Observational Study on Actual Cancer Screening Participation and Outcomes Among Patients with Lung Cancer Based on Linkage of Cancer Registry and Kyoto City Integrated Database Data from 2014 to 2018
Abstract
1. Introduction
2. Materials and Methods
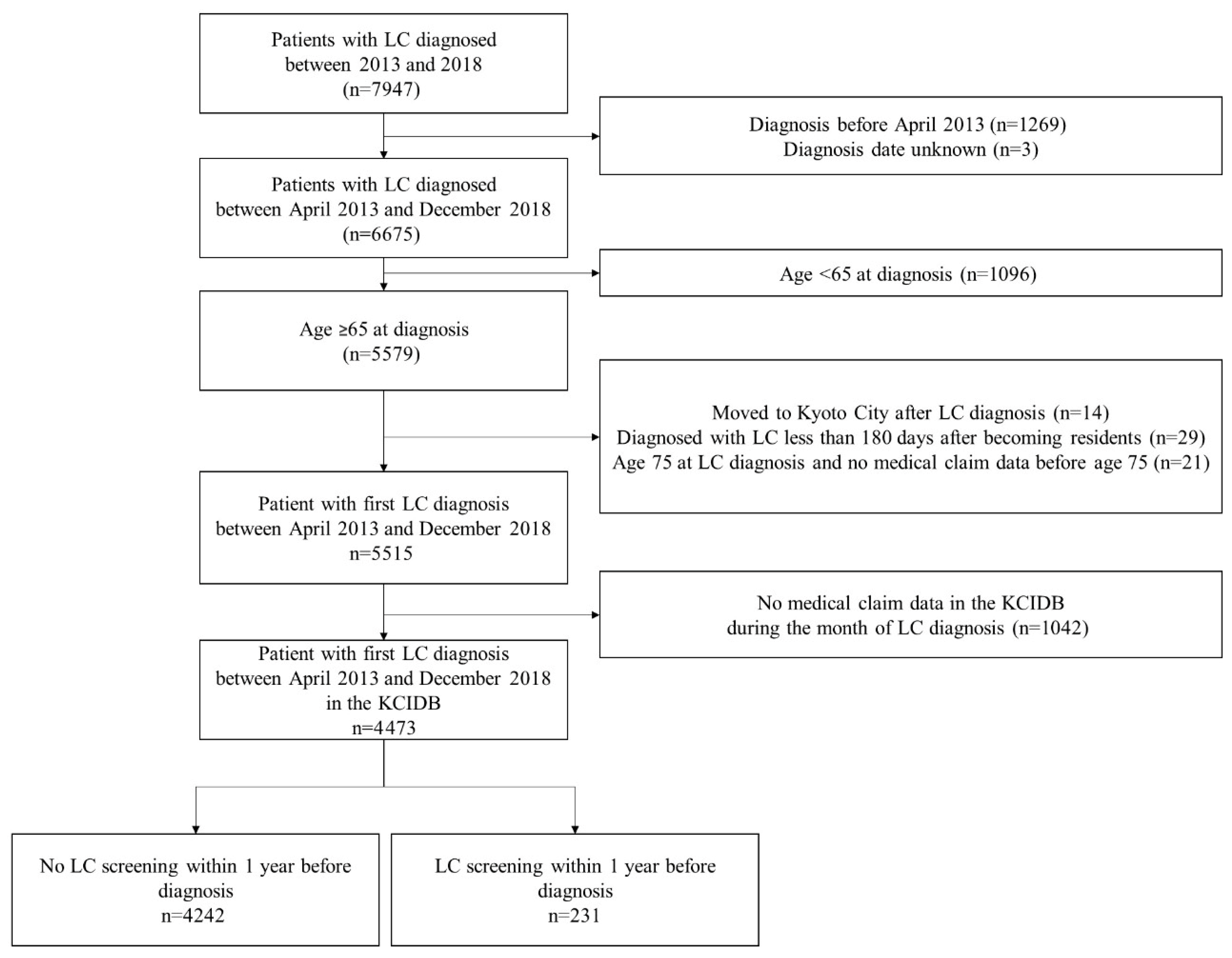
2.1. Study Design, Population, and Settings
2.2. Data Collection and Quality Control
2.3. Statistical Analysis
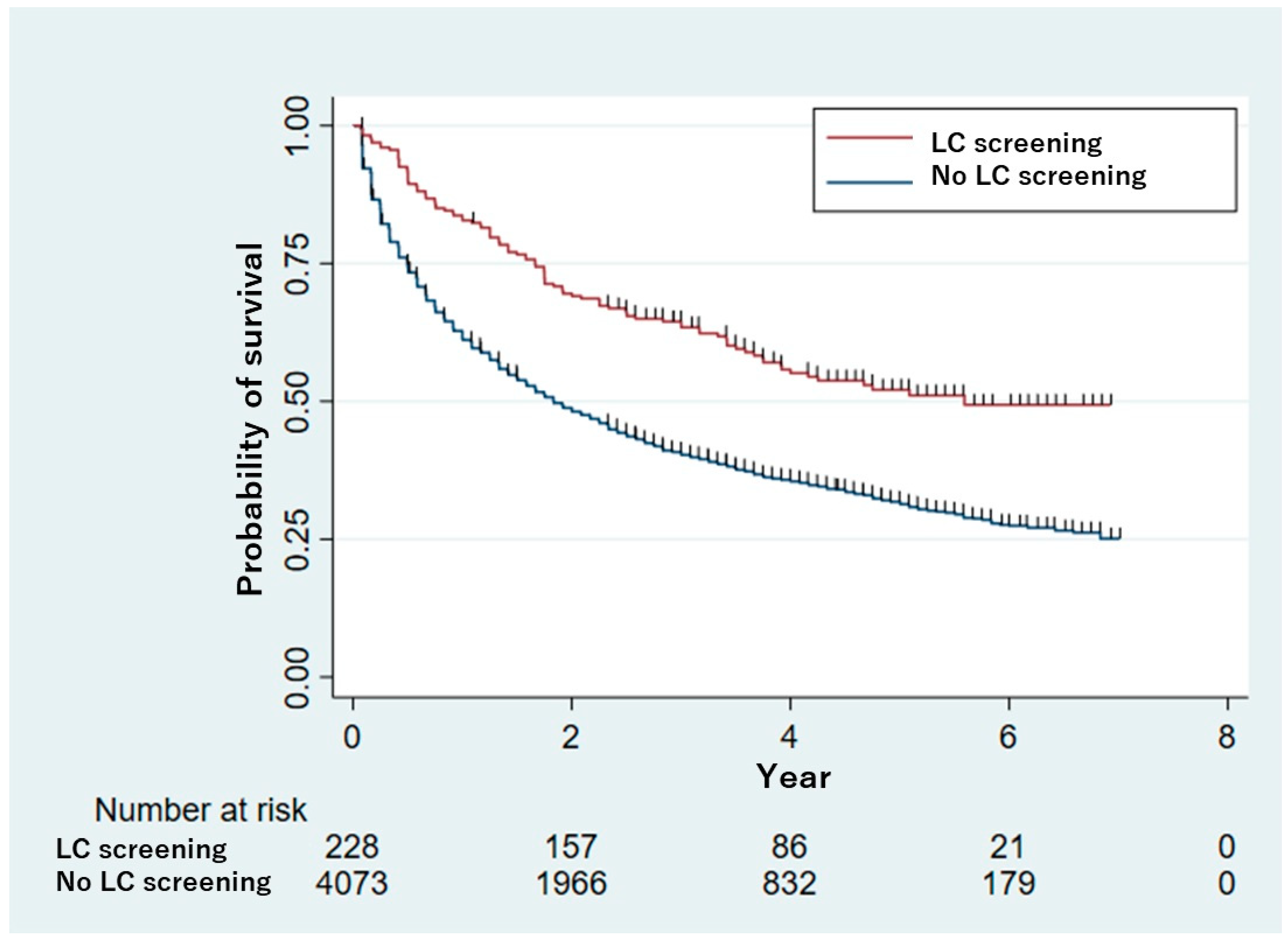
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Howlader, N.; Noone, A.M.; Krapcho, M.; Miller, D.; Brest, A.; Yu, M.; Ruhl, J.; Tatalovich, Z.; Mariotto, A.; Lewis, D.R.; et al. (Eds.) SEER Cancer Statistics Review, 1975–2016; National Cancer Institute: Bethesda, MD, USA, 2019. Available online: https://seer.cancer.gov/csr/1975_2016/ (accessed on 17 September 2025).
- Horinouchi, H.; Kusumoto, M.; Yatabe, Y.; Aokage, K.; Watanabe, S.I.; Ishikura, S. Lung Cancer in Japan. J. Thorac. Oncol. 2022, 17, 353–361. [Google Scholar] [CrossRef]
- Allemani, C.; Matsuda, T.; Di Carlo, V.; Harewood, R.; Matz, M.; Nikšić, M.; Bonaventure, A.; Valkov, M.; Johnson, C.J.; Estève, J.; et al. Global surveillance of trends in cancer survival 2000–14 (CONCORD-3): Analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet 2018, 391, 1023–1075. [Google Scholar] [CrossRef]
- Ministry of Health, Labor and Welfare. Guideline for Population Based Cancer Screening Program. Available online: https://www.mhlw.go.jp/stf/seisakunitsuite/bunya/0000059490.html (accessed on 17 September 2025). (In Japanese).
- National Cancer Center Institute for Cancer Control. Cancer Screening Performance Measures_2021 Data Book. 2022. Available online: https://ganjoho.jp/public/qa_links/report/pdf/Cancer_Screening_Performance_Measures_2021.pdf (accessed on 17 September 2025). (In Japanese).
- Fontana, R.S.; Sanderson, D.R.; Woolner, L.B.; Taylor, W.F.; Miller, W.E.; Muhm, J.R. Lung cancer screening: The Mayo program. J. Occup. Med. 1986, 28, 746–750. [Google Scholar] [PubMed]
- Oken, M.M.; Hocking, W.G.; Kvale, P.A.; Andriole, G.L.; Buys, S.S.; Church, T.R.; Crawford, E.D.; Fouad, M.N.; Isaacs, C.; Reding, D.J.; et al. Screening by chest radiograph and lung cancer mortality: The Prostate, Lung, Colorectal, and Ovarian (PLCO) randomized trial. JAMA 2011, 306, 1865–1873. [Google Scholar] [CrossRef]
- Kubik, A.; Parkin, D.M.; Khlat, M.; Erban, J.; Polak, J.; Adamec, M. Lack of benefit from semi-annual screening for cancer of the lung: Follow-up report of a randomized controlled trial on a population of high-risk males in Czechoslovakia. Int. J. Cancer 1990, 45, 26–33. [Google Scholar] [CrossRef]
- Melamed, M.R.; Flehinger, B.J.; Zaman, M.B.; Heelan, R.T.; Perchick, W.A.; Martini, N. Screening for early lung cancer. Results of the Memorial Sloan-Kettering study in New York. Chest 1984, 86, 44–53. [Google Scholar] [CrossRef]
- Sagawa, M.; Nakayama, T.; Tsukada, H.; Nishii, K.; Baba, T.; Kurita, Y.; Saito, Y.; Kaneko, M.; Sakuma, T.; Suzuki, T.; et al. The efficacy of lung cancer screening conducted in 1990s: Four case-control studies in Japan. Lung Cancer 2003, 41, 29–36. [Google Scholar] [CrossRef]
- Sadate, A.; Occean, B.V.; Beregi, J.P.; Hamard, A.; Addala, T.; de Forges, H.; Fabbro-Peray, P.; Frandon, J. Systematic review and meta-analysis on the impact of lung cancer screening by low-dose computed tomography. Eur. J. Cancer (Oxf. Engl. 1990) 2020, 134, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Field, J.K.; Vulkan, D.; Davies, M.P.A.; Baldwin, D.R.; Brain, K.E.; Devaraj, A.; Eisen, T.; Gosney, J.; Green, B.A.; Holemans, J.A.; et al. Lung cancer mortality reduction by LDCT screening: UKLS randomised trial results and international meta-analysis. Lancet Reg. Health—Eur. 2021, 10, 100179. [Google Scholar] [CrossRef]
- Jonas, D.E.; Reuland, D.S.; Reddy, S.M.; Nagle, M.; Clark, S.D.; Weber, R.P.; Enyioha, C.; Malo, T.L.; Brenner, A.T.; Armstrong, C.; et al. Screening for Lung Cancer with Low-Dose Computed Tomography: Updated Evidence Report and Systematic Review for the US Preventive Services Task Force. JAMA 2021, 325, 971–987. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.L.; Wang, S.Y.; Lu, W.C.; Chang, Y.-H.; Su, J.; Lu, Y.-T. Effects of low-dose computed tomography on lung cancer screening: A systematic review, meta-analysis, and trial sequential analysis. BMC Pulm. Med. 2019, 19, 126. [Google Scholar] [CrossRef]
- Wood, D.E.; Kazerooni, E.A.; Aberle, D.; Berman, A.; Brown, L.M.; Eapen, G.A.; Ettinger, D.S.; Ferguson, J.S.; Hou, L.; Kadaria, D.; et al. NCCN Guidelines® Insights: Lung Cancer Screening, Version 1.2022. J. Natl. Compr. Cancer Netw. 2022, 20, 754–764. [Google Scholar] [CrossRef]
- Moyer, V.A. US Preventive Services Task Force. Screening for lung cancer: U.S. Preventive Services Task Force recommendation statement. Ann. Intern. Med. 2014, 160, 330–338. [Google Scholar] [CrossRef]
- National Cancer Center. Lung Cancer Screening Guidelines 2025 Edition, Based on Effectiveness Evaluation. 2025. Available online: https://canscreen.ncc.go.jp/guideline/lung_guideline2025.pdf (accessed on 17 September 2025). (In Japanese).
- Dubin, S.; Griffin, D. Lung Cancer in Non-Smokers. Mo. Med. 2020, 117, 375–379. [Google Scholar]
- Zhou, F.; Zhou, C. Lung cancer in never smokers-the East Asian experience. Transl. Lung Cancer Res. 2018, 7, 450–463. [Google Scholar] [CrossRef]
- Shimamoto, T.; Tateyama, Y.; Kobayashi, D.; Yamamoto, K.; Takahashi, Y.; Ueshima, H.; Sasaki, K.; Nakayama, T.; Iwami, T. Temporal Trend in an Initial Treatment, Survival, and Medical Costs Among Patients with Lung Cancer Between 2013 and 2018 in Kyoto City, Japan. Value Health Reg. Issues 2022, 31, 163–168. [Google Scholar] [CrossRef]
- Shimamoto, T.; Tateyama, Y.; Kobayashi, D.; Yamamoto, K.; Takahashi, Y.; Ueshima, H.; Sasaki, K.; Nakayama, T.; Iwami, T. Survival and medical costs of non-small cell lung cancer patients according to the first-line treatment: An observational study using the Kyoto City Integrated Database. Thorac. Cancer 2023, 14, 1574–1580. (In Japanese) [Google Scholar] [CrossRef]
- Kyoto City Statistics Portal: Kyoto City. 2023. Available online: https://www2.city.kyoto.lg.jp/sogo/toukei/ (accessed on 17 September 2025). (In Japanese).
- Cancer Information Services National Cancer Center. Available online: https://ganjoho.jp/public/institution/registry/national.html (accessed on 17 September 2025). (In Japanese).
- Glasheen, W.P.; Cordier, T.; Gumpina, R.; Haugh, G.; Davis, J.; Renda, A. Charlson Comorbidity Index: ICD-9 Update and ICD-10 Translation. Am. Health Drug Benefits 2019, 12, 188–197. [Google Scholar] [PubMed]
- Iwagami, M.; Tamiya, N. The Long-Term Care Insurance System in Japan: Past, Present, and Future. JMA J. 2022, 2, 67–69. [Google Scholar]
- Tsutsui, T.; Muramatsu, N. Care-needs certification in the long-term care insurance system of Japan. J. Am. Geriatr. Soc. 2005, 53, 522–527. [Google Scholar] [CrossRef]
- Buys, S.S.; Partridge, E.; Black, A.; Johnson, C.C.; Lamerato, L.; Isaacs, C.; Reding, D.J.; Greenlee, R.T.; Yokochi, L.A.; Kessel, B.; et al. Effect of screening on ovarian cancer mortality: The Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Randomized Controlled Trial. JAMA 2011, 305, 2295–2303. [Google Scholar] [CrossRef] [PubMed]
- Patz, E.F., Jr.; Goodman, P.C.; Bepler, G. Screening for Lung Cancer. N. Engl. J. Med. 2000, 343, 1627–1633. [Google Scholar] [CrossRef] [PubMed]
- Kyoto City’s Initiatives on Cancer Screening. Available online: https://www.city.kyoto.lg.jp/templates/shingikai_kekka/cmsfiles/contents/0000336/336415/8.pdf (accessed on 17 September 2025). (In Japanese).
- Ministry of Health, Labor and Welfare. Comprehensive Survey of Living Conditions Summary Report. 2019. Available online: https://www.mhlw.go.jp/english/database/db-hss/cslc-report2019.htm (accessed on 17 September 2025).
- Sagawa, M.; Nakayama, T.; Tanaka, M.; Sakuma, T.; Sobue, T.; JECS Study Group. A randomized controlled trial on the efficacy of thoracic CT screening for lung cancer in non-smokers and smokers of <30 Pack-years Aged 50–64 Years (JECS Study): Research Design. Jpn. J. Clin. Oncol. 2012, 42, 1219–1221. [Google Scholar] [PubMed]
- Nawa, T.; Nakagawa, T.; Mizoue, T.; Kusano, S.; Chonan, T.; Hayashihara, K.; Suito, T.; Endo, K. A decrease in lung cancer mortality following the introduction of low-dose chest CT screening in Hitachi, Japan. Lung Cancer 2012, 78, 225–228. [Google Scholar] [CrossRef]
- Nawa, T.; Nakagawa, T.; Mizoue, T.; Kusano, S.; Chonan, T.; Fukai, S.; Endo, K. Long-term prognosis of patients with lung cancer detected on low-dose chest computed tomography screening. Lung Cancer 2012, 75, 197–202. [Google Scholar] [CrossRef]

| With LC Screening Within One Year Before the Diagnosis | Without LC Screening Within One Year Before the Diagnosis | p | |
|---|---|---|---|
| n = 231 | n = 4242 | ||
| Male, n (%) | 135 (58.4) | 2696 (63.6) | 0.13 |
| Age mean (SD) | 75.4 (6.1) | 77.8 (7.1) | <0.001 |
| Result of LC screening | |||
| Requires a detailed examination (not suspicion for LC) | 45 (19.5) | NA | |
| Requires a detailed examination (suspicion for LC) | 96 (41.6) | NA | |
| Detection circumstances, n (%) | |||
| Screening examination | 103 (44.6) | 231 (5.4) | <0.001 |
| Incidental detection in treatment of other diseases | 78 (33.8) | 2139 (50.4) | |
| Autopsy | 0 (0.0) | 3 (0.1) | |
| Others | 26 (11.3) | 1103 (26.0) | |
| Unknown | 24 (10.4) | 766 (18.1) | |
| Type of LC, n (%) | |||
| Small cell | 8 (3.5) | 317 (7.5) | <0.001 |
| Non-small cell | 192 (83.1) | 2833 (66.8) | |
| Unspecified | 31 (13.4) | 1092 (25.7) | |
| Need for long-term care, n (%) | 12 (5.2) | 955 (22.5) | <0.001 |
| CCI | |||
| ≤2 | 62 (26.8) | 373 (8.8) | <0.001 |
| 3~4 | 90 (39.0) | 1427 (33.6) | |
| 5≤ | 79 (34.2) | 2442 (57.6) |
| With LC Screening Within One Year Before the Diagnosis | Without LC Screening Within One Year Before the Diagnosis | p | ||
|---|---|---|---|---|
| n = 231 | n = 4242 | |||
| Tumor stage, n (%) | Intraepithelial | 2(0.9) | 29(0.7) | <0.001 |
| Localized | 120(51.9) | 1486(35.0) | ||
| Regional lymph node metastasis | 19(8.2) | 415(9.8) | ||
| Invasion to adjacent structures | 17(7.4) | 335(7.9) | ||
| Distant metastasis | 58(25.1) | 1552(36.6) | ||
| Unknown | 15(6.5) | 422(9.9) | ||
| Missing data | 0(0.0) | 3(0.1) | ||
| Surgical treatment, n (%) | Yes | 115(49.8) | 1287(30.3) | <0.001 |
| No | 114(49.4) | 2876(67.8) | ||
| Unknown | 2(0.9) | 68(1.6) | ||
| Missing data | 0(0.0) | 11(0.3) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shimamoto, T.; Tateyama, Y.; Kobayashi, D.; Yamamoto, K.; Nishioka, N.; Takahashi, Y.; Ueshima, H.; Sasaki, K.; Kiyohara, K.; Nakayama, T.; et al. Observational Study on Actual Cancer Screening Participation and Outcomes Among Patients with Lung Cancer Based on Linkage of Cancer Registry and Kyoto City Integrated Database Data from 2014 to 2018. Int. J. Environ. Res. Public Health 2025, 22, 1595. https://doi.org/10.3390/ijerph22101595
Shimamoto T, Tateyama Y, Kobayashi D, Yamamoto K, Nishioka N, Takahashi Y, Ueshima H, Sasaki K, Kiyohara K, Nakayama T, et al. Observational Study on Actual Cancer Screening Participation and Outcomes Among Patients with Lung Cancer Based on Linkage of Cancer Registry and Kyoto City Integrated Database Data from 2014 to 2018. International Journal of Environmental Research and Public Health. 2025; 22(10):1595. https://doi.org/10.3390/ijerph22101595
Chicago/Turabian StyleShimamoto, Tomonari, Yukiko Tateyama, Daisuke Kobayashi, Keiichi Yamamoto, Norihiro Nishioka, Yoshimitsu Takahashi, Hiroaki Ueshima, Kosuke Sasaki, Kosuke Kiyohara, Takeo Nakayama, and et al. 2025. "Observational Study on Actual Cancer Screening Participation and Outcomes Among Patients with Lung Cancer Based on Linkage of Cancer Registry and Kyoto City Integrated Database Data from 2014 to 2018" International Journal of Environmental Research and Public Health 22, no. 10: 1595. https://doi.org/10.3390/ijerph22101595
APA StyleShimamoto, T., Tateyama, Y., Kobayashi, D., Yamamoto, K., Nishioka, N., Takahashi, Y., Ueshima, H., Sasaki, K., Kiyohara, K., Nakayama, T., & Iwami, T. (2025). Observational Study on Actual Cancer Screening Participation and Outcomes Among Patients with Lung Cancer Based on Linkage of Cancer Registry and Kyoto City Integrated Database Data from 2014 to 2018. International Journal of Environmental Research and Public Health, 22(10), 1595. https://doi.org/10.3390/ijerph22101595







