Abstract
Introduction: Occupational allergic rhinitis (OAR) is a common respiratory condition that can lead to varying degrees of symptom severity, significantly impacting workers’ quality of life and productivity. While occupational risk factors are well established, the influence of nonoccupational factors, such as obesity, that contribute to OAR severity remains largely unexplored. Aims: This study aims to study the association between obesity and the severity of OAR. Methods: A cross-sectional analytical study was conducted among patients diagnosed with OAR at the Occupational Medicine Department of Farhat Hached University Hospital of Sousse. It combines a retrospective review of medical records (2013–2021) with prospective structured telephone interviews (January–March 2023). Data were collected from medical records and supplemented with telephone interviews. The severity of OAR was assessed via the PAREO score and rhinomanometry results. Results: A total of 196 patients were included. The mean age was 39.69 ± 7.92 years, with a sex ratio of 0.53. The most frequently reported symptoms were nasal obstruction (78.6%) and sneezing (88.8%). The mean PAREO score was 5.78 ± 1.61, with severe OAR reported in 59.2% of the patients. Obesity was significantly associated with increased severity of OAR symptoms (p < 0.001; OR = 5.4; 95% CI [2.6–11.1]), a finding confirmed after adjustment for variables such as age, sex, and occupational seniority. Conclusion: Obesity appears to be a modifiable risk factor influencing OAR severity. Integrating weight management strategies into the treatment of OAR patients may contribute to significant symptom relief and improved quality of life. Further longitudinal studies are needed to confirm these findings and explore the underlying mechanisms involved.
1. Introduction
Occupational rhinitis (OR) is an inflammatory disease of the nose. It is characterized by intermittent or persistent symptoms (e.g., nasal congestion, sneezing, rhinorrhea, and itching) and/or variable nasal airflow limitation and/or hypersecretion. Its underlying causes are attributable to the particular work environment and not to stimuli encountered outside the workplace. OR can be either allergic or nonallergic. Nonallergic ORs are triggered by irritants and nonimmunological reactions, whereas occupational allergic rhinitis (OAR) is caused by immunological sensitization to workplace allergens [1,2,3].
Allergic rhinitis affects 10 to 30% of the population in developed countries, and its prevalence is increasing in developing countries [4]. A substantial body of research has revealed that 15% of the working population is affected by OR, which constitutes 4% of occupational respiratory diseases [5]. Despite its higher prevalence than occupational asthma, OAR remains underdiagnosed and undertreated. Patients with mild-to-moderate symptoms are less likely to seek medical advice [6,7]. Consequently, OAR is associated with a negative impact on quality of life (QOL) and work ability [5], resulting in functional limitations, reduced productivity and substantial financial hardship, mainly in its severe clinical presentation [8,9,10,11,12].
While genetic susceptibility and family history of allergies are recognized as major risk factors for OAR, other factors should be considered, such as exposure to allergens, indoor and outdoor pollution, and dietary factors [4]. Therefore, further research is needed to identify OAR severity risk factors, which are mainly modifiable, such as obesity. This major global public health concern, whose prevalence has tripled since 1975 [13], is multifactorial. It is associated with several respiratory disorders, including asthma, obstructive sleep apnea, and chronic rhinosinusitis, largely due to systemic inflammation, mechanical airway obstruction, and altered immune responses [14].
While the impact of obesity on respiratory diseases has been extensively assessed, its specific role in exacerbating occupational allergic rhinitis (OAR) symptoms remains under investigation. The systemic inflammation and immune dysregulation linked to obesity suggest a potential mechanism for amplifying OAR. Specifically, obesity-induced alterations in airway physiology and chronic low-grade inflammation may contribute to increased nasal hyperreactivity and heightened allergic responses in occupational settings [15]. The interest in allergic rhinitis in the workplace is justified by its frequency, particularly due to occupational exposure, its repercussions for employees, and its impact on productivity. Identifying factors that can aggravate its progression, such as obesity, which is increasingly common, could lead to the identification of preventive targets. The present study therefore aimed to analyze the relationship between obesity and the severity of OAR in a cohort of Tunisian workers.
2. Materials and Methods
2.1. Study Design
This analytical cross-sectional study was conducted in 2023 at the Occupational Medicine Department of Farhat Hached University Hospital, Sousse. It combines a retrospective review of medical records (2013–2021) with prospective structured telephone interviews (January–March 2023).
2.2. Participants and Sampling
This study enrolled patients who were diagnosed with OAR between January 2013 and December 2021, who were active employees during data collection and who had undergone anterior rhinomanometry. OAR was diagnosed via a combination of clinical evaluation and occupational history. Specifically, participants were classified as having OAR if they met typical nasal symptoms (sneezing, rhinorrhea, congestion, and itching) occurring during work hours and improving on their days off or vacation. Individuals were excluded from the study if their medical records were incomplete, if they were unreachable despite three attempts to contact them via telephone, or if they withdrew their informed consent. Participants with a prior diagnosis of nasal polyposis, obstructive sleep apnea syndrome (OSAS), or chronic rhinosinusitis with nasal polyps were excluded from the study.
2.3. Data Collection
At the time of occupational health consultations, informed consent for research was not routinely sought, as this study was not planned during routine clinical practice. Subsequently, structured telephone interviews (January–March 2023) were conducted to obtain oral informed consent from all participants and supplement incomplete clinical data. The real-time data collected during the occupational visit included anthropometric measurements, rhinoscopy, rhinomanometry, and initial PAREO scores. The collected variables included sociodemographic characteristics (e.g., age, sex, educational qualifications, marital status), lifestyle habits (e.g., smoking, alcohol consumption, physical activity), occupational characteristics (e.g., sector of activity, occupation, occupational seniority, use of protective respiratory equipment), and medical data (e.g., personal and family allergy history, body mass index (BMI), OAR symptoms, clinical examination findings, rhinomanometry results). Anterior rhinoscopy was performed during the ENT examination via a nasal speculum and direct illumination; nasal endoscopy was not systematically performed. The severity of OAR was assessed through the P.A.R.E.O. score and rhinomanometry. P.A.R.E.O. stands for Pruritus, Anosmia, Rhinorrhea, Eternuation [sternutation], and Obstruction. It is a validated scale evaluating the severity of functional signs associated with OAR, with each symptom scored from 0 to 2 (0 = absent, 1 = mild or bothersome, 2 = present and severe), yielding a total score from 0 to 10. Rhinomanometry was performed on all patients to provide objective measurements.
2.4. Operational Variables
Severe OAR was defined as a PAREO score exceeding 5 or a rhinomanometry value less than 500 mL/s. Moderate OAR was defined as RNT 500–700 mL/s, mild OAR was defined as RNT between 700 and 870 mL/s, and normal nasal function was defined as RNT greater than 870 mL/s. The classification of obesity is based on the World Health Organization (WHO) criteria. Overweight corresponds to a BMI ranging between 25 and 29.9 kg/m2, and obesity is defined as a BMI ≥ 30 kg/m2.
2.5. Data Analysis
The data were analyzed via SPSS version 25.0. Qualitative variables are reported as numbers and percentages. Quantitative variables are reported as the means ± standard deviations. The chi-square test and Fisher’s exact test were applied to compare categorical variables, whereas Student’s t test or the Mann–Whitney U test was used for continuous variables. Multivariate logistic regression was performed to estimate adjusted odds ratios (aORs) with 95% confidence intervals (CIs), controlling for potential confounders. The dependent factor was OAR severity. Spearman’s rank correlation coefficient (ρ) was computed to assess the association between the subjective PAREO symptom score and objective rhinomanometric values. Statistical significance was set at p ≤ 0.05.
2.6. Ethical Considerations
This study was conducted in accordance with the ethical principles of the Declaration of Helsinki. Participation was voluntary, and informed consent was obtained from all participants during the initial telephone contact (January–March 2023) prior to conducting the interviews.
Anonymity and confidentiality were ensured throughout the data collection and analysis process. The study was approved by the institutional ethics committee of the Sahloul University Hospital.
3. Results
A total of 196 patients were included in this study from the 264 eligible patients (see Figure 1), representing a participation rate of 74.2%.
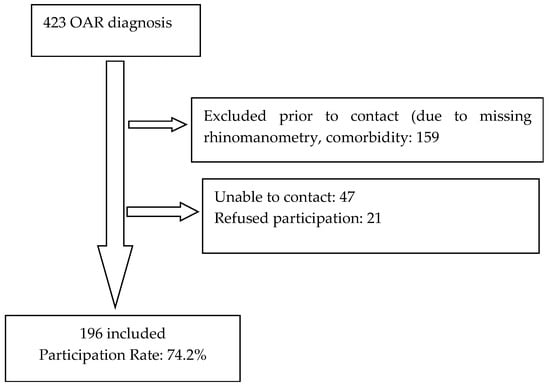
Figure 1.
Flow chart.
The mean age of the participants was 39.7 ± 7.9 years. The sex ratio was 0.53 (65.3% were female). The participants were predominantly married (75%) and had completed primary education in 69.4% of the cases. Among the participants, 19.4% were active smokers, and 27% reported engaging in regular physical activity. The most common occupational sectors were textile manufacturing (49.5%), electronics (14.8%), and food production (10.2%). The most prevalent occupational categories were blue-collar workers (69.4%), welders (5.6%) and technicians (3.6%). The average occupational seniority was 15.3 ± 8.1 years, and only 14.8% used respiratory protective equipment (Table 1).

Table 1.
Sociodemographic, lifestyle, and occupational characteristics of the study population.
Among the participants, 14.3% (n = 28) reported a familial history of allergic disease dominated by asthma (n = 17). A personal history of allergic disease was reported by 12.8% of the participants. The primary reported condition was asthma (n = 6). The mean body mass index (BMI) was 28.07 ± 4.95 kg/m2, and 35.2% of the participants were classified as obese (Table 2).

Table 2.
Medical characteristics of the study population.
The most frequently reported OAR symptoms were nasal obstruction (78.6%), sneezing (88.8%), rhinorrhea (67.8%), and pruritus (85.2%) (Table 3).

Table 3.
Severity of functional signs among the study population.
The mean PAREO score was 5.8 ± 1.6, and severe OAR was reported in 59.2% of the patients. Rhinomanometry revealed normal findings in 43.4% of the patients. Severe nasal obstruction was reported in 27% of the patients (Table 4). However, a weak but significant inverse correlation was observed between the PAREO score and rhinomanometric values (Spearman’s ρ = −0.24, p = 0.001).

Table 4.
Clinical examination and rhinomanometry findings.
Table 5 shows the prevalence of different degrees of OAR in the different BMI groups.

Table 5.
Prevalence of different degrees of OAR in the different BMI groups.
A significant association was observed between obesity and the clinical severity of OAR (p < 0.001; OR = 5.4; 95% CI [2.6–11.1]). However, no significant association was found between obesity and severe nasal obstruction, as measured by rhinomanometry (p = 0.82).
Other significant risk factors included female sex (p = 0.027; OR = 1.9; 95% CI [1.07–3.5]), marital status (p = 0.003; OR = 2.7; 95% CI [1.4–5.2]), lack of physical activity (p = 0.006; OR = 2.4; 95% CI [1.2–4.6]), occupational seniority (p = 0.024), and delay in symptom onset (p = 0.036) (Table 6).

Table 6.
Factors associated with the clinical severity of occupational allergic rhinitis.
After multivariate analysis, obesity remained the strongest independent predictor of severe OAR (p < 0.001; aOR = 5.47; 95% CI [2.6–11.1]) (Table 7).

Table 7.
Clinical severity of occupational allergic rhinitis and independent factors in multivariate analysis.
4. Discussion
This study suggests that obesity may exacerbate OAR symptoms, providing valuable insights into this under-researched area. In fact, few studies have assessed the association between obesity and OAR in the general population, and fewer have investigated this relationship in an occupational setting.
Our findings align with available research conducted in general populations and report a link between obesity and allergic rhinitis (AR) and/or a link between higher BMI and a greater prevalence and severity of AR [16,17,18,19,20]. These studies highlight the potential of obesity to modulate immune responses and promote inflammation, which are key factors in the development of AR.
However, other studies reported no association between BMI and AR [21,22]. These discrepancies may result from variations in study methodologies, population characteristics, and the criteria used to define AR severity. Notably, these investigations focused primarily on nonoccupational AR, where the triggers and environmental context differ significantly.
Distinguishing between general AR and OAR is imperative, as our study focused exclusively on the occupational field. OAR is a respiratory condition precipitated by an IgE-mediated immune hypersensitivity reaction to a particular occupational allergen [1]. A latency period ranging from several months to several years is typically required to achieve sensitization to this agent. Re-exposure to the allergen serves as a trigger, prompting an inflammatory immune response that is frequently accompanied by the infiltration of eosinophils into the airways. However, notably, OAR is not invariably associated with a predominant IgE response. In certain instances, it can be facilitated by IgG antibodies or alternative adaptive immune mechanisms. The inflammation underlying AR, irrespective of its etiology (occupational or environmental allergens), is a multifaceted process. Its intensity can be increased by various factors, including systemic conditions such as obesity.
The lack of association between obesity and severe nasal obstruction as measured by rhinomanometry (p = 0.82), despite a strong association with subjective symptom severity (PAREO; p < 0.001; OR = 5.47), suggests that obesity exacerbates OAR primarily through systemic inflammatory and neurosensory pathways rather than via anatomical narrowing of the nasal airway. Obesity induces a state of chronic low-grade inflammation characterized by elevated adipokines (e.g., leptin) and proinflammatory cytokines (e.g., TNF-α and IL-6) [15], which promote airway hyperreactivity, increase mucosal permeability, and amplify IgE-mediated responses to occupational allergens. Concurrently, obesity is associated with systemic immune dysregulation and altered microbiome composition, including reduced diversity and increased colonization by Staphylococcus aureus in the nasal cavity [23], which may disrupt local immune homeostasis and lower the threshold for allergic inflammation. Emerging evidence further supports the concept of the “gut–airway axis,” wherein obesity-driven gut dysbiosis and systemic metabolic disturbances propagate distant inflammatory signals to the upper airway mucosa [24,25,26,27]. These interconnected mechanisms of systemic inflammation, immune deviation, and microbiome dysbiosis likely converge to intensify symptom perception and neurogenic signaling (e.g., via trigeminal nerve sensitization), thereby explaining the pronounced discordance between objective airflow measurements and patient-reported burden in obese individuals with OAR. Thus, while obesity does not appear to cause structural obstruction, it significantly amplifies the subjective experience of OAR through multifactorial, systemically mediated pathways.
Other factors, such as age and exposure to different allergens, may influence the severity of AR. Jabri H et al. [28] highlighted the impact of persistent allergen exposure, poor treatment adherence, humidity, and smoking on AR management. A recent Tunisian comparative study [29] involving patients with AR reported that persistent allergic exposure, poor treatment adherence, and uncontrolled asthma were associated with the development of severe forms of AR. Consistent and correctly fitted respiratory protection serves as a vital barrier against inhaled occupational allergens, directly reducing nasal exposure, dampening local inflammation, and lowering the risk of sensitization and symptom development in workers at risk for OAR. Its integration into comprehensive occupational health programs is essential for primary prevention. Therefore, further multivariate analyses, particularly in occupational settings, are needed to better understand the real impact of each of these factors.
Our study also revealed that female sex and lack of physical activity were independent risk factors for severe OAR. These findings align with the available literature and suggest that hormonal influences and immune system variations may underlie sex disparities due to hormonal modulation of immune responses in women [15,28,30,31]. The association between sedentary behavior and heightened allergic inflammation has also been previously described, suggesting that regular physical activity may be a protective factor [25,32].
Finally, it is important to acknowledge the limitations of our study before any definitive conclusions can be drawn. The cross-sectional design precludes causal inference and raises the possibility of reverse causation (severe OAR leading to reduced activity and subsequent obesity). Other limitations include lack of allergen quantification, reliance on self-reported behaviors, potential recall bias from telephone interviews and survivorship/availability bias. The single-center nature of the study may limit the generalizability of the findings. OAR severity was assessed by a validated score and objective rhinomanometry, whereas other factors, such as the specific types of allergens encountered in the workplace, should have been considered. Consequently, further research, particularly longitudinal studies with larger populations, is necessary to validate our findings and elucidate the interactions of different factors contributing to OAR severity. Additionally, the dominance of textile workers in our sample may introduce potential biases. such as the healthy worker effect and distinct occupational exposures in the textile sector from those in other manufacturing or industrial sectors.
Owing to the absence of quantitative data on allergen type and exposure intensity, our findings lack specificity regarding particular occupational exposures. Future research should include a detailed assessment of workplace allergens.
Despite these limitations, our results have important clinical implications. Considering obesity as a potentially modifiable risk factor for OAR severity could lead to more targeted interventions. Furthermore, our study highlights the need for a multidisciplinary approach to OAR management involving collaboration between occupational physicians, allergists, nutritionists, and other healthcare professionals.
5. Conclusions
This study identified obesity as a modifiable factor associated with increased clinical severity of OAR. The observed association suggests the potential importance of incorporating obesity into OAR management strategies. However, given the cross-sectional design of this study, causal inferences cannot be drawn. Future longitudinal research is therefore essential to confirm these findings, elucidate the underlying biological and behavioral mechanisms, and evaluate the impact of targeted interventions aimed at reducing the obesity-related burden in populations at risk for OAR.
Author Contributions
Conceptualization, I.K., A.M. and M.K.; methodology, I.K. and A.M.; software, I.K.; validation, I.K., A.M., C.S., M.K. and N.M.; formal analysis, I.K.; investigation, I.K., A.M., C.S. and A.F.; resources, M.A. and M.T.; data curation, I.K.; writing—original draft preparation, I.K. and A.M.; writing—review and editing, O.E.M. and M.K.; visualization, A.M.; supervision, N.M., M.K. and O.E.M.; project administration, N.M.; Revision: I.K., M.K., A.M. and M.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of Sahloul Sousse University Hospital Center (protocol code HS 01-2022 and date of approval 6 January 2022).
Informed Consent Statement
Informed consent was obtained from all the subjects involved in the study.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors upon request.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
References
- Zamora-Sifuentes, J.; Poole, J.A. Occupational Rhinitis: An Update. Curr. Allergy Asthma Rep. 2023, 23, 579–587. [Google Scholar] [CrossRef]
- Vandenplas, O.; Hox, V.; Bernstein, D. Occupational Rhinitis. J. Allergy Clin. Immunol. Pract. 2020, 8, 3311–3321. [Google Scholar] [CrossRef] [PubMed]
- Grammer, L.C. Occupational Rhinitis. Immunol. Allergy Clin. N. Am. 2016, 36, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Alnahas, S.; Abouammoh, N.; Althagafi, W.; Elsayed Abd-Ellatif, E. Prevalence, severity, and risk factors for allergic rhinitis among schoolchildren in Saudi Arabia: A national cross-sectional study. World Allergy Organ. J. 2023, 16, 100824. [Google Scholar] [CrossRef] [PubMed]
- Maoua, M.; Maalel, O.; Kacem, I.; Guedri, S.; Ben Kacem, M.; Aissa, S.; Ghammem, M.; Brahem, A.; Kalboussi, H.; Debbabi, F.; et al. Quality of Life and Work Productivity Impairment of Patients with Allergic Occupational Rhinitis. Tanaffos 2019, 18, 58–65. [Google Scholar]
- Inserm. Asthme et Rhinite D’origine Professionnelle. Rapport; Les Éditions Inserm: Paris, France, 2000; p. 95, (Expertise Collective); Available online: http://hdl.handle.net/10608/180 (accessed on 15 September 2024).
- Sublett, J.W.; Bernstein, D.I. Occupational rhinitis. Immunol. Allergy Clin. N. Am. 2011, 31, 787–796. [Google Scholar] [CrossRef]
- Garnier, R. Rhinites allergiques professionnelles. EMC—Pathol. Prof. L’environnement 2012, 7, 1–12. [Google Scholar] [CrossRef]
- Liva, G.A.; Karatzanis, A.D.; Prokopakis, E.P. Review of Rhinitis: Classification, Types, Pathophysiology. J. Clin. Med. 2021, 10, 3183. [Google Scholar] [CrossRef]
- de la Hoz Caballer, B.; Rodríguez, M.; Fraj, J.; Cerecedo, I.; Antolín-Amérigo, D.; Colás, C. Allergic rhinitis and its impact on work productivity in primary care practice and a comparison with other common diseases: The Cross-sectional study to evAluate work Productivity in allergic Rhinitis compared with other common dIseases (CAPRI) study. Am. J. Rhinol. Allergy 2012, 26, 390–394. [Google Scholar] [CrossRef]
- Groenewoud, G.C.M.; de Groot, H.; van Wijk, R.G. Impact of occupational and inhalant allergy on rhinitis-specific quality of life in employees of bell pepper greenhouses in the Netherlands. Ann. Allergy Asthma Immunol. 2006, 96, 92–97. [Google Scholar] [CrossRef]
- Lamb, C.E.; Ratner, P.H.; Johnson, C.E.; Ambegaonkar, A.J.; Joshi, A.V.; Day, D.; Sampson, N.; Eng, B. Economic impact of workplace productivity losses due to allergic rhinitis compared with select medical conditions in the United States from an employer perspective. Curr. Med. Res. Opin. 2006, 22, 1505–1512. [Google Scholar] [CrossRef]
- Boutari, C.; Mantzoros, C.S. A 2022 update on the epidemiology of obesity and a call to action: As its twin COVID-19 pandemic appears to be receding, the obesity and dysmetabolism pandemic continues to rage on. Metabolism 2022, 133, 155217. [Google Scholar] [CrossRef]
- Vatankhah, V.; Khazraei, H.; Iranpoor, H.; Lotfizadeh, M. Impact of high body mass index on allergic rhinitis patients. Rev. Française D’allergologie 2017, 57, 370–374. [Google Scholar] [CrossRef]
- Han, M.W.; Kim, S.H.; Oh, I.; Kim, Y.H.; Lee, J. Obesity Can Contribute to Severe Persistent Allergic Rhinitis in Children through Leptin and Interleukin-1β. Int. Arch. Allergy Immunol. 2021, 182, 546–552. [Google Scholar] [CrossRef] [PubMed]
- Kilpeläinen, M.; Terho, E.O.; Helenius, H.; Koskenvuo, M. Body mass index and physical activity in relation to asthma and atopic diseases in young adults. Respir. Med. 2006, 100, 1518–1525. [Google Scholar] [CrossRef] [PubMed]
- Irei, A.V.; Takahashi, K.; Le, D.S.N.T.; Ha, P.T.N.; Hung, N.T.K.; Kunii, D.; Sakai, T.; Matoba, T.; Yamamoto, S. Obesity is associated with increased risk of allergy in Vietnamese adolescents. Eur. J. Clin. Nutr. 2005, 59, 571–577. [Google Scholar] [CrossRef]
- Irei, A.V.; Sato, Y.; Lin, T.-L.; Wang, M.-F.; Chan, Y.-C.; Hung, N.T.K.; Kunii, D.; Sakai, T.; Kaneda, M.; Yamamoto, S. Overweight is associated with allergy in school children of Taiwan and Vietnam but not Japan. J. Med. Investig. 2005, 52, 33–40. [Google Scholar] [CrossRef][Green Version]
- Al Rasyid, H.; McManus, A.; Mallon, D.; Claire, N. Elevated Body Mass Index is associated with severity of Allergic Rhinitis: Results from a cross sectional study. Australas. Med. J. 2008, 1, 114–119. [Google Scholar] [CrossRef]
- Kim, J.-L.; Brisman, J.; Aberg, M.A.; Forslund, H.B.; Winkvist, A.; Torén, K. Trends in the prevalence of asthma, rhinitis, and eczema in 15-year-old adolescents over an 8 year period. Respir. Med. 2014, 108, 701–708. [Google Scholar] [CrossRef]
- Bråbäck, L.; Hjern, A.; Rasmussen, F. Body mass index, asthma and allergic rhinoconjunctivitis in Swedish conscripts—A national cohort study over three decades. Respir. Med. 2005, 99, 1010–1014. [Google Scholar] [CrossRef]
- Mai, X.M.; Nilsson, L.; Axelson, O.; Bråbäck, L.; Sandin, A.; Kjellman, N.I.M.; Björkstén, B. High body mass index, asthma and allergy in Swedish schoolchildren participating in the International Study of Asthma and Allergies in Childhood: Phase II. Acta Paediatr. 2003, 92, 1144–1148. [Google Scholar] [CrossRef]
- Olsen, K.; Danielsen, K.; Wilsgaard, T.; Sangvik, M.; Sollid, J.U.E.; Thune, I.; Eggen, A.E.; Simonsen, G.S.; Furberg, A.-S.; Wertheim, H.F.L. Obesity and Staphylococcus aureus nasal colonization among women and men in a general population. PLoS ONE 2013, 8, e63716. [Google Scholar] [CrossRef] [PubMed]
- Rawls, M.; Ellis, A.K. The microbiome of the nose. Ann. Allergy Asthma Immunol. 2019, 122, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Ruokolainen, L.; Paalanen, L.; Karkman, A.; Laatikainen, T.; von Hertzen, L.; Vlasoff, T.; Markelova, O.; Masyuk, V.; Auvinen, P.; Paulin, L.; et al. Significant disparities in allergy prevalence and microbiota between the young people in Finnish and Russian Karelia. Clin. Exp. Allergy 2017, 47, 665–674. [Google Scholar] [CrossRef] [PubMed]
- Choi, C.H.; Poroyko, V.; Watanabe, S.; Jiang, D.; Lane, J.; deTineo, M.; Baroody, F.M.; Naclerio, R.M.; Pinto, J.M. Seasonal Allergic Rhinitis Affects Sinonasal Microbiota. Am. J. Rhinol. Allergy 2014, 28, 281–286. [Google Scholar] [CrossRef]
- Lal, D.; Keim, P.; Delisle, J.; Barker, B.; Rank, M.A.; Chia, N.; Schupp, J.M.; Gillece, J.D.; Cope, E.K. Mapping and comparing bacterial microbiota in the sinonasal cavity of healthy, allergic rhinitis, and chronic rhinosinusitis subjects. Int. Forum Allergy Rhinol. 2017, 7, 561–569. [Google Scholar] [CrossRef]
- Jabri, H.; El Khattabi, W.; Aichane, A.; Afif, H.; Bouayad, Z. Profil allergique de la rhinite allergique sévère. Rev. Française D’allergologie 2014, 54, 4–7. [Google Scholar] [CrossRef]
- Boubaker, N.; Mejri, I.; M’hamdi, S.; Daboussi, S.; Aichaouia, C.; Moetemri, Z. Facteurs prédictifs de sévérité de la rhinite allergique. Rev. Française D’allergologie 2022, 62, 351–357. [Google Scholar] [CrossRef]
- Ciprandi, G.; Pistorio, A.; Tosca, M.; Ferraro, M.R.; Cirillo, I. Body mass index, respiratory function and bronchial hyperreactivity in allergic rhinitis and asthma. Respir. Med. 2009, 103, 289–295. [Google Scholar] [CrossRef][Green Version]
- Zhou, J.; Luo, F.; Han, Y.; Lou, H.; Tang, X.; Zhang, L. Obesity/overweight and risk of allergic rhinitis: A meta-analysis of observational studies. Allergy 2020, 75, 1272–1275. [Google Scholar] [CrossRef]
- Ali, Z.; Ulrik, C.S. Obesity and asthma: A coincidence or a causal relationship? A systematic review. Respir. Med. 2013, 107, 1287–1300. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).