Effects of a Hypocaloric Diet and Physical Training on Ventilatory Efficiency in Women with Metabolic Syndrome: A Prospective Interventional Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Study Design and Ethical Approval
2.3. Body Composition Assessment
2.4. Laboratory Tests
2.5. Cardiopulmonary Exercise Test
2.6. Oxygen Uptake Efficiency Slope
2.7. Ventilatory Anaerobic Threshold
2.8. Physical Training Program
2.9. Hypocaloric Diet Protocol
2.10. Statistical Analysis
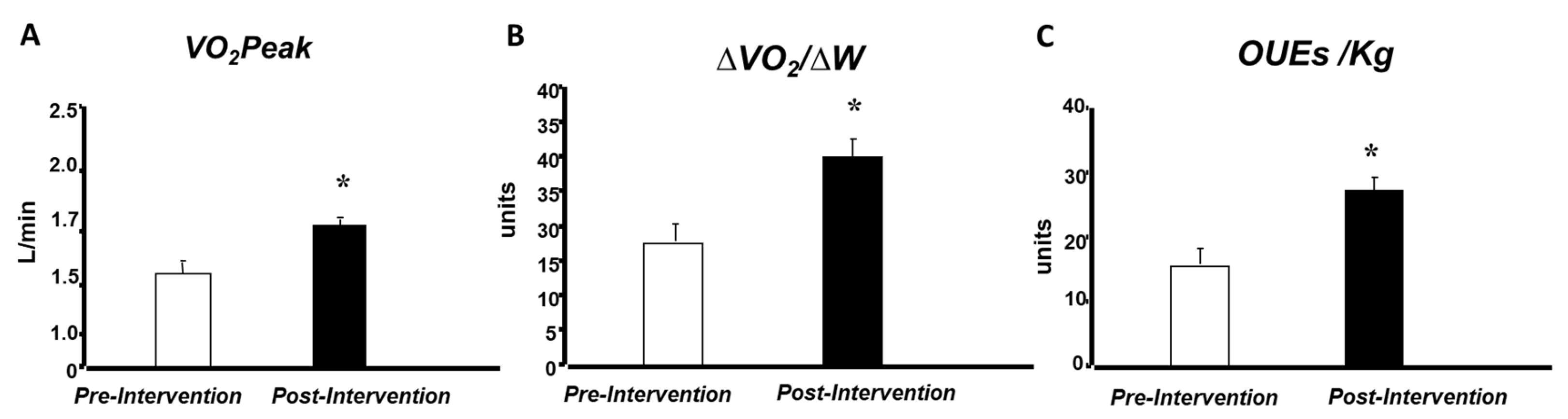
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| MetS | Metabolic syndrome |
| HD | Hypocaloric diet |
| PT | Physical training |
| CPET | Cardiopulmonary exercise test |
| OUES | Oxygen uptake efficiency slope |
| VO2/W | Oxygen uptake-to-workload ratio |
References
- Garg, G.K. The Alarming Rise of Lifestyle Diseases and Their Impact on Public Health: A Comprehensive Overview and Strategies for Overcoming the Epidemic. J. Res. Med. Sci. 2025, 30, 30–31. [Google Scholar] [CrossRef]
- Riley, L.; Guthold, R.; Cowan, M.; Savin, S.; Bhatti, L.; Armstrong, T.; Bonita, R. The World Health Organization STEPwise Approach to Noncommunicable Disease Risk-Factor Surveillance: Methods, Challenges, and Opportunities. Am. J. Public Health 2016, 106, 74–78. [Google Scholar] [CrossRef] [PubMed]
- Arshad, M.S.; Khan, U.; Patel, K.; Butt, M.N.; Khan, S. The Interplay of Factors in Metabolic Syndrome: Understanding Its Pathophysiology and Diagnostic Criteria. Mol. Med. 2024, 30, 19. [Google Scholar]
- Roberts, C.K.; Hevener, A.L.; Barnard, R.J. Metabolic Syndrome and Insulin Resistance: Underlying Causes and Modification by Exercise Training. Compr. Physiol. 2013, 3, 1–58. [Google Scholar] [CrossRef]
- Rask-Madsen, C.; Kahn, C.R. Tissue-Specific Insulin Signaling, Metabolic Syndrome and Cardiovascular Disease. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 2052–2059. [Google Scholar] [CrossRef]
- Trombetta, I.C.; Somers, V.K.; Maki-Nunes, C.; Drager, L.F.; Toschi-Dias, E.; Alves, M.J.; Fraga, R.F.; Rondon, M.U.P.B.; Bechara, M.G.; Lorenzi-Filho, G.; et al. Consequences of Comorbid Sleep Apnea in the Metabolic Syndrome—Implications for Cardiovascular Risk. Sleep 2010, 33, 1193–1199. [Google Scholar] [CrossRef]
- Wu, Y.S.; Tzeng, W.C.; Chu, C.M.; Wang, W.Y. Metabolic Syndrome and Its Related Factors among Hospital Employees: A Population-Based Cohort Study. Int. J. Environ. Res. Public Health 2021, 18, 9826. [Google Scholar] [CrossRef]
- Church, T. Exercise in Obesity, Metabolic Syndrome, and Diabetes. Prog. Cardiovasc. Dis. 2011, 53, 412–418. [Google Scholar] [CrossRef]
- Teixeira, C.S.; Corrêa, M.A.; Rocco, D.D.F.M.; Gardenghi, G.; Carvalho, J.C.; Medeiros, A.; da Silva, A.G. Patients with Metabolic Syndrome Present Decreased Cardiorespiratory Fitness Facing Maximum Progressive Exercise. Rev. Bras. Fisiol. Exerc. 2021, 20, 304–314. [Google Scholar] [CrossRef]
- Dieli-Conwright, C.M.; Courneya, K.S.; Demark-Wahnefried, W.; Sami, N.; Lee, K.; Buchanan, T.A.; Spicer, D.V.; Tripathy, D.; Bernstein, L.; Mortimer, J.E. Effects of Aerobic and Resistance Exercise on Metabolic Syndrome in Overweight or Obese Survivors of Breast Cancer: A Randomized Controlled Trial. J. Clin. Oncol. 2018, 36, 875–883. [Google Scholar] [CrossRef]
- Drinkard, B.; Roberts, M.D.; Ranzenhofer, L.M.; Han, J.C.; Yanoff, L.B.; Merke, D.P.; Savastano, D.M.; Brady, S.; Yanovski, J.A. Oxygen-Uptake Efficiency Slope as a Determinant of Fitness in Overweight Adolescents. Med. Sci. Sports Exerc. 2007, 39, 1811–1816. [Google Scholar] [CrossRef]
- Masenga, S.K.; Kabwe, L.S.; Chakulya, M.; Kirabo, A. Mechanisms of Oxidative Stress in Metabolic Syndrome. Int. J. Mol. Sci. 2023, 24, 7898. [Google Scholar] [CrossRef]
- Phillips, D.B.; Collins, S.É.; Stickland, M.K. Measurement and Interpretation of Exercise Ventilatory Efficiency. Front. Physiol. 2020, 11, 659. [Google Scholar] [CrossRef] [PubMed]
- Said, M.A.; Abdelmoneem, M.; Alibrahim, M.C.; Elsebee, M.A.; Kotb, A.A.H. Effects of Diet versus Diet Plus Aerobic and Resistance Exercise on Metabolic Syndrome in Obese Young Men. J. Exerc. Sci. Fit. 2020, 18, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Niezgoda, N.; Chomiuk, T.; Kasiak, P.; Mamcarz, A.; Śliż, D. The Impact of Physical Activity on Weight Loss in Relation to the Pillars of Lifestyle Medicine—A Narrative Review. Nutrients 2025, 17, 1095. [Google Scholar] [CrossRef] [PubMed]
- Valsdottir, T.D.; Øvrebø, B.; Falck, T.M.; Litleskare, S.; Johansen, E.I.; Henriksen, C.; Jensen, J. Low-Carbohydrate High-Fat Diet and Exercise: Effect of a 10-Week Intervention on Body Composition and CVD Risk Factors in Overweight and Obese Women—A Randomized Controlled Trial. Nutrients 2020, 13, 110. [Google Scholar]
- Schulz, K.F.; Altman, D.G.; Moher, D.; CONSORT Group. CONSORT 2010 Statement: Updated Guidelines for Reporting Parallel Group Randomised Trials. Trials 2010, 11, 32. [Google Scholar] [CrossRef]
- Barroso, W.K.S.; Rodrigues, C.I.S.; Bortolotto, L.A.M.; Mota-Gomes, M.A.; Brandão, A.A.; Feitosa, A.D.M.; Machado, C.A.; Poli-de-Figueiredo, C.E.; Amodeo, C.; Mion Júnior, D.; et al. Brazilian Guidelines on Hypertension—2020. Arq. Bras. Cardiol. 2021, 116, 516–658. [Google Scholar]
- National Cholesterol Education Program (NCEP) Expert Panel. Third Report of the NCEP Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Circulation 2002, 106, 3143–3421. [Google Scholar] [CrossRef]
- São Paulo State Cardiology Society (SOCESP). National Guidelines and Cardiovascular Health; São Paulo State Cardiology Society (SOCESP): São Paulo, Brazil, 2021. [Google Scholar]
- Skinner, J.S.; McLellan, T.M. The Transition from Aerobic to Anaerobic Metabolism. Res. Q. Exerc. Sport 1980, 51, 234–248. [Google Scholar] [CrossRef]
- Baba, R.; Nagashima, M.; Goto, M.; Nagano, Y.; Yokota, M.; Tauchi, N.; Nishibata, K. Oxygen Uptake Efficiency Slope: A New Index of Cardiorespiratory Functional Reserve Derived from the Relation Between Oxygen Uptake and Minute Ventilation During Incremental Exercise. J. Am. Coll. Cardiol. 1996, 28, 1567–1572. [Google Scholar] [CrossRef] [PubMed]
- Hollenberg, M.; Tager, I.B. Oxygen Uptake Efficiency Slope: An Index of Exercise Performance and Cardiopulmonary Reserve Requiring Only Submaximal Exercise. J. Am. Coll. Cardiol. 2000, 36, 194–201. [Google Scholar] [CrossRef] [PubMed]
- Van Laethem, C.; Bartunek, J.; Goethals, M.; Nellens, P.; Andries, E.; Vanderheyden, M. Oxygen Uptake Efficiency Slope, a New Submaximal Parameter in Evaluating Exercise Capacity in Chronic Heart Failure Patients. Am. Heart J. 2005, 149, 175–180. [Google Scholar] [CrossRef] [PubMed]
- Hansen, J.E.; Sue, D.Y.; Wasserman, K. Relation of Oxygen Uptake to Work Rate in Normal Men and Men with Circulatory Disorders. Am. J. Cardiol. 1987, 57, 669–674. [Google Scholar] [CrossRef]
- Beaver, W.L.K.; Wasserman, K.; Whipp, B.J. A New Method for Detecting Anaerobic Threshold by Gas Exchange. J. Appl. Physiol. 1986, 60, 2020–2027. [Google Scholar] [CrossRef]
- Reinhard, W.; Hoffmann, G.; Wünsche, H. Pulmonary Ventilation in Relation to Oxygen Uptake and Carbon Dioxide Production During Incremental Load Work. Eur. J. Appl. Physiol. Occup. Physiol. 1979, 42, 173–183. [Google Scholar]
- Ramos, R.P.; Ota-Arakaki, J.S.; Alencar, M.C.; Ferreira, A.V.M.; Nery, L.E.; Neder, J.A. Exercise Oxygen Uptake Efficiency Slope Independently Predicts Poor Outcome in Pulmonary Arterial Hypertension. Eur. Respir. J. 2014, 43, 1507–1510. [Google Scholar] [CrossRef]
- Després, J.P. Body Fat Distribution and Risk of Cardiovascular Disease: An Update. Circulation 2012, 126, 1301–1313. [Google Scholar] [CrossRef]
- Singh, S.; Singh, S.K. Prevalence of Metabolic Syndrome in India: A Systematic Review and Meta-Analysis. PLoS ONE 2021, 16, e0240971. [Google Scholar]
- Ross, R.; Neeland, I.J.; Yamashita, S.; Shai, I.; Seidell, J.; Magni, P.; Santos, R.D.; Arsenault, B.; Cuevas, A.; Hu, F.B.; et al. Waist Circumference as a Vital Sign in Clinical Practice: A Consensus Statement from the IAS and ICCR Working Group on Visceral Obesity. Nat. Rev. Endocrinol. 2020, 16, 177–189. [Google Scholar] [CrossRef]
- Kodama, S.; Saito, K.; Tanaka, S.; Maki, M.; Yachi, Y.; Asumi, M.; Sugawara, A.; Totsuka, K.; Shimano, H.; Ohashi, Y.; et al. Cardiorespiratory Fitness as a Quantitative Predictor of All-Cause Mortality and Cardiovascular Events in Healthy Men and Women: A Meta-Analysis. JAMA 2009, 301, 2024–2035. [Google Scholar] [CrossRef]
- Onofre, A.S.; da Silva Santos, A.V.; Vitorino, P.V.O.; de Castro, C.L.B.; Moreira, S.R.; Lima, R.M. Oxygen Uptake Efficiency Slope in Obese Women: Responses to Maximal and Submaximal Exercise Testing. Clin. Physiol. Funct. Imaging 2017, 37, 393–398. [Google Scholar]
- Hansen, J.E.; Sue, D.Y.; Wasserman, K. Predicted Values for Clinical Exercise Testing. Am. Rev. Respir. Dis. 1987, 129, S49–S55. [Google Scholar] [CrossRef]
- Prado, D.M.; Rocco, E.A.; Silva, A.G.; Silva, P.F.; Lazzari, J.M.; Assumpção, G.L.; Thies, S.B.; Suzaki, C.Y.; Puig, R.S.; Furlan, V. The influence of aerobic fitness status on ventilatory efficiency in patients with coronary artery disease. Clinics 2015, 70, 46–51. [Google Scholar]
- Sun, X.G.; Hansen, J.E.; Garatachea, N.; Storer, T.W.; Wasserman, K. Ventilatory Efficiency During Exercise in Healthy Subjects. Am. J. Respir. Crit. Care Med. 2002, 166, 1443–1448. [Google Scholar] [CrossRef]
| Variable | Pre-Intervention | Post-Intervention |
|---|---|---|
| Weight (kg) | 86.6 ± 3.3 | 78.2 ± 3.3 * |
| BMI (kg/m2) | 31.9 ± 0.6 | 28.9 ± 0.6 * |
| Fat (%) | 40.1 ± 0.6 | 33.4 ± 1.6 * |
| Lean mass (%) | 59.9 ± 6.5 | 66.6 ± 1.7 * |
| WC (cm) | 105.1 ± 1.2 | 95.7 ± 1.9 * |
| FG (mmol/L) | 6.5 ± 0.2 | 5.3 ± 0.2 * |
| TC (mmol/L) | 5.4 ± 0.3 | 4.7 ± 0.3 |
| LDL-c (mmol/L) | 3.4 ± 0.3 | 2.9 ± 0.2 |
| HDL-c (mmol/L) | 1.0 ± 0.1 | 1.2 ± 0.1 |
| TG (mg/dL) | 1.8 ± 0.1 | 1.3 ± 0.91 * |
| Variables | Pre-Intervention | Post-Intervention |
|---|---|---|
| SBP (mmHg) | 145.4 ± 3.9 | 140.1 ± 2.7 * |
| DBP (mmHg) | 80.2 ± 3.0 | 75.2 ± 1.6 * |
| HR (bpm) | 69.9 ± 2.0 | 64.9 ± 1.8 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teixeira, C.S.; Rocco, D.D.F.M.; Pinto, R.d.S.; da Silva, A.G.; Medeiros, A. Effects of a Hypocaloric Diet and Physical Training on Ventilatory Efficiency in Women with Metabolic Syndrome: A Prospective Interventional Study. Int. J. Environ. Res. Public Health 2025, 22, 1520. https://doi.org/10.3390/ijerph22101520
Teixeira CS, Rocco DDFM, Pinto RdS, da Silva AG, Medeiros A. Effects of a Hypocaloric Diet and Physical Training on Ventilatory Efficiency in Women with Metabolic Syndrome: A Prospective Interventional Study. International Journal of Environmental Research and Public Health. 2025; 22(10):1520. https://doi.org/10.3390/ijerph22101520
Chicago/Turabian StyleTeixeira, Caroline Simões, Débora Dias Ferraretto Moura Rocco, Raphael de Souza Pinto, Alexandre Galvão da Silva, and Alessandra Medeiros. 2025. "Effects of a Hypocaloric Diet and Physical Training on Ventilatory Efficiency in Women with Metabolic Syndrome: A Prospective Interventional Study" International Journal of Environmental Research and Public Health 22, no. 10: 1520. https://doi.org/10.3390/ijerph22101520
APA StyleTeixeira, C. S., Rocco, D. D. F. M., Pinto, R. d. S., da Silva, A. G., & Medeiros, A. (2025). Effects of a Hypocaloric Diet and Physical Training on Ventilatory Efficiency in Women with Metabolic Syndrome: A Prospective Interventional Study. International Journal of Environmental Research and Public Health, 22(10), 1520. https://doi.org/10.3390/ijerph22101520







