From Trust to Choice: A Cross-Sectional Survey of How Patient Trust in Pharmacists Shapes Willingness and Vaccination Decision Control Preferences
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design, Sample and Setting
2.2. Data Collection and Measures
2.3. Statistical Analysis
3. Results
3.1. Characteristics of the Study Participants (N = 924)
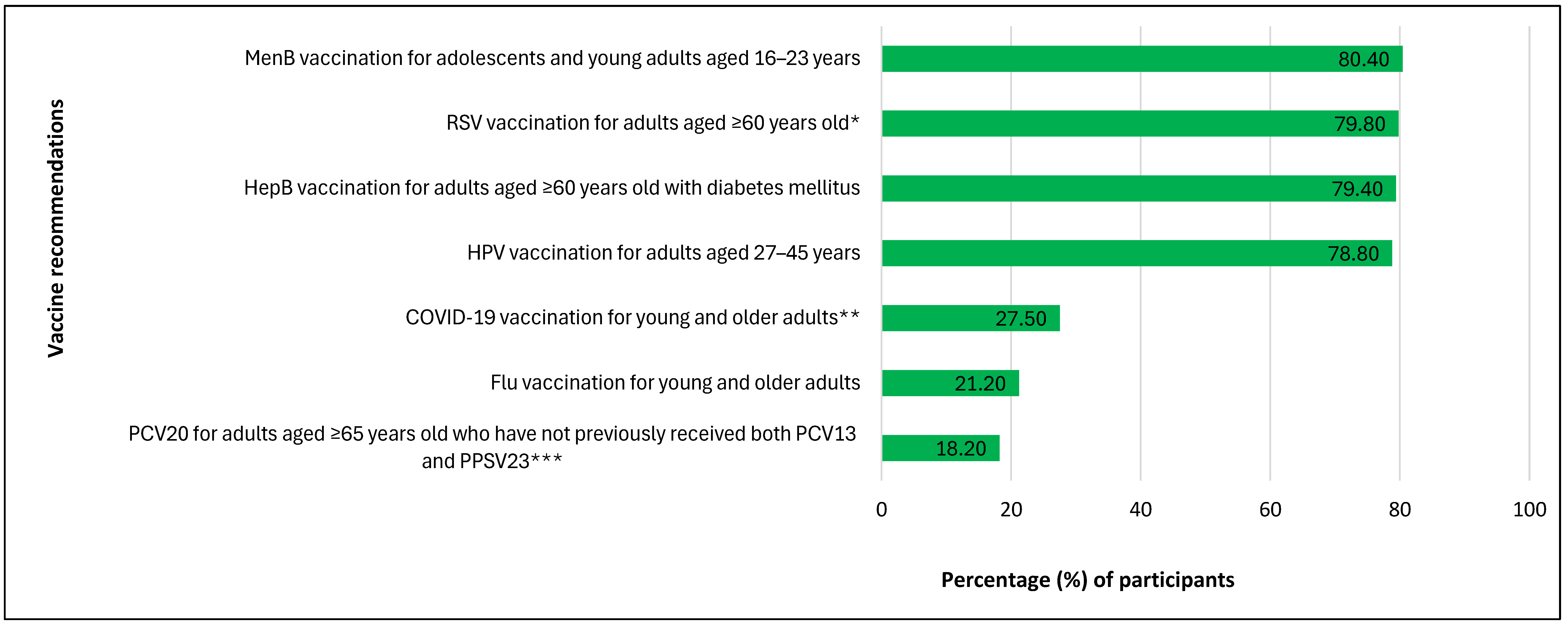
3.2. Participants’ Awareness and Knowledge of SCDM for Vaccines, Willingness to Engage in SCDM with Community Pharmacists and Vaccination Decision Control Preference (N = 924)
3.3. Factors Influencing Participants’ Willingness to Engage in Shared Clinical Decision-Making with Community Pharmacists (N = 924)
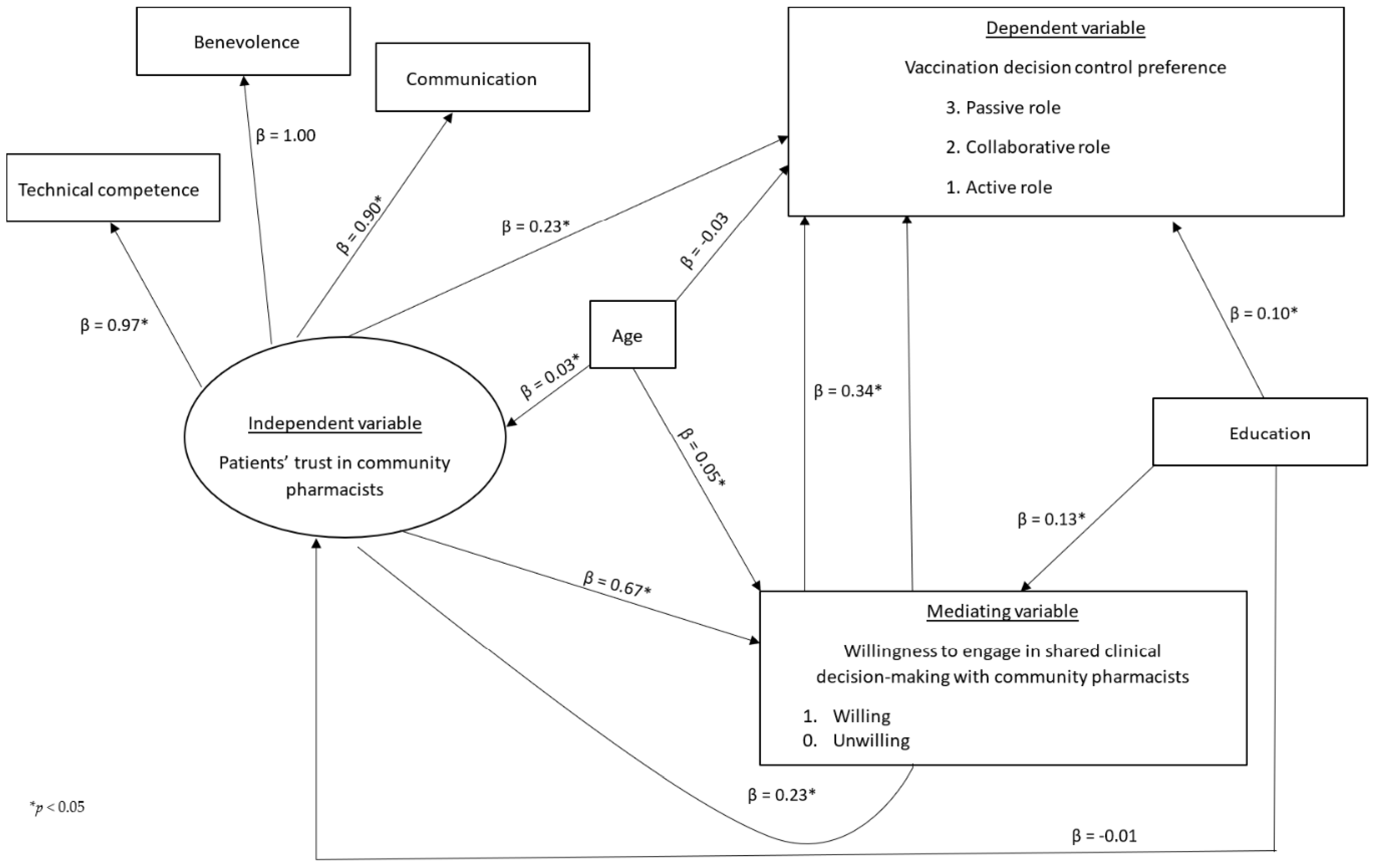
3.4. Structural Equation Modeling Results (N = 924)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| U.S. | United States |
| SCDM | Shared Clinical Decision-Making |
| ACIP | Advisory Committee on Immunization Practices |
| CDC | Centers for Disease Control and Prevention |
| MenB | Meningococcal B |
| HepB | Hepatitis B |
| HPV | Human Papillomavirus |
| PCV20 | 20-valent Pneumococcal Conjugate Vaccine |
| PCV13 | 13-valent Pneumococcal Conjugate Vaccine |
| PPSV23 | 23-valent Pneumococcal Polysaccharide Vaccine |
| RSV | Respiratory Syncytial Virus |
| PCV21 | 21-valent Pneumococcal Conjugate Vaccine |
| COVID-19 | Coronavirus Disease 2019 |
| TRUST-Ph | Trust in Community Pharmacists |
References
- Centers for Disease Control and Prevention. ACIP Shared Clinical Decision-Making Recommendations. 2025. Available online: https://www.cdc.gov/acip/vaccine-recommendations/shared-clinical-decision-making.html (accessed on 14 February 2025).
- Elwyn, G.; Durand, M.A.; Song, J.; Aarts, J.; Barr, P.J.; Berger, Z.; Cochran, N.; Frosch, D.; Galasiński, D.; Gulbrandsen, P.; et al. A three-talk model for shared decision making: Multistage consultation process. BMJ 2017, 359, j4891. [Google Scholar] [CrossRef] [PubMed]
- Ommen, O.; Thuem, S.; Pfaff, H.; Janssen, C. The relationship between social support, shared decision-making and patient’s trust in doctors: A cross-sectional survey of 2,197 inpatients using the Cologne Patient Questionnaire. Int. J. Public Health 2011, 56, 319–327. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. CDC Updates RSV Vaccination Recommendation for Adults. Available online: https://www.cdc.gov/media/releases/2024/s-0626-vaccination-adults.html (accessed on 21 April 2025).
- Champions for Vaccine Education Equity + Progress (CVEEP). Shared Clinical Decision-Making for Vaccines: Challenges and Implication for Vaccination Awarenness, Administration and Uptake. Available online: https://cveep.org/wp-content/uploads/2024/06/CVEEP_SCDM.pdf (accessed on 1 October 2024).
- MedPage Today. ACIP Makes Changes to Adult RSV Vaccine Recommendations. 2024. Available online: https://www.medpagetoday.com/meetingcoverage/acip/110845 (accessed on 28 August 2024).
- Kobayashi, M.; Leidner, A.J.; Gierke, R.; Farrar, J.L.; Morgan, R.L.; Campos-Outcalt, D.; Schechter, R.; Poehling, K.A.; Long, S.S.; Loehr, J.; et al. Use of 21-Valent Pneumococcal Conjugate Vaccine Among U.S. Adults: Recommendations of the Advisory Committee on Immunization Practices-United States, 2024. MMWR Morb. Mortal. Wkly Rep. 2024, 73, 793–798. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Advisory Committee on Immunization Practices (ACIP) Evidence to Recommendations (EtR) for Use of Additional Doses of 2024-2025 COVID-19 Vaccines in Older Adults and People Who Are Moderately or Severely Immunocompromised. Available online: https://www.cdc.gov/acip/evidence-to-recommendations/covid-19-2024-2025-additional-dose.html (accessed on 21 April 2025).
- Centers for Disease Control and Prevention. Child and Adolescent Immunization Schedule by Age. Available online: https://www.cdc.gov/vaccines/hcp/imz-schedules/child-adolescent-age.html (accessed on 4 June 2025).
- Xiao, L.; Peng, M.; Liu, Y.; Zhang, L. Information, deliberation, and decisional control preferences for participation in medical decision-making and its influencing factors among Chinese cancer patients. Health Expect. 2021, 24, 1725–1736. [Google Scholar] [CrossRef]
- Singh, J.A.; Sloan, J.A.; Atherton, P.J.; Smith, T.; Hack, T.F.; Huschka, M.M.; Rummans, T.A.; Clark, M.M.; Diekmann, B.; Degner, L.F. Preferred roles in treatment decision making among patients with cancer: A pooled analysis of studies using the Control Preferences Scale. Am. J. Manag. Care 2010, 16, 688–696. [Google Scholar]
- Sankar, S.D.; Dhanapal, B.; Shankar, G.; Krishnaraj, B.; Karra, S.; Natesan, V. Desire for Information and Preference for Participation in Treatment Decisions in Patients With Cancer Presenting to the Department of General Surgery in a Tertiary Care Hospital in India. J. Glob. Oncol. 2018, 4, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Whitney, R.L.; White, A.E.C.; Rosenberg, A.S.; Kravitz, R.L.; Kim, K.K. Trust and shared decision-making among individuals with multiple myeloma: A qualitative study. Cancer Med. 2021, 10, 8040–8057. [Google Scholar] [CrossRef]
- Kempe, A.; Lindley, M.C.; O’Leary, S.T.; Crane, L.A.; Cataldi, J.R.; Brtnikova, M.; Beaty, B.L.; Matlock, D.D.; Gorman, C.; Hurley, L.P. Shared clinical decision-making recommendations for adult immunization: What do physicians think? J. Gen. Intern. Med. 2021, 36, 2283–2291. [Google Scholar] [CrossRef]
- Kricorian, K.; Rachel, C.; Equils, O. COVID-19 vaccine hesitancy: Misinformation and perceptions of vaccine safety. Hum. Vaccines Immunother. 2022, 18, 1950504. [Google Scholar] [CrossRef]
- Gatwood, J.; McKnight, M.; Frederick, K.; Hohmeier, K.; Kapan, S.; Chiu, C.Y.; Renfro, C.; Hagemann, T. Extent of and reasons for vaccine hesitancy in adults at high-risk for pneumococcal disease. Am. J. Health Promot. 2021, 35, 908–916. [Google Scholar] [CrossRef] [PubMed]
- Resnicow, K.; Catley, D.; Goggin, K.; Hawley, S.; Williams, G.C. Shared Decision Making in Health Care: Theoretical Perspectives for Why It Works and For Whom. Med. Decis. Mak. 2022, 42, 755–764. [Google Scholar] [CrossRef] [PubMed]
- IQVIA Trends in Vaccine Administration in the United States. 2023. Available online: https://www.iqvia.com/insights/the-iqvia-institute/reports-and-publications/reports/trends-in-vaccine-administration-in-the-united-states (accessed on 30 July 2024).
- Berenbrok, L.A.; Tang, S.; Gabriel, N.; Guo, J.; Sharareh, N.; Patel, N.; Dickson, S.; Hernandez, I. Access to community pharmacies: A nationwide geographic information systems cross-sectional analysis. J. Am. Pharm. Assoc. 2022, 62, 1816–1822.e2. [Google Scholar] [CrossRef]
- Berenbrok, L.A.; Gabriel, N.; Coley, K.C.; Hernandez, I. Evaluation of frequency of encounters with primary care physicians vs visits to community pharmacies among Medicare beneficiaries. JAMA Netw. Open 2020, 3, e209132. [Google Scholar] [CrossRef]
- Yue, Z.; McCormick, N.P.; Ezeala, O.M.; Durham, S.H.; Westrick, S.C. EMSIG: Uncovering Factors Influencing COVID-19 Vaccination Across Different Subgroups Characterized by Embedding-Based Spatial Information Gain. Vaccines 2024, 12, 1253. [Google Scholar] [CrossRef] [PubMed]
- USAFacts. Alabama Coronavirus Vaccination Progress. Available online: https://usafacts.org/visualizations/covid-vaccine-tracker-states/state/alabama/ (accessed on 4 June 2025).
- QualtricsXM Understand Customers and Employees. Act When It Counts. Available online: https://www.qualtrics.com/ (accessed on 2 January 2025).
- Ngorsuraches, S.; Lerkiatbundit, S.; Li, S.C.; Treesak, C.; Sirithorn, R.; Korwiwattanakarn, M. Development and validation of the patient trust in community pharmacists (TRUST-Ph) scale: Results from a study conducted in Thailand. Res. Soc. Adm. Pharm. 2008, 4, 272–283. [Google Scholar] [CrossRef] [PubMed]
- Peek, M.E.; Gorawara-Bhat, R.; Quinn, M.T.; Odoms-Young, A.; Wilson, S.C.; Chin, M.H. Patient Trust in Physicians and Shared Decision-Making Among African-Americans with Diabetes. Health Commun. 2013, 28, 616–623. [Google Scholar] [CrossRef]
- Kraetschmer, N.; Sharpe, N.; Urowitz, S.; Deber, R.B. How does trust affect patient preferences for participation in decision-making? Health Expect. 2004, 7, 317–326. [Google Scholar] [CrossRef]
- Davison, B.J.; Breckon, E. Factors influencing treatment decision making and information preferences of prostate cancer patients on active surveillance. Patient Educ. Couns. 2012, 87, 369–374. [Google Scholar] [CrossRef]
- Croker, J.E.; Swancutt, D.R.; Roberts, M.J.; Abel, G.A.; Roland, M.; Campbell, J.L. Factors affecting patients’ trust and confidence in GPs: Evidence from the English national GP patient survey. BMJ Open 2013, 3, e002762. [Google Scholar] [CrossRef] [PubMed]
- Salt, E.; Rayens, M.K.; Kerr, A.M.; Alikhan, M.; Crofford, L.J. Examining rheumatoid arthritis patients’ trust in their provider over time: The effects of demographic factors and accessing sources of information. Orthop. Nurs. 2015, 34, 159–165. [Google Scholar] [CrossRef]
- Kim, J.H. Multicollinearity and misleading statistical results. Korean J. Anesth. 2019, 72, 558–569. [Google Scholar] [CrossRef]
- Hu, L.T.; Bentler, P.M. Cutoff criteria for fit indexes in covariance structure analysis: Conventional criteria versus new alternatives. Struct. Equ. Model. A Multidiscip. J. 1999, 6, 1–55. [Google Scholar] [CrossRef]
- West, S.G.; Taylor, A.B.; Wu, W. Model fit and model selection in structural equation modeling. In Handbook of Structural Equation Modeling; The Guilford Press: Guilford, NY, USA, 2012. [Google Scholar]
- Kim, H.; Ku, B.; Kim, J.Y.; Park, Y.J.; Park, Y.B. Confirmatory and Exploratory Factor Analysis for Validating the Phlegm Pattern Questionnaire for Healthy Subjects. Evid. Based Complement. Altern. Med. 2016, 2016, 2696019. [Google Scholar] [CrossRef] [PubMed]
- Akmatov, M.K.; Rübsamen, N.; Deyneko, I.V.; Karch, A.; Mikolajczyk, R.T. Poor knowledge of vaccination recommendations and negative attitudes towards vaccinations are independently associated with poor vaccination uptake among adults—Findings of a population-based panel study in Lower Saxony, Germany. Vaccine 2018, 36, 2417–2426. [Google Scholar] [CrossRef]
- Tan, L. Adult vaccination: Now is the time to realize an unfulfilled potential. Hum. Vaccin. Immunother. 2015, 11, 2158–2166. [Google Scholar] [CrossRef]
- Kaljee, L.M.; Kilgore, P.; Prentiss, T.; Lamerato, L.; Moreno, D.; Arshad, S.; Zervos, M. “You need to be an advocate for yourself”: Factors associated with decision-making regarding influenza and pneumococcal vaccine use among US older adults from within a large metropolitan health system. Hum. Vaccin. Immunother. 2017, 13, 206–212. [Google Scholar] [CrossRef][Green Version]
- Shen, A.K.; Michel, J.J.; Langford, A.T.; Sobczyk, E.A. Shared clinical decision-making on vaccines: Out of sight, out of mind. J. Am. Med. Inf. Assoc. 2021, 28, 2523–2525. [Google Scholar] [CrossRef] [PubMed]
- Chido-Amajuoyi, O.G.; Osaghae, I.; Onyeaka, H.K.; Shete, S. Barriers to the assessment and recommendation of HPV vaccination among healthcare providers in Texas. Vaccine X 2024, 18, 100471. [Google Scholar] [CrossRef]
- Gellin, B.G.; Shen, A.K.; Fish, R.; Zettle, M.A.; Uscher-Pines, L.; Ringel, J.S. The National Adult Immunization Plan: Strengthening Adult Immunization Through Coordinated Action. Am. J. Prev. Med. 2016, 51, 1079–1083. [Google Scholar] [CrossRef]
- Feemster, K.A. Building vaccine acceptance through communication and advocacy. Hum. Vaccines Immunother. 2020, 16, 1004–1006. [Google Scholar] [CrossRef]
- Labbé, S.; Bacon, S.L.; Wu, N.; Ribeiro, P.A.; Boucher, V.G.; Stojanovic, J.; Voisard, B.; Deslauriers, F.; Tremblay, N.; Hébert-Auger, L.; et al. Addressing vaccine hesitancy: A systematic review comparing the efficacy of motivational versus educational interventions on vaccination uptake. Transl. Behav. Med. 2025, 15, ibae069. [Google Scholar] [CrossRef]
- Kane, H.L.; Halpern, M.T.; Squiers, L.B.; Treiman, K.A.; McCormack, L.A. Implementing and evaluating shared decision making in oncology practice. CA Cancer J. Clin. 2014, 64, 377–388. [Google Scholar] [CrossRef]
- Cuevas, A.G.; O’Brien, K.; Saha, S. African American experiences in healthcare:“I always feel like I’m getting skipped over”. Health Psychol. 2016, 35, 987. [Google Scholar] [CrossRef]
- Alsan, M.; Wanamaker, M. Tuskegee and the health of black men. Q. J. Econ. 2018, 133, 407–455. [Google Scholar] [CrossRef]
- Thompson, H.S.; Manning, M.; Mitchell, J.; Kim, S.; Harper, F.W.; Cresswell, S.; Johns, K.; Pal, S.; Dowe, B.; Tariq, M.; et al. Factors associated with racial/ethnic group–based medical mistrust and perspectives on COVID-19 vaccine trial participation and vaccine uptake in the US. JAMA Netw. Open 2021, 4, e2111629. [Google Scholar] [CrossRef] [PubMed]
- Mhaimeed, N.; Mhaimeed, N.; Mhaimeed, O.; Alanni, J.; Burney, Z.; Elshafeey, A.; Laws, S.A.; Choi, J.J. Shared decision making with black patients: A scoping review. Patient Educ. Couns. 2023, 110, 107646. [Google Scholar] [CrossRef] [PubMed]
- Institute of Medicine (US) Committee on Understanding and Eliminating Racial and Ethnic Disparities in Health Care. Unequal Treatment: Confronting Racial and Ethnic Disparities in Health Care; Smedley, B.D., Stith, A.Y., Nelson, A.R., Eds.; National Academies Press (US): Washington DC, USA, 2003. [Google Scholar]
- ASPE. Health Care Access and Coverage. In HHS ASPE Reports; Office of the Assistant Secretary for Planning and Evaluation (ASPE): Washington, DC, USA, 2024. [Google Scholar]
- Reuter, M.; Herke, M.; Richter, M.; Diehl, K.; Hoffmann, S.; Pischke, C.R.; Dragano, N. Young people’s health and well-being during the school-to-work transition: A prospective cohort study comparing post-secondary pathways. BMC Public Health 2022, 22, 1823. [Google Scholar] [CrossRef]
- Deeks, A.; Lombard, C.; Michelmore, J.; Teede, H. The effects of gender and age on health related behaviors. BMC Public Health 2009, 9, 213. [Google Scholar] [CrossRef]
- Newman, T.V.; San-Juan-Rodriguez, A.; Parekh, N.; Swart, E.C.; Klein-Fedyshin, M.; Shrank, W.H.; Hernandez, I. Impact of community pharmacist-led interventions in chronic disease management on clinical, utilization, and economic outcomes: An umbrella review. Res. Soc. Adm. Pharm. 2020, 16, 1155–1165. [Google Scholar] [CrossRef] [PubMed]
- Pot, M.; Paulussen, T.G.; Ruiter, R.A.; Eekhout, I.; De Melker, H.E.; Spoelstra, M.E.; Van Keulen, H.M. Effectiveness of a web-based tailored intervention with virtual assistants promoting the acceptability of HPV vaccination among mothers of invited girls: Randomized controlled trial. J. Med. Internet Res. 2017, 19, e312. [Google Scholar] [CrossRef]
- Geana, M.V.; Rabb, N.; Sloman, S. Walking the party line: The growing role of political ideology in shaping health behavior in the United States. SSM-Popul. Health 2021, 16, 100950. [Google Scholar] [CrossRef]
- Parikh, R.K. Fighting for the Reputation of Vaccines: Lessons From American Politics. Pediatrics 2008, 121, 621–622. [Google Scholar] [CrossRef]
- Facciani, M.; Lazić, A.; Viggiano, G.; McKay, T. Political network composition predicts vaccination attitudes. Soc. Sci. Med. 2023, 328, 116004. [Google Scholar] [CrossRef]
- Muscat, D.M.; Shepherd, H.L.; Nutbeam, D.; Trevena, L.; McCaffery, K.J. Health literacy and shared decision-making: Exploring the relationship to enable meaningful patient engagement in healthcare. J. Gen. Intern. Med. 2021, 36, 521–524. [Google Scholar] [CrossRef]
- Brabers, A.E.; Rademakers, J.J.; Groenewegen, P.P.; Van Dijk, L.; De Jong, J.D. What role does health literacy play in patients’ involvement in medical decision-making? PLoS ONE 2017, 12, e0173316. [Google Scholar] [CrossRef]
- Elwyn, G. Shared decision making: What is the work? Patient Educ. Couns. 2021, 104, 1591–1595. [Google Scholar] [CrossRef]
- Politi, M.C.; Street, R.L., Jr. The importance of communication in collaborative decision making: Facilitating shared mind and the management of uncertainty. J. Eval. Clin. Pract. 2011, 17, 579–584. [Google Scholar] [CrossRef] [PubMed]
- Dennison Himmelfarb, C.R.; Beckie, T.M.; Allen, L.A.; Commodore-Mensah, Y.; Davidson, P.M.; Lin, G.; Lutz, B.; Spatz, E.S. and American Heart Association Council on Cardiovascular and Stroke Nursing. Shared Decision-Making and Cardiovascular Health: A Scientific Statement From the American Heart Association. Circulation 2023, 148, 912–931. [Google Scholar] [CrossRef] [PubMed]
- Gurtner, C.; Lohrmann, C.; Schols, J.; Hahn, S. Shared Decision Making in the Psychiatric Inpatient Setting: An Ethnographic Study about Interprofessional Psychiatric Consultations. Int. J. Environ. Res. Public Health 2022, 19, 3644. [Google Scholar] [CrossRef]
- Waddell, A.; Lennox, A.; Spassova, G.; Bragge, P. Barriers and facilitators to shared decision-making in hospitals from policy to practice: A systematic review. Implement. Sci. 2021, 16, 74. [Google Scholar] [CrossRef] [PubMed]
- Oerlemans, A.J.M.; Knippenberg, M.L.; Olthuis, G.J. Learning shared decision-making in clinical practice. Patient Educ. Couns. 2021, 104, 1206–1212. [Google Scholar] [CrossRef] [PubMed]
- Keij, S.M.; Lie, H.C.; Laidsaar-Powell, R.; Kunneman, M.; de Boer, J.E.; Moaddine, S.; Stiggelbout, A.M.; Pieterse, A.H. Patient-related characteristics considered to affect patient involvement in shared decision making about treatment: A scoping review of the qualitative literature. Patient Educ. Couns. 2023, 111, 107677. [Google Scholar] [CrossRef] [PubMed]
- Becker, C.; Gross, S.; Gamp, M.; Beck, K.; Amacher, S.A.; Mueller, J.; Bohren, C.; Blatter, R.; Schaefert, R.; Schuetz, P.; et al. Patients’ Preference for Participation in Medical Decision-Making: Secondary Analysis of the BEDSIDE-OUTSIDE Trial. J. Gen. Intern. Med. 2023, 38, 1180–1189. [Google Scholar] [CrossRef]
- Edgman-Levitan, S.; Schoenbaum, S.C. Patient-centered care: Achieving higher quality by designing care through the patient’s eyes. Isr. J. Health Policy Res. 2021, 10, 21. [Google Scholar] [CrossRef] [PubMed]

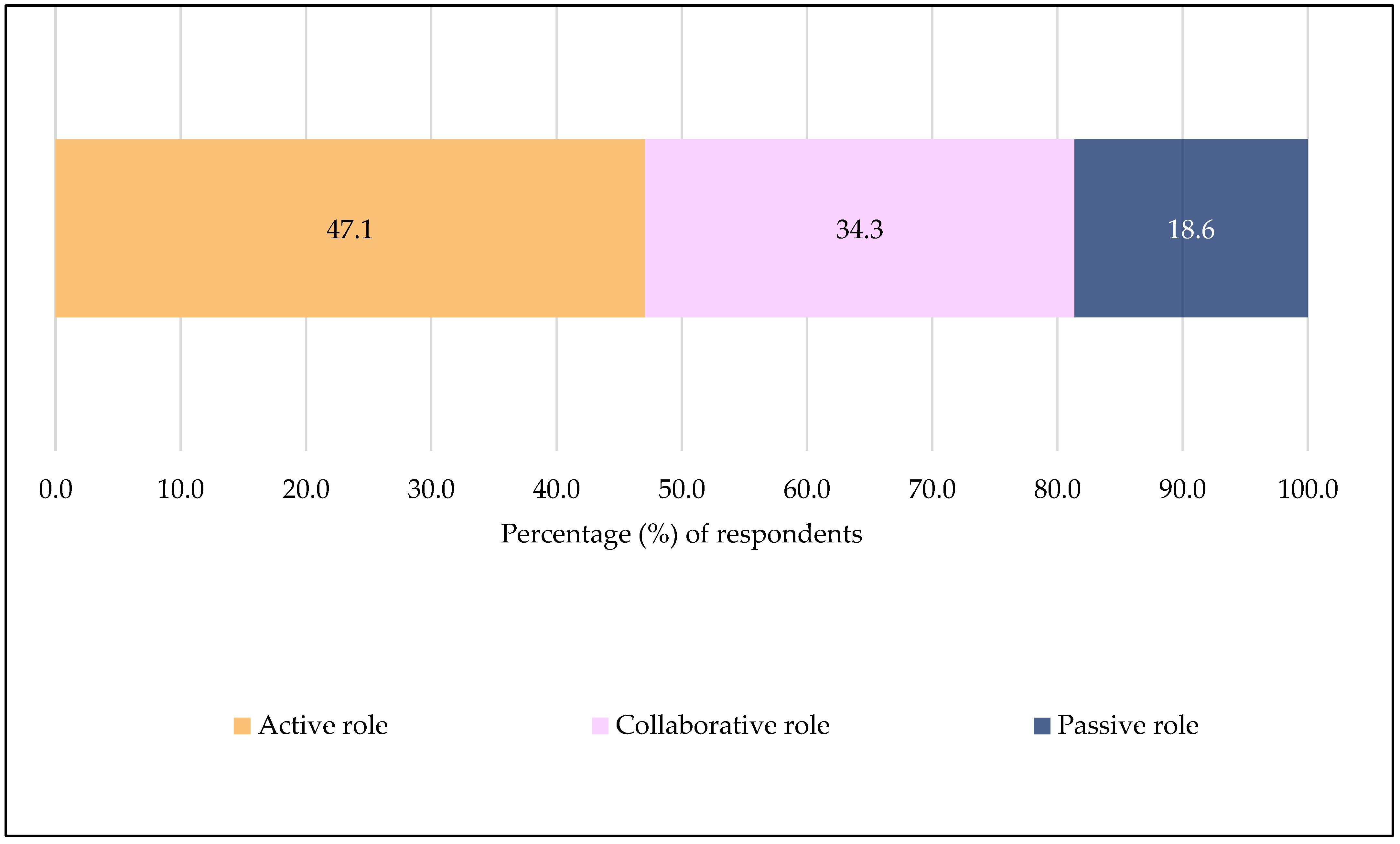
| Variable | n (%) |
|---|---|
| Sex | |
| Male | 279 (30.2) |
| Female | 645 (69.8) |
| Race | |
| Asian | 10 (1.1) |
| American Indian or Alaskan Native | 12 (1.3) |
| Black | 194 (21.0) |
| White | 648 (70.1) |
| Multi-racial | 37 (4.0) |
| Other | 23 (2.5) |
| Ethnicity | |
| Hispanic or Latino | 60 (6.5) |
| Not Hispanic or Latino | 864 (93.5) |
| Age | |
| 18–24 | 104 (11.3) |
| 25–34 | 134 (14.5) |
| 35–44 | 172 (18.6) |
| 45–54 | 184 (19.9) |
| 55–64 | 149 (16.1) |
| 65+ | 181 (19.6) |
| Highest Degree Obtained | |
| Less than high school | 58 (6.3) |
| High school diploma or Graduate Equivalency Degree (GED) | 451 (48.8) |
| Associate’s degree or vocational certificate | 218 (23.6) |
| 4-year bachelor’s degree or higher | 197 (21.3) |
| Marital Status | |
| Married | 382 (41.3) |
| Not Married | 542 (58.7) |
| Employment Status | |
| Disabled, not able to work | 143 (15.5) |
| Not Employed | 177 (19.2) |
| Retired | 198 (21.4) |
| Employed | 406 (43.9) |
| Household Income Level | |
| $0–$30,000 | 344 (37.2) |
| $30,001–$60,000 | 289 (31.3) |
| $60,001–$90,000 | 131 (14.2) |
| $90,001–$120,000 | 54 (5.8) |
| $120,000+ | 56 (6.1) |
| I choose not to say | 50 (5.4) |
| Political Affiliation | |
| Democrat | 197 (21.3) |
| Independent | 220 (23.8) |
| Republican | 391 (42.3) |
| Other | 116 (12.6) |
| Insurance Status | |
| Not Insured | 75 (8.1) |
| Insured | 849 (91.9) |
| Residence a | |
| Rural | 379 (41.0) |
| Urban | 538 (58.2) |
| Other b | 7 (0.8) |
| Level of confidence in understanding health-related information | |
| Highly confident | 516 (55.8) |
| Moderately confident | 292 (31.6) |
| Slightly/not at all confident | 116 (12.6) |
| Frequency of healthcare provider visits regarding health concerns in 2023 | |
| 0 | 143 (15.5) |
| 1 | 128 (13.9) |
| 2–4 | 396 (42.9) |
| 5–7 | 133 (14.4) |
| 8 or more | 124 (13.4) |
| Median (range) | |
| Total number of chronic conditions | 1.0 (22.0) |
| Variable | n (%) |
|---|---|
| Awareness of SCDM vaccination recommendations | |
| Not aware | 423 (45.8) |
| Aware | 501 (54.2) |
| Degree of willingness to engage in SCDM with a pharmacist | |
| Totally willing | 131 (14.2) |
| Moderately willing | 143 (15.5) |
| Somewhat willing | 288 (31.2) |
| Somewhat unwilling | 136 (14.7) |
| Moderately unwilling | 71 (7.7) |
| Totally unwilling | 155 (16.8) |
| Factor | Effects | Unadjusted Odds Ratio (95% Confidence Interval) | p-Value | Adjusted Odds Ratio (95% Confidence Interval) a | p-Value a |
|---|---|---|---|---|---|
| Sex | Female vs. male | 1.060 (0.795, 1.412) | 0.692 | ||
| Race | Asian vs. White | 0.906 (0.253, 3.242) | 0.879 | 0.994 (0.250, 3.948) | 0.994 |
| American Indian or Alaskan Native vs. White | 1.208 (0.360, 4.054) | 0.760 | 1.541 (0.395, 6.006) | 0.534 | |
| Black vs. White | 0.698 (0.505, 0.965) | 0.030 | 0.499 (0.326, 0.763) | 0.001 | |
| Multiracial vs. White | 1.258 (0.621, 2.550) | 0.524 | 1.433(0.643, 3.192) | 0.379 | |
| Other vs. White | 1.132 (0.473, 2.710) | 0.780 | 1.133 (0.430, 2.984) | 0.800 | |
| Ethnicity | Hispanic or Latino vs. not Hispanic or Latino | 1.039 (0.606, 1.779) | 0.890 | ||
| Age | 25–34 vs. 18–24 | 1.827 (1.087, 3.071) | 0.023 | 1.913 (1.070, 3.419) | 0.029 |
| 35–44 vs. 18–24 | 1.353 (0.830, 2.204) | 0.225 | 1.350 (0.774, 2.354) | .290 | |
| 45–54 vs. 18–24 | 1.746 (1.074, 2.838) | 0.025 | 1.760 (0.999, 3.100) | 0.050 | |
| 55–64 vs. 18–24 | 2.362 (1.410, 3.957) | 0.001 | 1.760 (0.939, 3.299) | 0.078 | |
| 65+ vs. 18–24 | 2.380 (1.450, 3.908) | 0.001 | 1.355 (0.654, 2.806) | 0.414 | |
| Highest degree obtained | High school diploma or Graduate Equivalency Degree (GED) vs. less than high school | 2.580 (1.463, 4.551) | 0.001 | 2.855 (1.518, 5.369) | 0.001 |
| Associate’s degree or vocational certificate vs. less than high school | 3.292 (1.800, 6.019) | 0.000 | 3.472 (1.753, 6.880) | 0.000 | |
| 4-year bachelor’s degree or higher vs. less than high school | 3.497 (1.897, 6.447) | <0.0001 | 3.179 (1.562, 6.471) | 0.001 | |
| Marital status | Married vs. not married | 1.133 (0.866, 1.483) | 0.362 | ||
| Employment status | Disabled, not able to work vs. employed | 1.164 (0.788, 1.720) | 0.445 | 1.091 (0.675, 1.766) | 0.722 |
| Not Employed vs. employed | 0.990 (0.692, 1.415) | 0.954 | 1.090 (0.727, 1.634) | 0.675 | |
| Retired vs. employed | 1.634 (1.140, 2.343) | 0.008 | 1.365 (0.782, 2.384) | 0.274 | |
| Household income level | $30,001–$60,000 vs. $0–$30,000 | 1.203 (0.874, 1.657) | 0.257 | ||
| $60,001–$90,000 vs. $0–$30,000 | 1.393 (0.916, 2.118) | 0.122 | |||
| $90,001–$120,000 vs. $0–$30,000 | 1.457 (0.796, 2.668) | 0.222 | |||
| $120,000+ vs. $0–$30,000 | 1.126 (0.632, 2.006) | 0.687 | |||
| I choose not to say vs. $0–$30,000 | 0.855 (0.471, 1.552) | 0.607 | |||
| Political affiliation | Democrat vs. republican | 1.586 (1.100, 2.286) | 0.014 | 2.133 (1.331, 3.421) | 0.002 |
| Independent vs. republican | 1.077 (0.768, 1.511) | 0.667 | 1.441 (0.968, 2.146) | 0.072 | |
| Other vs. republican | 0.633 (0.417, 0.960) | 0.031 | 1.085 (0.671, 1.757) | 0.738 | |
| insurance | Not insured vs. insured | 0.675 (0.421, 1.084) | 0.104 | ||
| Residence b,c | Rural vs. urban | 1.018 (0.778, 1.333) | 0.895 | ||
| Confidence in understanding health-related information | Highly confident vs. (low) slightly/not at all confident | 2.451 (1.626, 3.695) | <0.0001 | 1.655 (1.052, 2.605) | 0.029 |
| Moderately confident vs. (low) slightly/not at all confident | 2.523 (1.625, 3.917) | <0.0001 | 2.118 (1.314, 3.416) | 0.002 | |
| Frequency of healthcare provider visits regarding health concerns in 2023 | 1 vs. 0 | 1.290 (0.800, 2.080) | 0.297 | 1.052 (0.620, 1.786) | 0.850 |
| 2–4 vs. 0 | 2.017 (1.369, 2.971) | 0.000 | 1.489 (0.965, 2.297) | 0.072 | |
| 5–7 vs. 0 | 2.157 (1.326, 3.508) | 0.002 | 1.578 (0.913, 2.728) | 0.102 | |
| 8 or more vs. 0 | 2.078 (1.267, 3.406) | 0.004 | 1.395 (0.787, 2.472) | 0.254 | |
| Total number of chronic conditions | 1.088 (0.986, 1.199) | 0.092 | |||
| Benevolence | 2.443 (1.970, 3.030) | <0.0001 | 1.780 (1.254, 2.524) | 0.001 | |
| Technical competence | 2.489 (1.973, 3.139) | <0.0001 | 1.163 (0.765, 1.769) | 0.480 | |
| Communication | 2.387 (1.916, 2.975) | <0.0001 | 1.420 (1.009, 1.999) | 0.045 | |
| Effect Type | Hypothesis | β (95% CI) | Standard Error | p Value |
|---|---|---|---|---|
| Direct effect of patient’s trust in community pharmacists on vaccination decision control preference | Ha1 | 0.23 (0.09, 0.37) | 0.07 | 0.001 |
| Direct effect of patient’s trust in community pharmacists on willingness to engage in SCDM with community pharmacists | Ha2 | 0.67 (0.54, 0.79) | 0.07 | 0.000 |
| Direct effect of willingness to engage in SCDM with community pharmacists on vaccination decision control preference | Ha3 | 0.34 (0.24, 0.43) | 0.05 | 0.000 |
| Indirect effect of patient’s trust in community pharmacists on vaccination decision control preference through willingness to engage in SCDM with community pharmacists | Ha4 = Ha2 × Ha3 | 0.23 (0.16, 0.29) | 0.04 | 0.000 |
| Total effect of patient’s trust in community pharmacists on vaccination decision control preference | Ha1 + Ha4 | 0.46 (0.33, 0.58) | 0.06 | 0.000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ezeala, O.M.; McCormick, N.P.; Ezeja, L.; Jaradat, S.K.; Durham, S.H.; Westrick, S.C. From Trust to Choice: A Cross-Sectional Survey of How Patient Trust in Pharmacists Shapes Willingness and Vaccination Decision Control Preferences. Int. J. Environ. Res. Public Health 2025, 22, 1525. https://doi.org/10.3390/ijerph22101525
Ezeala OM, McCormick NP, Ezeja L, Jaradat SK, Durham SH, Westrick SC. From Trust to Choice: A Cross-Sectional Survey of How Patient Trust in Pharmacists Shapes Willingness and Vaccination Decision Control Preferences. International Journal of Environmental Research and Public Health. 2025; 22(10):1525. https://doi.org/10.3390/ijerph22101525
Chicago/Turabian StyleEzeala, Oluchukwu M., Nicholas P. McCormick, Lotanna Ezeja, Sara K. Jaradat, Spencer H. Durham, and Salisa C. Westrick. 2025. "From Trust to Choice: A Cross-Sectional Survey of How Patient Trust in Pharmacists Shapes Willingness and Vaccination Decision Control Preferences" International Journal of Environmental Research and Public Health 22, no. 10: 1525. https://doi.org/10.3390/ijerph22101525
APA StyleEzeala, O. M., McCormick, N. P., Ezeja, L., Jaradat, S. K., Durham, S. H., & Westrick, S. C. (2025). From Trust to Choice: A Cross-Sectional Survey of How Patient Trust in Pharmacists Shapes Willingness and Vaccination Decision Control Preferences. International Journal of Environmental Research and Public Health, 22(10), 1525. https://doi.org/10.3390/ijerph22101525







