R. Exposure to air pollutants in English homes. J. Expo. Anal. Environ. Epidemiol. 2004, 14, S85–S94. [Google Scholar] [CrossRef] [PubMed]
- Fairs, A.W.; Thompson, J.R.; Pashley, C.H. Guidelines on Ambient Intramural Airborne Fungal Spores. J. Investig. Allergol. Clin. Immunol. 2010, 20, 490–498. [Google Scholar]
- ECRHS.II. INDOOR QUESTIONNAIRE. 1998. Available online: https://www.ecrhs.org/_files/ugd/4aa474_019d8378b1e44875a09a832bb2a50dba.pdf (accessed on 22 January 2025).
- Ethnic Group Classifications: Census. 2021. Available online: https://www.ons.gov.uk/census/census2021dictionary/variablesbytopic/ethnicgroupnationalidentitylanguageandreligionvariablescensus2021/ethnicgroup/classifications (accessed on 26 October 2023).
- Religion Classifications: Census. 2021. Available online: https://www.ons.gov.uk/census/census2021dictionary/variablesbytopic/ethnicgroupnationalidentitylanguageandreligionvariablescensus2021/religion/classifications (accessed on 26 October 2023).
- Lai, H.K.; Kendall, M.; Ferrier, H.; Lindup, I.; Alm, S.; Hänninen, O.; Jantunen, M.; Mathys, P.; Colvile, R.; Ashmore, M.R.; et al. Personal exposures and microenvironment concentrations of PM2.5, VOC, NO2 and CO in Oxford, UK. Atmos. Environ. 2004, 38, 6399–6410. [Google Scholar] [CrossRef]
- Bari, M.A.; Kindzierski, W.B.; Wheeler, A.J.; Héroux, M.; Wallace, L.A. Source apportionment of indoor and outdoor volatile organic compounds at homes in Edmonton, Canada. Build. Environ. 2015, 90, 114–124. [Google Scholar] [CrossRef]
- Rumchev, K.; Spickett, J.; Bulsara, M.; Phillips, M.; Stick, S. Association of domestic exposure to volatile organic compounds with asthma in young children. Thorax 2004, 59, 746–751. [Google Scholar] [CrossRef]
- Hulin, M.; Caillaud, D.; Annesi-Maesano, I. Indoor air pollution and childhood asthma: Variations between urban and rural areas. Indoor Air 2010, 20, 502–514. [Google Scholar] [CrossRef] [PubMed]
- Ricklund, N.; Bryngelsson, I.L.; Hagberg, J. Occupational Exposure to Volatile Organic Compounds (VOCs), Including Aldehydes for Swedish Hairdressers. Ann. Work Expo. Health 2023, 67, 366–378. [Google Scholar] [CrossRef] [PubMed]
- Norback, D.; Bjornsson, E.; Janson, C.; Widstrom, J.; Boman, G. Asthmatic symptoms and volatile organic compounds, formaldehyde, and carbon dioxide in dwelling. Occup. Environ. Med. 1995, 52, 388–395. [Google Scholar] [CrossRef]
- Chin, J.Y.; Godwin, C.; Parker, E.; Robins, T.; Lewis, T.; Harbin, P.; Batterman, S. Levels and sources of volatile organic compounds in homes of children with asthma. Indoor Air 2014, 24, 403–415. [Google Scholar] [CrossRef]
- Billionnet, C.; Gay, E.; Kirchner, S.; Leynaert, B.; Annesi-Maesano, I. Quantitative assessments of indoor air pollution and respiratory health in a population-based sample of French dwellings. Environ. Res. 2011, 111, 425–434. [Google Scholar] [CrossRef] [PubMed]
- Norback, D.W.; Nordstrom, K.; Walinder, R. Asthma symptoms in relation to measured building dampness in upper concrete floor construction, and 2-ethyl-1-hexanol in indoor air. Int. J. Tuberc Lung Dis. 2000, 4, 1016–1025. [Google Scholar]
- Chew, G.L.; Rogers, C.; Burge, H.A.; Muilenberg, M.L.; Gold, D.R. Dustborne and airborne fungal propagules represent a different spectrum of fungi with differing relations to home characteristics. Allergy 2003, 58, 13–20. [Google Scholar] [CrossRef]
- Wang, J.; Janson, C.; Gislason, T.; Gunnbjörnsdottir, M.; Jogi, R.; Orru, H.; Norbäck, D. Volatile organic compounds (VOC) in homes associated with asthma and lung function among adults in Northern Europe. Environ. Pollut. 2023, 321, 121103. [Google Scholar] [CrossRef]
- Rive, S.; Hulin, M.; Baiz, N.; Hassani, Y.; Kigninlman, H.; Toloba, Y.; Caillaud, D.; Annesi-Maesano, I. Urinary S-PMA related to indoor benzene and asthma in children. Inhal. Toxicol. 2013, 25, 373–382. [Google Scholar] [CrossRef]
- Wilde, M.J.; Cordell, R.L.; Salman, D.; Zhao, B.; Ibrahim, W.; Bryant, L.; Ruszkiewicz, D.; Singapuri, A.; Free, R.C.; Gaillard, E.A.; et al. Breath analysis by two-dimensional gas chromatography with dual flame ionisation and mass spectrometric detection—Method optimisation and integration within a large-scale clinical study. J. Chromatogr. A 2019, 1594, 160–172. [Google Scholar] [CrossRef]
- Wilde, M.J.; Zhao, B.; Cordell, R.L.; Ibrahim, W.; Singapuri, A.; Greening, N.J.; Brightling, C.E.; Siddiqui, S.; Monks, P.S.; Free, R.C. Automating and Extending Comprehensive Two-Dimensional Gas Chromatography Data Processing by Interfacing Open-Source and Commercial Software. Anal. Chem. 2020, 92, 13953–13960. [Google Scholar] [CrossRef] [PubMed]
- US.EPA. Compendium of Methods for the Determination of Toxic Organic Compounds in Ambient Air, Method TO-11A—Determination of Formaldehyde in Ambient Air Using Adsorbent Cartridge Followed by High Performance Liquid Chromatography (HPLC) [Active Sampling Methodology]. 1999. Available online: https://www3.epa.gov/ttnamti1/files/ambient/airtox/to-11ar.pdf (accessed on 22 January 2025).
- Shelton, J.M.G.; Rhodes, J.; Uzzell, C.B.; Hemmings, S.; Brackin, A.P.; Sewell, T.R.; Alghamdi, A.; Dyer, P.S.; Fraser, M.; Borman, A.M.; et al. Landscape-scale exposure to multiazole-resistant Aspergillus fumigatus bioaerosols. bioRxiv 2022, 7, 2022-11. [Google Scholar] [CrossRef]
- Sterling, M.; Rogers, C.; Levetin, E. An evaluation of two methods used for microscopic analysis of airborne fungal spore concentrations from the Burkard Spore Trap. Aerobiologia 1999, 15, 9–18. [Google Scholar] [CrossRef]
- Lacey, M.E.; West, J.S. The Air Spora: A Manual for Catching and Identifying Airborne Biological Particles; Springer: Dordrecht, The Netherlands, 2006; Volume 156. [Google Scholar]
- Vesper, S.J.; Wymer, L.J.; Meklin, T.; Varma, M.; Stott, R.; Richardson, M.; Haugland, R.A. Comparison of populations of mould species in homes in the UK and USA using mould-specific quantitative PCR. Lett. Appl. Microbiol. 2005, 41, 367–373. [Google Scholar] [CrossRef]
- Marczylo, E.L.; Macchiarulo, S.; Gant, T.W. Metabarcoding of Soil Fungi from Different Urban Greenspaces Around Bournemouth in the UK. EcoHealth 2021, 18, 315–330. [Google Scholar] [CrossRef]
- Fairs, A.; Agbetile, J.; Bourne, M.; Hargadon, B.; Monteiro, W.R.; Morley, J.P.; Edwards, R.E.; Wardlaw, A.J.; Pashley, C.H. Isolation of Aspergillus fumigatus from sputum is associated with elevated airborne levels in homes of patients with asthma. Indoor Air 2013, 23, 275–284. [Google Scholar] [CrossRef]
- Dingle, J.H.; Kohl, L.; Khan, N.; Meng, M.; Shi, Y.A.; Pedroza-Brambila, M.; Chow, C.W.; Chan, A.W.H. Sources and composition of metals in indoor house dust in a mid-size Canadian city. Environ. Pollut. 2021, 289, 117867. [Google Scholar] [CrossRef]
- Weiss, J.M.; Gustafsson, Å.; Gerde, P.; Bergman, Å.; Lindh, C.H.; Krais, A.M. Daily intake of phthalates, MEHP, and DINCH by ingestion and inhalation. Chemosphere 2018, 208, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.K.; Kim, T.; Choi, J.; Joo, Y.E.; Park, H.; Lee, H.; Lee, C.; Jang, S.; Vasseghian, Y.; Joo, S.W.; et al. Analysis of semi-volatile organic compounds in indoor dust and organic thin films by house type in South Korea. Environ. Res. 2022, 214, 113782. [Google Scholar] [CrossRef]
- Kong, W.J.; Liu, S.Y.; Qiu, F.; Xiao, X.H.; Yang, M.H. Simultaneous multi-mycotoxin determination in nutmeg by ultrasound-assisted solid-liquid extraction and immunoaffinity column clean-up coupled with liquid chromatography and on-line post-column photochemical derivatization-fluorescence detection. Analyst 2013, 7, 2729–2739. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.F.; Wu, M.W.; Ting, M.H.; Crane, J.; Siebers, R. Cat, dog and house dust mite allergen levels on children’s soft toys. J. Asthma Off. J. Assoc. Care Asthma 2014, 51, 75–78. [Google Scholar] [CrossRef]
- Belanger, K.; Holford, T.R.; Gent, J.F.; Hill, M.E.; Kezik, J.M.; Leaderer, B.P. Household levels of nitrogen dioxide and pediatric asthma severity. Epidemiology 2013, 24, 320–330. [Google Scholar] [CrossRef]
- Simons, E.; Curtin-Brosnan, J.; Buckley, T.; Breysse, P.; Eggleston, P.A. Indoor environmental differences between inner city and suburban homes of children with asthma. J. Urban Health 2007, 84, 577–590. [Google Scholar] [CrossRef][Green Version]
- Caporaso, J.G.; Lauber, C.L.; Walters, W.A.; Berg-Lyons, D.; Lozupone, C.A.; Turnbaugh, P.J.; Fierer, N.; Knight, R. Global patterns of 16S rRNA diversity at a depth of millions of sequences per sample. Proc. Natl. Acad. Sci. USA 2011, 108 (Suppl. S1), 4516–4522. [Google Scholar] [CrossRef]
- SIST EN 12341:2023; Ambient Air—Standard Gravimetric Measurement Method for the Determination of the PM10 or PM2.5 Mass Concentration of Suspended Particulate Matter. European Standard, Department of Air Quality: Brussels, Belgium, 2023.
- Wickham, H.; Averick, M.; Bryan, J.; Chang, W.; McGowan, L.D.; François, R.; Grolemund, G.; Hayes, A.; Henry, L.; Hester, J.; et al. Welcome to the tidyverse. J. Open Source Softw. 2019, 4, 1686. [Google Scholar] [CrossRef]
- Hlavac, M. Stargazer: Well-Formatted Regression and Summary Statistics Tables, R Package Version 5.2.3; Social Policy Institute: Bratislava, Slovakia, 2022.
- Pedersen, T. Patchwork: The Composer of Plots. 2023. Available online: https://cir.nii.ac.jp/crid/1360020310293806336 (accessed on 13 August 2025).
- Wickham, H.; François, R.; Henry, L.; Müller, K.; Vaughan, D. dplyr: A Grammar of Data Manipulation. 2023. Available online: https://cran.r-project.org/web/packages/dplyr/index.html (accessed on 13 August 2025).
- Venables, W.N.; Ripley, B.D. Modern Applied Statistics with S, 4th ed.; Springer: New York, NY, USA, 2002. [Google Scholar]
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016. [Google Scholar]
- IUPAC. Limit of Detection, 3.0.1 ed.; International Union of Pure and Applied Chemistry (IUPAC): Research Triangle Park, NC, USA, 2019. [Google Scholar] [CrossRef]
- Mishra, P.; Singh, U.; Pandey, C.M.; Mishra, P.; Pandey, G. Application of student’s t-test, analysis of variance, and covariance. Ann. Card. Anaesth. 2019, 22, 407–411. [Google Scholar] [CrossRef]
- Wei, T.; Simko, V. R Package ‘Corrplot’: Visualization of a Correlation Matrix, Version 0.92; R Package: Madison, WI, USA, 2021.
- Redlands, Esri. ArcGIS Desktop: Release 10, 2011. Available online: https://www.esri.com/news/arcnews/spring12articles/introducing-arcgis-101.html (accessed on 13 August 2025).
- WHO. WHO Global Air Quality Guidelines: Particulate Matter (PM2.5 and PM10), Ozone, Nitrogen Dioxide, Sulfur Dioxide and Carbon Monoxide. 2021. Available online: https://www.who.int/publications/i/item/9789240034228 (accessed on 22 January 2025).
- ATSDR. Calculating Hazard Quotients and Cancer Risk Estimates. 2014. Available online: https://www.atsdr.cdc.gov/pha-guidance/conducting_scientific_evaluations/epcs_and_exposure_calculations/hazardquotients_cancerrisk.html#:~:text=The%20hazard%20quotient%20(HQ)%20is,MRLs%2C%20RfDs%2C%20RfCs) (accessed on 22 January 2025).
- Drusch, M.; Del Bello, U.; Carlier, S.; Colin, O.; Fernandez, V.; Gascon, F.; Hoersch, B.; Isola, C.; Laberinti, P.; Martimort, P.; et al. SENTINEL-2: ESA’s Optical High-Resolution Mission for GMES Operational Services. Remote Sens. Environ. 2012, 120, 25–36. [Google Scholar] [CrossRef]
- Marston, C.; Rowland, C.S.; O’Neil, A.W.; Morton, R.D. Land Cover Map 2021 (10 m Classified Pixels, GB); NERC EDS Environmental Information Data Centre: Lancaster, UK, 2022. [Google Scholar]
- Morley, D.W.; Gulliver, J. Methods to improve traffic flow and noise exposure estimation on minor roads. Environ. Pollut. 2016, 216, 746–754. [Google Scholar] [CrossRef]
- English Indices of Deprivation. 2019. Available online: https://imd-by-postcode.opendatacommunities.org/imd/2019 (accessed on 13 August 2025).
- Kephalopoulos, S.; Bopp, S.K.; Costa, S.D.; Cusinato, A.; Lipsa, D.; Geiss, O. Indoor air monitoring: Sharing and accessing data via the Information Platform for chemical monitoring (IPCHEM). Int. J. Hyg. Environ. Health 2020, 227, 1438–4639. [Google Scholar] [CrossRef] [PubMed]
- Heard, D.; Mudge, S.; Turner, D.; Dimitroulopoulou, S.; Hilborne, V.; Pfrang, C.; Clave, S.A.; Barron, L.; Beevers, S.; Birch, H.; et al. Indoor Air Quality: A Workshop Report on the Contribution Chemical Sciences Can Make to Improving Indoor Air Quality in the UK. 2023. Available online: https://research.birmingham.ac.uk/en/publications/indoor-air-quality-a-workshop-report-on-the-contribution-chemical/ (accessed on 27 August 2025).
- UKHSA. Understanding and Addressing the Health Risks of Damp and Mould in the Home. 2023. Available online: https://www.gov.uk/government/publications/damp-and-mould-understanding-and-addressing-the-health-risks-for-rented-housing-providers/understanding-and-addressing-the-health-risks-of-damp-and-mould-in-the-home--2 (accessed on 22 January 2025).
- Awaab’s Law: Consultation on Timescales for Repairs in the Social Rented Sector. Available online: https://www.gov.uk/government/consultations/awaabs-law-consultation-on-timescales-for-repairs-in-the-social-rented-sector/awaabs-law-consultation-on-timescales-for-repairs-in-the-social-rented-sector (accessed on 25 May 2025).
- PHE. Indoor Air Quality Guidelines for Selected Volatile Organic Compounds (VOCs) in the UK. 2019. Available online: https://assets.publishing.service.gov.uk/media/5d7a2912ed915d522e4164a5/VO__statement_Final_12092019_CS__1_.pdf (accessed on 22 January 2025).
- Diário da República, 1.ª série—N.º 235—4 de Dezembro de 2013z. 2013. Available online: https://files.diariodarepublica.pt/1s/2013/12/23500/0663806638.pdf (accessed on 22 January 2025).

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Riveron, T.P.; Cordell, R.L.; Hansell, A.L. INdoor Home Air Level Exploration (INHALE) Study: Protocol to Monitor Indoor Pollution in British Dwellings. Int. J. Environ. Res. Public Health 2025, 22, 1635. https://doi.org/10.3390/ijerph22111635
Riveron TP, Cordell RL, Hansell AL. INdoor Home Air Level Exploration (INHALE) Study: Protocol to Monitor Indoor Pollution in British Dwellings. International Journal of Environmental Research and Public Health. 2025; 22(11):1635. https://doi.org/10.3390/ijerph22111635
Chicago/Turabian StyleRiveron, Thiphanie P., Rebecca L. Cordell, and Anna L. Hansell. 2025. "INdoor Home Air Level Exploration (INHALE) Study: Protocol to Monitor Indoor Pollution in British Dwellings" International Journal of Environmental Research and Public Health 22, no. 11: 1635. https://doi.org/10.3390/ijerph22111635
APA StyleRiveron, T. P., Cordell, R. L., & Hansell, A. L. (2025). INdoor Home Air Level Exploration (INHALE) Study: Protocol to Monitor Indoor Pollution in British Dwellings. International Journal of Environmental Research and Public Health, 22(11), 1635. https://doi.org/10.3390/ijerph22111635





