Interoperability as a Catalyst for Digital Health and Therapeutics: A Scoping Review of Emerging Technologies and Standards (2015–2025)
Abstract
1. Plain Language Summary
2. Introduction
2.1. Background
2.1.1. Interoperability as a Foundational Enabler of Digital Health
2.1.2. Policy Push and International Frameworks
2.1.3. Standards-Based Interoperability Infrastructure
2.1.4. Emerging Technologies and Interoperability
2.1.5. Interoperability in Digital Therapeutics and Chronic Care
2.2. Rationale
2.3. Objectives and Research Questions
- Emerging technologies: Which new technologies (e.g., AI, blockchain, IoT, federated learning) are being integrated to support digital health interoperability?
- Standards and Governance: What standards for data (e.g., HL7 FHIR, open APIs) and governance models (global, national, vendor-led) are implemented to enable interoperability?
- Enablers and Barriers: What are the most important enablers and inhibitors of interoperable HIS implementation, particularly in LMIC contexts?
3. Methods
3.1. Study Design
3.2. Objectives and Design
- Protocol development (Appendix A) and team calibration;
- A systematic search was conducted on five databases;
- Screening Title, abstracts, and full texts;
- Random selection of a 10% sample for detailed extraction;
- Thematic synthesis and statistical representativeness testing.
3.3. Eligibility
- Population: Health system users and digital governance entities;
- Concept: Interoperability frameworks, standards (e.g., HL7 and FHIR), and new technologies (e.g., blockchain and artificial intelligence);
- Context: Global studies with a focus on LMICs or inclusive settings.
- Peer-reviewed journal or conference papers;
- Addressing technical or semantic interoperability in digital/uHealth;
- Implementation of at least one emerging technology (AI, blockchain, federated learning, and IoT);
- Discussion about governance, standards, or equity implications;
- January 2015 through June 2025 published writings;
- English language.
- Preprints, grey literature, or non-peer-reviewed work;
- Single studies about radiology or imaging-related artificial intelligence;
- Articles limited to high-income countries without relevance to LMIC or global contexts;
- Concept papers without implementation or technical details.
3.4. Information Sources and Search Strategy
- PubMed;
- Scopus;
- IEEE Xplore;
- ACM Digital Library;
- Google Scholar (hand-searched).
- “digital health” OR “uHealth” OR “mobile health” OR “digital therapeutics”
- “interoperability” OR “FHIR” OR “HL7” OR “TEFCA” OR “data governance”
- “blockchain” OR “AI” OR “artificial intelligence” OR “federated learning” OR “IoT”
- “LMIC” OR “low- and middle-income country” OR “global health”
3.5. Selection Process
- Title and abstract screening was conducted by [K.A.] and [T.K.] individually;
- The full-text assessment was conducted on eligible or questionable records;
- Disagreements were resolved by consensus with [D.D., A.B.].
3.6. Sample Selection and Data Charting
- Study title, author(s), and year;
- Country/region;
- Intervention and the digital health domain;
- Technology used (e.g., AI, blockchain, and IoT);
- Interoperability standard(s) (e.g., HL7 FHIR, and TEFCA);
- Governance or regulatory frameworks;
- Barriers and enablers;
- Mention of equity or inclusion;
- Additional observations.
3.7. Synthesis and Analysis
- Governance and Policy Frameworks;
- Interoperability Standards and Technical Architectures;
- Emerging Technologies;
- Barriers and Enablers to Implementation;
- Equity and LMIC Representation.
3.8. Statistical Justification and Representativeness
3.8.1. Distributional Comparison
- Full dataset: 255 studies (2006–2025); Sample: 26 (2019–2025).
- Journal articles: 92.3% in sample vs. 72.9% in full dataset.
- Conference papers: 7.7% in sample vs. 27.1% in full dataset.
- Noted overrepresentation of journal articles in the sample.
3.8.2. Chi-Square Test
3.9. Limitations
- Subsampling: Only 10% of the eligible studies were included because of feasibility constraints. Although random sampling was statistically validated (χ2 = 1.25, p = 0.535), thematic saturation could not be guaranteed.
- Language Restriction: Only English-language publications were included, excluding potentially relevant studies in French, Spanish, or local LMIC.
- Source Restrictions: Gray literature and non-indexed implementation reports were excluded, which may have resulted in the underrepresentation of field-based and NGO-led initiatives.
- Database and Year Limits: Although the databases were diverse, some relevant publications indexed after the search (June 2025) may have been missing.
4. Results
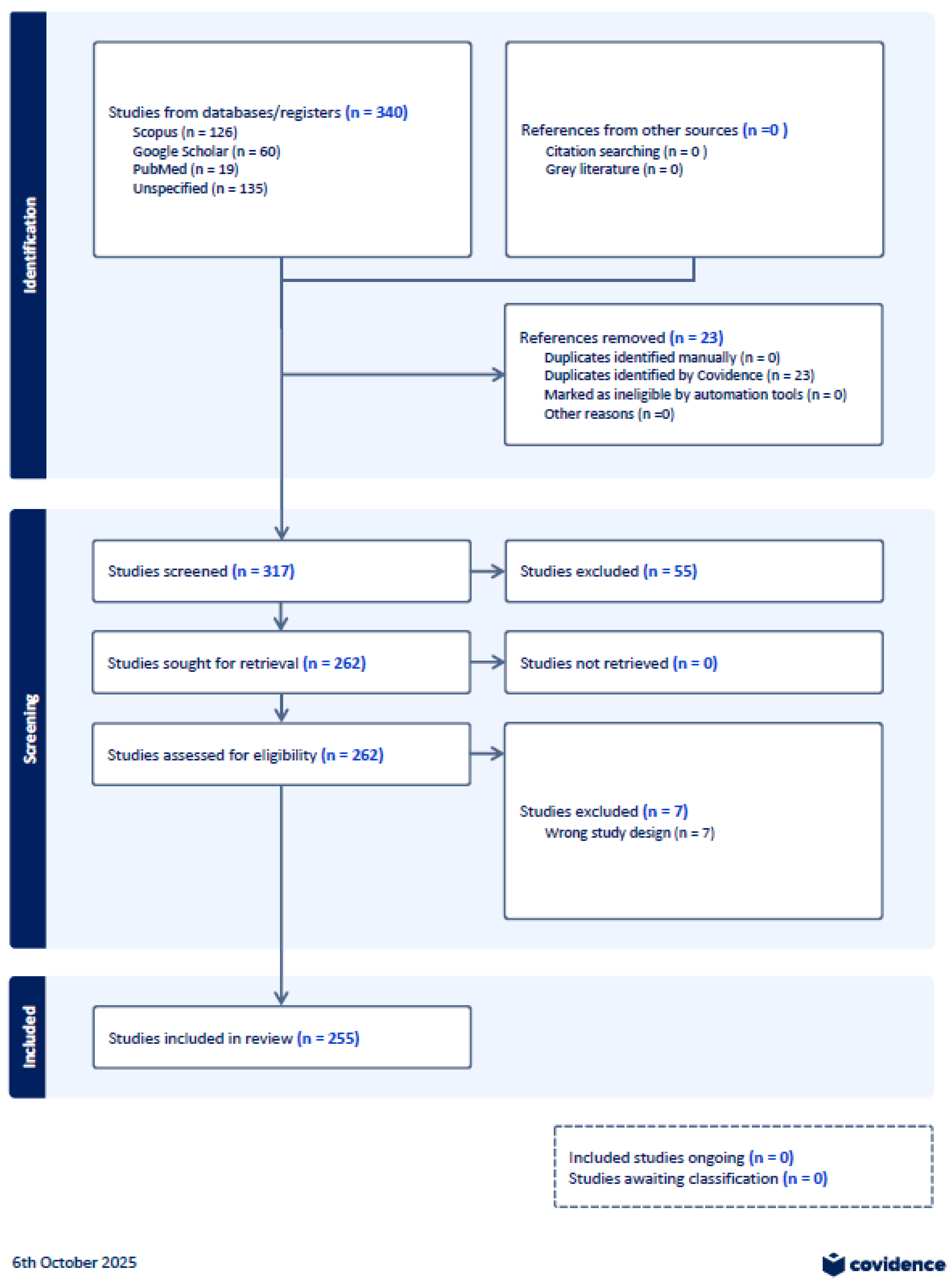
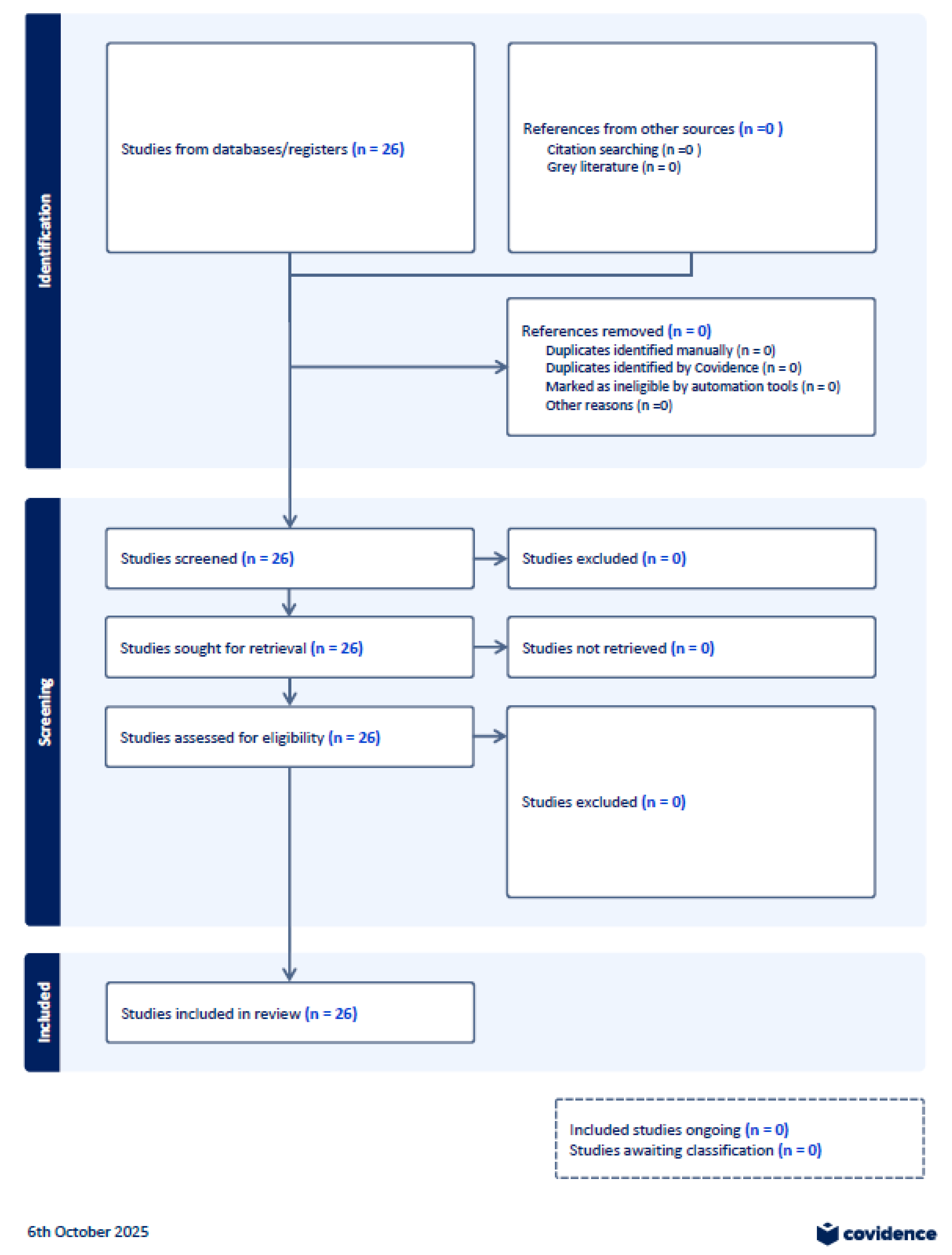
4.1. Selection of Sources of Evidence
4.2. Characteristics of Included Studies
- Scoping/Systematic Reviews: 9;
- Conceptual or Framework Papers: 3;
- Prototype/Pilot Studies: 3;
- Viewpoint or Qualitative Analyses: 11.
- Title, authorship, year, and region;
- Technology focus and interoperability standard;
- Barriers, enablers, and equity considerations;
- Governance/policy and implementation themes.
4.3. Critical Appraisal Within Sources of Evidence
4.4. Results of Individual Sources of Evidence
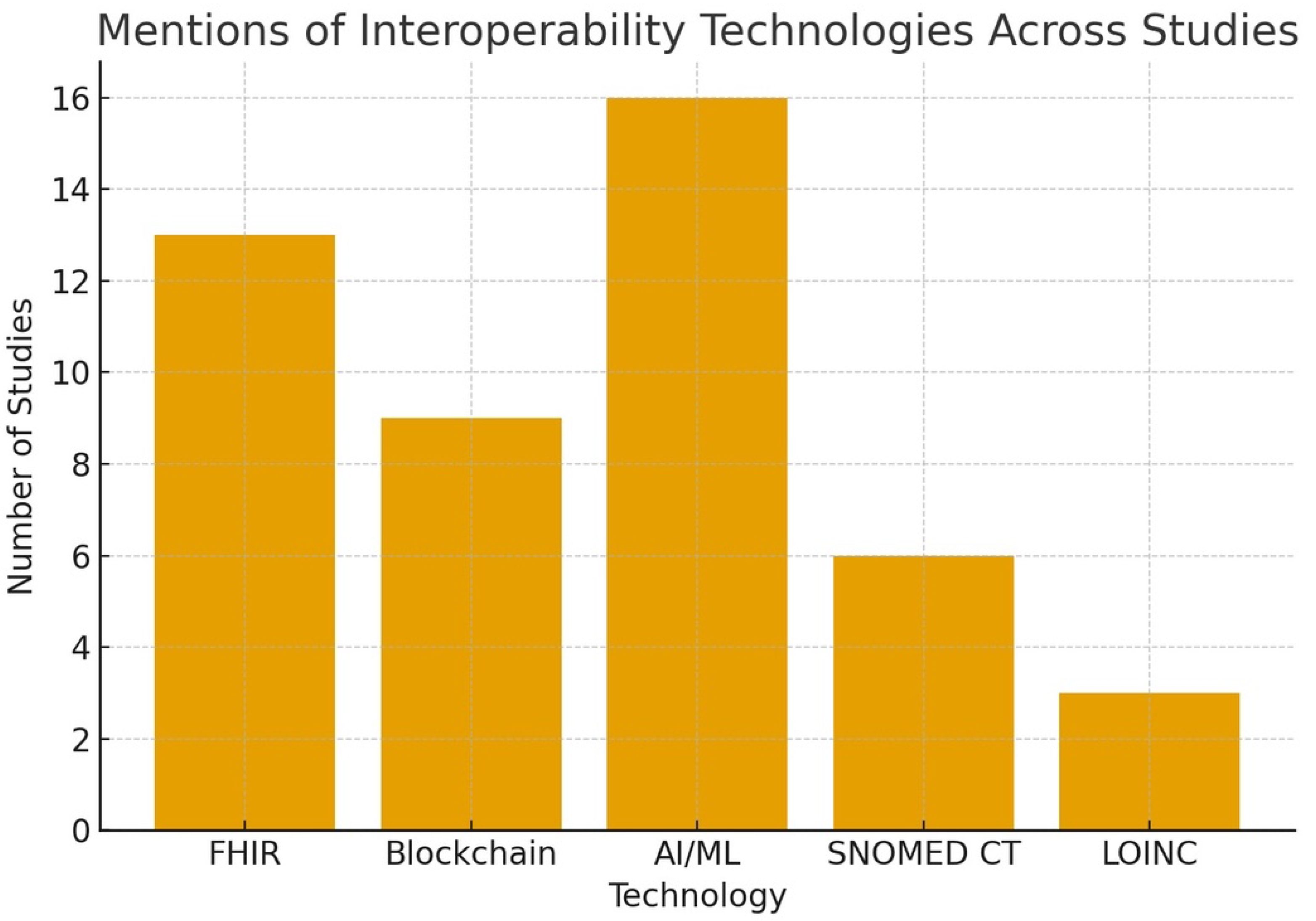
- AI/ML appeared in 16;
- Blockchain in 9;
- HL7 FHIR in 13;
- Equity considerations in 9;
- Barriers/enablers in 13.
- Governance (4.1) → 6 studies;
- Standards (4.2) → 8 studies;
- Emerging Tech (4.3) → 14 studies;
- Barriers/Enablers (4.4) → 13 studies;
- Equity (4.5) → 9 studies.
4.5. Synthesis of Results
4.5.1. Governance and Standards
4.5.2. Emerging Technologies and Innovation
4.5.3. Implementation Barriers and Enablers
4.5.4. Equity and Inclusion
4.6. Summary Statistics and Patterns
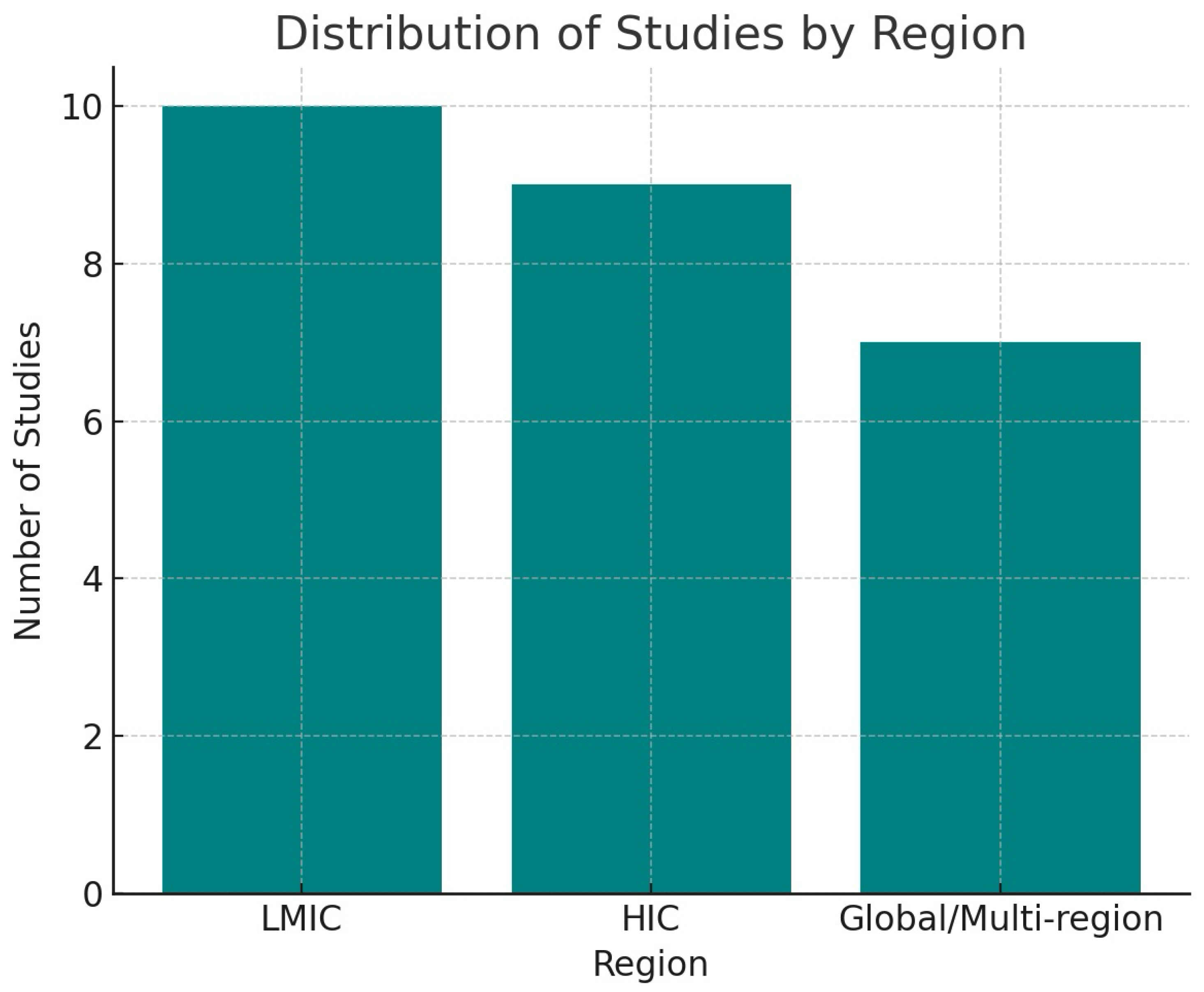
- Regions: 10 LMIC, 9 HIC, 7 Global.
- Equity mentioned: 9 studies.
- The most frequent themes were implementation barriers (n = 13), governance gaps (n = 6), and integration of emerging technologies (n = 14).
- Legend Summary:
5. Discussion
5.1. Summary of Key Findings
5.2. Contribution to the Literature
5.3. Policy & Practice Implications
- LMICs require contextualized standards; for example, Botswana’s adoption of HL7 FHIR and SNOMED demonstrates that national standardization is feasible even in resource-constrained settings but necessitates governance alignment and capacity building [25].
- Developers must design with integration and scalability in mind: As highlighted in Wong et al.’s enterprise architecture framework [31], design decisions will need to factor in regulatory realities, legacy systems, and multilingual environments to avoid siloed implementations.
5.4. Future Research Agenda
5.5. Limitations
- Although a 10% sample was statistically representative, it would not necessarily fully reflect the regional or thematic richness, particularly for novel or emerging technologies, such as federated learning, or less common standards, such as FHIR.
- Omitting gray literature could underestimate ground-level implementation efforts, particularly in LMIC settings where documentation is frequently located outside academic publishing.
- The English-only inclusion criteria may have excluded studies that recorded context-related interoperability efforts in Francophone Africa, Latin America, or Southeast Asia.
- The bulk of the published work is conceptual or prototype-based in nature; therefore, it limits inferences regarding real-world scalability and long-term consequences.
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Abbreviation | Meaning |
| AI | Artificial Intelligence |
| FHIR | Fast Healthcare Interoperability Resources |
| HL7 | Health Level Seven International |
| LMICs | Low- and Middle-Income Countries |
| mHealth | Mobile Health |
| TEFCA | Trusted Exchange Framework and Common Agreement |
| uHealth | Ubiquitous Health |
| QHINs | Qualified Health Information Networks |
| SNOMED CT | Systematized Nomenclature of Medicine—Clinical Terms |
| OMOP CDM | Observational Medical Outcomes Partnership Common Data Model |
| LOINC | Logical Observation Identifiers, Names and Codes |
Appendix A
- Description:
- Outline the methodological framework guiding the review, including objectives, research questions, inclusion/exclusion criteria, and review procedures. The JBI and PRISMA-ScR guidelines have been developed to ensure rigor and reproducibility.
- Description:
- Detailed database search strings, Boolean logic, inclusion criteria (e.g., English, peer-reviewed), date ranges (2015–2025), and deduplication/screening methods. This included the commands used in Stata for stratified random sampling.
- Description:
- Two PRISMA diagrams visualizing the identification, screening, eligibility, and inclusion of studies
- C1: Full PRISMA for all 255 screened studies
- C2: PRISMA for the 10% stratified sample (n = 26)
- Description:
- Structured extraction matrix with bibliographic details, study type, region, technology focus, interoperability standards, and equity relevance. This was used to generate the sampling frame and for analytical mapping.
- Description:
- Thematic extraction of the 10% sample analyzed in depth. It contains detailed notes on barriers, enablers, governance frameworks, standards adoption, and technology implementation.
- The Stata commands were used to generate a 10% random subsample (n = 26) from a full dataset of 255 eligible studies, stored in a CSV file titled Appendix_F_Stata_Command.pdf.
References
- Lehne, M.; Sass, J.; Essenwanger, A.; Schepers, J.; Thun, S. Why digital medicine depends on interoperability. npj Digit Med. 2019, 2, 79. [Google Scholar] [CrossRef]
- Ndlovu, K.; Mars, M.; Scott, R.E. Interoperability opportunities and challenges in linking mHealth applications and eRecord systems: Botswana as an exemplar. BMC Med. Inform. Decis. Mak. 2021, 21, 246. [Google Scholar] [CrossRef]
- World Health Organization. Global Strategy on Digital Health 2020–2025; WHO: Geneva, Switzerland, 2021; Available online: https://www.who.int/docs/default-source/documents/gs4dhdaa2a9f352b0445bafbc79ca799dce4d.pdf (accessed on 25 September 2025).
- Office of the National Coordinator for Health IT (ONC). Trusted Exchange Framework and Common Agreement (TEFCA); U.S. Department of Health and Human Services: Washington, DC, USA, 2021. Available online: https://www.healthit.gov/tefca (accessed on 25 September 2025).
- World Health Organization. WHO Guideline: Recommendations on Digital Interventions for Health System Strengthening; World Health Organization; Geneva, Switzerland, 2019. Available online: https://www.ncbi.nlm.nih.gov/books/NBK541905/ (accessed on 25 September 2025).
- Gazzarata, R.; Almeida, J.; Lindsköld, L.; Cangioli, G. HL7 Fast Healthcare Interoperability Resources (HL7 FHIR) in digital healthcare ecosystems for chronic disease management: Scoping review. Int. J. Med. Inform. 2024, 185, 105507. [Google Scholar] [CrossRef]
- Amar, F.; April, A.; Abran, A. Electronic health record and semantic issues using Fast Healthcare Interoperability Resources: Systematic mapping review. JMIR Med. Inform. 2024, 12, e45209. [Google Scholar] [CrossRef] [PubMed]
- Braunstein, M.L. Health Informatics on FHIR: How HL7’s API is Transforming Healthcare, 2nd ed.; Springer Nature Switzerland AG: Cham, Switzerland, 2022. [Google Scholar] [CrossRef]
- Agbo, C.C.; Mahmoud, Q.H.; Eklund, J.M. Blockchain Technology in Healthcare: A Systematic Review. Healthcare 2019, 7, 56. [Google Scholar] [CrossRef] [PubMed]
- Sheller, M.J.; Edwards, B.; Reina, G.A.; Martin, J.; Pati, S.; Kotrotsou, A.; Milchenko, M.; Xu, W.; Marcus, D.; Colen, R.R.; et al. Federated learning in medicine: Facilitating multi-institutional collaborations without sharing patient data. Sci. Rep. 2020, 10, 12598. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, S.; Mathias, R.; Schönfelder, A.; Wekenborg, M.; Steinigen-Fuchs, J.; Dillenseger, A.; Ziemssen, T. A roadmap for safe, regulation-compliant living labs for AI and digital health development. Sci. Adv. 2025, 11, eadn9348. [Google Scholar] [CrossRef]
- Zhang, X.; Saltman, R. Impact of electronic health record interoperability on telehealth service outcomes. JMIR Med. Inform. 2022, 10, e31837. [Google Scholar] [CrossRef]
- Rohaj, A.; Bulaj, G. Digital Therapeutics (DTx) Expand Multimodal Treatment Options for Chronic Low Back Pain: The Nexus of Precision Medicine, Patient Education, and Public Health. Healthcare 2023, 11, 1469. [Google Scholar] [CrossRef]
- World Health Organization. Ethics and Governance of Artificial Intelligence for Health; WHO: Geneva, Switzerland, 2021; Available online: https://www.who.int/publications/i/item/9789240029200 (accessed on 25 September 2025).
- Arksey, H.; O’Malley, L. Scoping studies: Towards a methodological framework. Int. J. Soc. Res. Methodol. 2005, 8, 19–32. [Google Scholar] [CrossRef]
- Levac, D.; Colquhoun, H.; O’Brien, K.K. Scoping studies: Advancing the methodology. Implement Sci. 2010, 5, 69. [Google Scholar] [CrossRef]
- Peters, M.D.J.; Marnie, C.; Tricco, A.C.; Pollock, D.; Munn, Z.; Alexander, L.; McInerney, P.; Godfrey, C.M.; Khalil, H. Updated methodological guidance for the conduct of scoping reviews. JBI Evid. Synth. 2020, 18, 2119–2126. [Google Scholar] [CrossRef]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef]
- Sharp, C.A. Qualitative research and evaluation methods. 3rd ed. Eval. J. Australas. 2003, 3, 60–61. [Google Scholar] [CrossRef]
- Palinkas, L.A.; Horwitz, S.M.; Green, C.A.; Wisdom, J.P.; Duan, N.; Hoagwood, K. Purposeful sampling for qualitative data collection and analysis in mixed method implementation research. Adm. Policy Ment. Health 2015, 42, 533–544. [Google Scholar] [CrossRef]
- Bene, B.A.; O’Connor, S.; Mastellos, N.; Majeed, A.; Fadahunsi, K.P.; O’Donoghue, J. Regulatory standards and guidance for the use of health apps for self-management in Sub-Saharan Africa: Scoping review. J. Med. Internet Res. 2024, 26, e49163. Available online: https://www.jmir.org/2024/1/e49163 (accessed on 25 September 2025). [CrossRef]
- Fassbender, A.; Donde, S.; Silva, M.; Friganovic, A.; Stievano, A.; Costa, E.; Winders, T.; van Vugt, J. Adoption of digital therapeutics in Europe. Ther. Clin. Risk Manag. 2024, 20, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Ambalavanan, R.; Snead, R.S.; Marczika, J.; Towett, G.; Malioukis, A.; Mbogori-Kairichi, M. Challenges and strategies in building a foundational digital health data integration ecosystem: A systematic review and thematic synthesis. Front Health Serv. 2025, 5, 1600689. [Google Scholar] [CrossRef] [PubMed]
- Curioso, W.H. Building capacity and training for digital health: Challenges and opportunities in Latin America. JMIR Med. Inform. 2020, 8, e16513. [Google Scholar] [CrossRef] [PubMed]
- Ndlovu, K.; Mars, M.; Scott, R.E. Interoperability frameworks linking mHealth applications to electronic record systems. BMC Health Serv. Res. 2021, 21, 1177. [Google Scholar] [CrossRef]
- Gupta, R.; Tanwar, S.; Tyagi, S.; Kumar, N.; Obaidat, M.S. HaBiTs: Blockchain-based telesurgery framework for healthcare 4.0. IEEE Access 2019, 7, 168636–168655. [Google Scholar] [CrossRef]
- Hasselgren, A.; Kralevska, K.; Gligoroski, D.; Pedersen, S.A.; Faxvaag, A. Blockchain in healthcare and health sciences—A scoping review. Int. J. Med. Inform. 2020, 134, 104040. [Google Scholar] [CrossRef] [PubMed]
- Sylla, B.; Ouedraogo, I.; Diallo, G. 25 years of digital health toward universal health coverage in low- and middle-income countries: Rapid systematic review. JMIR Public Health Surveill. 2022, 8, e35437. [Google Scholar] [CrossRef]
- Yung, A.; Shaw, T.; Kay, J.; Janssen, A. Examining how technology supports shared decision-making in oncology consultations: Qualitative thematic analysis. JMIR Cancer 2025, 11, e70827. [Google Scholar] [CrossRef]
- Mamun, A.A.; Azam, S.; Gritti, C. Blockchain-based electronic health records management: A comprehensive review and future research direction. IEEE Access 2022, 10, 1251–1275. [Google Scholar] [CrossRef]
- Wong, Z.S.-Y.; Gong, Y.; Ushiro, S. A pathway from fragmentation to interoperability through standards-based enterprise architecture to enhance patient safety. npj Digit Med. 2025, 8, 82. [Google Scholar] [CrossRef]
- Ayaz, M.; Pasha, M.F.; Alzahrani, M.Y.; Budiarto, R.; Stiawan, D. The Fast Health Interoperability Resources (FHIR) standard: Systematic literature review of implementations, applications, challenges and opportunities. JMIR Med. Inform. 2021, 9, e21929. [Google Scholar] [CrossRef]
- Tabari, P.; Costagliola, G.; De Rosa, M.; Boeker, M. State-of-the-art FHIR-based data model and structure implementations: Systematic scoping review. JMIR Med. Inform. 2022, 10, e38686. [Google Scholar] [CrossRef]
- Torab-Miandoab, A.; Samad-Soltani, T.; Jodati, A.; Rezaei-Hachesu, P. Interoperability of heterogeneous health information systems: A systematic literature review. BMC Med. Inform. Decis. Mak. 2023, 23, 18. [Google Scholar] [CrossRef]
- Gyrard, A.; Abedian, S.; Gribbon, P.; Manias, G.; van Nuland, R.; Zatloukal, K.; Nicolae, I.E.; Danciu, G.; Nechifor, S.; Marti-Bonmati, L.; et al. Lessons learned from European health data projects with cancer use cases: Implementation of health standards and internet of things semantic interoperability. JMIR Med. Inform. 2025, 13, e54501. [Google Scholar] [CrossRef]
- Brant, E.B.; Kennedy, J.N.; King, A.J.; Gerstley, L.D.; Mishra, P.; Schlessinger, D.; Shalaby, J.; Escobar, G.J.; Angus, D.C.; Seymour, C.W.; et al. Developing a shared sepsis data infrastructure: A systematic review and concept map to FHIR. npj Digit. Med. 2022, 5, 80. [Google Scholar] [CrossRef]
- Elangovan, D.; Long, C.S.; Bakrin, F.S.; Tan, C.S.; Goh, K.W.; Yeoh, S.F.; Loy, M.J.; Hussain, Z.; Lee, K.S.; Idris, A.C.; et al. The use of blockchain technology in the health care sector: Systematic review. JMIR Med. Inform. 2022, 10, e17278. [Google Scholar] [CrossRef] [PubMed]
- Firouzi, F.; Farahani, B.; Daneshmand, M.; Grise, K.; Song, J.; Saracco, R.; Wang, L.L.; Lo, K.; Angelov, P.; Soares, E.; et al. Harnessing the power of smart and connected health to tackle COVID-19: IoT, AI, robotics, and blockchain for a better world. IEEE Internet Things J. 2021, 8, 12826–12846. [Google Scholar] [CrossRef] [PubMed]
- Ghadi, Y.Y.; Shah, S.F.A.; Waheed, W.; Mazhar, T.; Ahmad, W.; Saeed, M.M.; Hamam, H. Integration of wearable technology and artificial intelligence in digital health for remote patient care. J. Cloud Comput. 2025, 14, 59. [Google Scholar] [CrossRef]
- Nankya, M.; Mugisa, A.; Usman, Y.; Upadhyay, A.; Chataut, R. Security and privacy in e-health systems: A review of AI and machine learning techniques. IEEE Access 2024, 12, 85697–85718. [Google Scholar] [CrossRef]
- Bout, N.; Ghizlane, G.; Belhadaoui, H.; Afifi, N.; Abik, M. Integrating emotional AI, IoT, and robotics for patient-centered healthcare: Challenges and future directions. Discov. Internet Things 2025, 5, 1–19. [Google Scholar] [CrossRef]
- Pourpanah, F.; Etemad, A. Exploring the landscape of ubiquitous in-home health monitoring: A comprehensive survey. ACM Trans. Comput. Healthc. 2024, 5, 1–43. [Google Scholar] [CrossRef]
- Cunha, J.; Duarte, R.; Guimarães, T.; Santos, M.F. A permissioned blockchain using open data to support healthcare. Procedia Comput. Sci. 2022, 203, 534–541. [Google Scholar] [CrossRef]
- Chandrakar, M. Telehealth and digital tools enhancing healthcare access in rural systems. Discov. Public Health 2024, 2, 27. [Google Scholar] [CrossRef]
- Glinkowski, W.M.; Cedro, T.; Wołk, A.; Doniec, R.; Wołk, K.; Wilk, S. Telemedicine, eHealth, and digital transformation in Poland (2014–2024): Trends, specializations, and systemic implications. Appl. Sci. 2025, 15, 8793. [Google Scholar] [CrossRef]
- Osebe, S.; Wachira, C.M.; Matu, F.; Bore, N.; Kaguma, D.; Mutahi, J.; Ogallo, W.; Cintas, C.; Remy, S.L.; Walcott, A.; et al. Enabling care continuity using a digital health wallet. In Proceedins of the 2019 IEEE International Conference on Healthcare Informatics (ICHI), Xi’an, China, 21 November 2019. [Google Scholar] [CrossRef]

| Characteristic | n (%) |
|---|---|
| Publication Year | |
| 2006–2010 | 1 (0.4) |
| 2011–2015 | 2 (0.8) |
| 2016–2020 | 30 (11.8) |
| 2021 | 14 (5.5) |
| 2022 | 34 (13.3) |
| 2023 | 29 (11.4) |
| 2024 | 60 (23.5) |
| 2025 | 85 (33.3) |
| Publication Type | |
| Journal Article | 180 (70.6) |
| Conference Paper | 62 (24.3) |
| Unknown/Not classified | 13 (5.1) |
| Domain Focus | |
| Digital/mHealth/Other | 122 (47.8) |
| Artificial Intelligence/ML | 63 (24.7) |
| Blockchain | 56 (22.0) |
| FHIR/Standards | 7 (2.7) |
| Internet of Things (IoT) | 6 (2.4) |
| Digital Therapeutics | 1 (0.4) |
| Metric | Value |
|---|---|
| χ2 | 1.25 |
| df | 2 |
| p | 0.535 |
| Category | n (%) |
|---|---|
| Digital/mHealth Tools | 13 (50.0%) |
| Policy/Structural | 8 (30.8%) |
| Community/Education | 5 (19.2%) |
| Domain | No. of Studies |
|---|---|
| Governance & Policy (4.1) | 6 |
| Interop Standards (4.2) | 8 |
| Emerging Tech (4.3) | 14 |
| Implementation (4.4) | 13 |
| Equity (4.5) | 9 |
| Study | Region | Themes | Barriers | Enablers | Equity Mention |
|---|---|---|---|---|---|
| Bene (2024) [21] | SSA | 4.1, 4.4 | Policy gaps | — | No |
| Fassbender (2024) [22] | EU | 4.1, 4.4 | Legal fragmentation | GDPR | No |
| Ambalavanan (2025) [23] | Global | 4.1, 4.3, 4.4 | No standards | Co-design | No |
| Curioso (2020) [24] | LATAM | 4.1, 4.3, 4.4, 4.5 | Capacity gaps | Regional collab | Yes |
| Ndlovu (2021) [25] | Botswana | 4.1, 4.2, 4.4, 4.5 | Legacy tech | HL7/FHIR/OpenHIE | Yes |
| Gupta (2019) [26] | Asia | 4.2, 4.3 | Data integrity | Smart contracts | No |
| Hasselgren (2020) [27] | Global | 4.2, 4.3 | Risk | Blockchain + FHIR | No |
| Sylla (2022) [28] | LMIC | 4.4, 4.5 | Donor dependence | Local adaptation | Yes |
| Yung (2025) [29] | Global | 4.3, 4.5 | Language bias | AI localization | Yes |
| Mamun (2022) [30] | NZ | 4.2, 4.3 | EHR heterogeneity | HL7 v2/3, ISO | No |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adegoke, K.; Adegoke, A.; Dawodu, D.; Adekoya, A.; Bayowa, A.; Kayode, T.; Singh, M. Interoperability as a Catalyst for Digital Health and Therapeutics: A Scoping Review of Emerging Technologies and Standards (2015–2025). Int. J. Environ. Res. Public Health 2025, 22, 1535. https://doi.org/10.3390/ijerph22101535
Adegoke K, Adegoke A, Dawodu D, Adekoya A, Bayowa A, Kayode T, Singh M. Interoperability as a Catalyst for Digital Health and Therapeutics: A Scoping Review of Emerging Technologies and Standards (2015–2025). International Journal of Environmental Research and Public Health. 2025; 22(10):1535. https://doi.org/10.3390/ijerph22101535
Chicago/Turabian StyleAdegoke, Kola, Abimbola Adegoke, Deborah Dawodu, Akorede Adekoya, Ayoola Bayowa, Temitope Kayode, and Mallika Singh. 2025. "Interoperability as a Catalyst for Digital Health and Therapeutics: A Scoping Review of Emerging Technologies and Standards (2015–2025)" International Journal of Environmental Research and Public Health 22, no. 10: 1535. https://doi.org/10.3390/ijerph22101535
APA StyleAdegoke, K., Adegoke, A., Dawodu, D., Adekoya, A., Bayowa, A., Kayode, T., & Singh, M. (2025). Interoperability as a Catalyst for Digital Health and Therapeutics: A Scoping Review of Emerging Technologies and Standards (2015–2025). International Journal of Environmental Research and Public Health, 22(10), 1535. https://doi.org/10.3390/ijerph22101535








