Association of Diarrhea Outcomes with Drinking Water Factors, Sanitation, Hygiene, and Malaria Practices in the Population of Béré, Chad
Abstract
1. Introduction
2. Materials and Methods
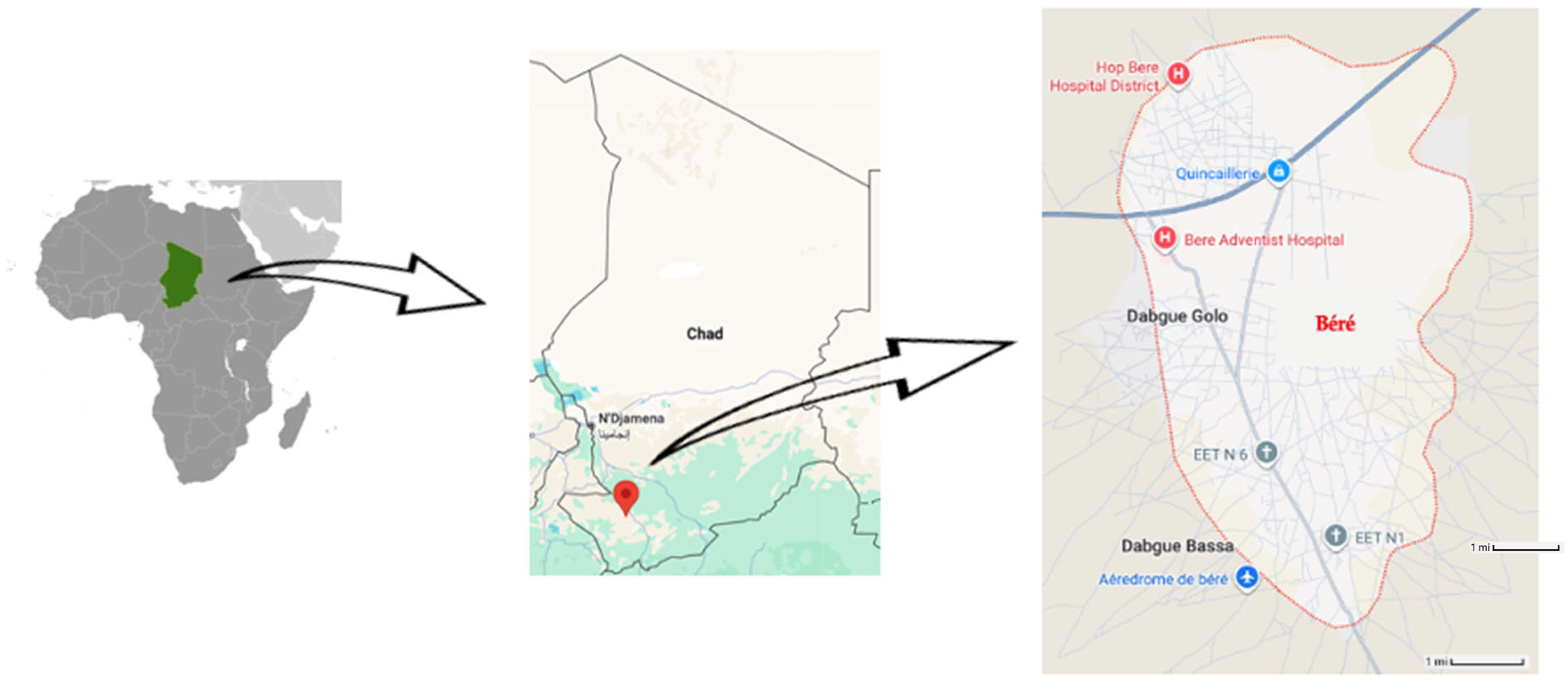
2.1. Study Setting and Design
2.2. Survey Respondents
2.3. Sample Size Calculation
2.4. Data Collection
2.5. Human Subjects’ Protection
2.6. Wells Mapping
2.7. Variables Included in the Study
2.8. Data Analysis
3. Results
3.1. Respondents’ Demographics
3.2. Mapped Pumped Wells
3.3. Water Sources Used by Respondents
3.4. Water Transport for Respondents
3.4.1. Number of Times per Day
3.4.2. Time Spent to Fetch Water
3.4.3. Water Carrier
3.5. Water Treatment Used by Respondents
3.6. Water Storage Conditions of Respondents
3.7. Sources of Health Advice
3.8. Sanitation and Hygiene Practices of Respondents
3.8.1. Toilet Facilities Used
3.8.2. Disposal of Children Stools
3.8.3. Hand Washing Practices
3.9. Deworming in Respondents’ Households
3.10. Malaria Indicators
3.10.1. Malaria Infections
3.10.2. Malaria Prevention
3.11. Diarrhea in Respondents’ Households
3.12. Predictors of Diarrhea Outcomes
3.13. Odds Ratios for Diarrhea Outcomes in Adults and Children
3.14. Covariate Analysis
4. Discussion
Limitations of the Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| TBA | Traditional birth attendant |
| CHW | Community health worker |
| CDC | Centers for Disease Control and Prevention |
| WHO | World Health Organization |
References
- World Health Organization. Diarrhoeal Disease. 2024. Available online: https://www.who.int/news-room/fact-sheets/detail/diarrhoeal-disease (accessed on 25 July 2025).
- Bain, R.; Cronk, R.; Hossain, R.; Bonjour, S.; Onda, K.; Wright, J.; Yang, H.; Slaymaker, T.; Hunter, P.; Pruss-Ustun, A.; et al. Global assessment of exposure to faecal contamination through drinking water based on a systematic review. Trop. Med. Int. Health 2014, 19, 917–927. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Country Disease Outlook-Chad, in African Region; World Health Organization: Geneva, Switzerland, 2023. [Google Scholar]
- World Health Organization. Chad-Health at a Glance; World Health Organization: Geneva, Switzerland, 2024. [Google Scholar]
- UNICEF. Water, Sanitation and Hygiene. Chad. 2022. Available online: https://www.unicef.org/chad/water-sanitation-and-hygiene (accessed on 25 July 2025).
- The World Bank. Data. 2021. Available online: https://data.worldbank.org/indicator/SP.DYN.LE00.IN?locations=TD (accessed on 25 July 2025).
- UNICEF. Country Profiles: Chad. 2019. Available online: https://data.unicef.org/country/tcd/ (accessed on 1 January 2023).
- Institute for Health Metrics and Evaluation. GBD Profile: Chad. Available online: https://www.healthdata.org/research-analysis/health-by-location/profiles/chad (accessed on 19 July 2025).
- Institute for Health Metrics and Evaluation. Child Health. University of Washington. 2025. Available online: https://www.healthdata.org/research-analysis/health-topics/child-health (accessed on 1 July 2025).
- Water and Sanitation Program. Tchad: Impacts Economiques d’un Mauvais Assainissement en Afrique. 2012. Available online: https://afwasakm.afwasa.org/wp-content/uploads/2025/04/2-1527-wsp-esi-chad-fr.pdf (accessed on 24 July 2016).
- Institut National de la Statistique des Études Économiques et Démographiques—INSEED; Tchad, Ministère de la Santé Publique—MSP; Tchad et ICF International. Enquete Demographique et de Sante et a Indicateurs Multiples (Eds-Mics) 2014–2015; INSEED: Rockville, MA, USA, 2015. [Google Scholar]
- Worldometer. Chad Population. 2025. Available online: https://www.worldometers.info/world-population/chad-population/ (accessed on 4 September 2025).
- Djaskano, M.I.; Cissoko, M.; Diar, M.S.I.; Israel, D.K.; Clément, K.H.; Ali, A.M.; Dormbaye, M.; Souleymane, I.M.; Batrane, A.; Sagara, I. Stratification and Adaptation of Malaria Control Interventions in Chad. Trop. Med. Infect. Dis. 2023, 8, 450. [Google Scholar] [CrossRef] [PubMed]
- Global Data Lab. Area Database; Average Household Size—Area Database-Table-Global Data Lab; Global Data Lab: Oslo, Norway, 2016; Available online: https://globaldatalab.org/demographics/table/hhsize/IND/ (accessed on 13 December 2024).
- Bandoumal, O.; Kostelngar, N.; Tchobkréo, B.; Riradjim, M.; Joël-Sibaye, S.; Ningam, N.; Joël-Nodjimbatem, N.; Caman, B.; Donato, K.; Bernard, B.; et al. Enquête Démographique et de Santé Tchad 2004. 2004. Available online: https://dhsprogram.com/pubs/pdf/FR170/FR170-TD04.pdf (accessed on 1 July 2025).
- Bechir, M.; Schelling, E.; Hamit, M.A.; Tanner, M.; Zinsstag, J. Parasitic infections, anemia and malnutrition among rural settled and mobile pastoralist mothers and their children in Chad. Ecohealth 2012, 9, 122–131. [Google Scholar] [CrossRef]
- Moyou-Somo, R.; Essomba, P.; Songue, E.; Tchoubou, N.N.; Ntambo, A.; Hiol, H.N.; Kemajou, J.P.; Essi, M.J.; Millet, P. A public private partnership to fight against malaria along the Chad-Cameroon pipeline corridor: I. Baseline data on socio-anthropological aspects, knowledge, attitudes and practices of the population concerning malaria. BMC Public Health 2013, 13, 1023. [Google Scholar] [CrossRef]
- Ntouda, J.; Sikodf, F.; Ibrahim, M.; Abba, I. Access to drinking water and health of populations in Sub-Saharan Africa. Comptes. Rendus. Biol. 2013, 336, 305–309. [Google Scholar] [CrossRef]
- Roka, M.; Goñi, P.; Rubio, E.; Clavel, A. Intestinal parasites in HIV-seropositive patients in the Continental Region of Equatorial Guinea: Its relation with socio-demographic, health and immune systems factors. Trans. R. Soc. Trop. Med. Hyg. 2013, 107, 502–510. [Google Scholar] [CrossRef]
- Sorlini, S.; Palazzini, D.; Mbawala, A.; Ngassoum, M.B.; Collivignarelli, M.C. Is drinking water from ‘improved sources’ really safe? A case study in the Logone valley (Chad-Cameroon). J. Water Health 2013, 11, 748–761. [Google Scholar] [CrossRef]
- Shaheed, A.; Orgill, J.; Ratana, C.; Montgomery, M.A.; Jeuland, M.A.; Brown, J. Water quality risks of ‘improved’ water sources: Evidence from Cambodia. Trop. Med. Int. Health 2014, 19, 186–194. [Google Scholar] [CrossRef]
- Soleimani-Ahmadi, M.; Vatandoost, H.; Zare, M.; Alizadej, A.; Salehi, M. Community knowledge and practices regarding malaria and long-lasting insecticidal nets during malaria elimination programme in an endemic area in Iran. Malar. J. 2014, 13, 511. [Google Scholar] [CrossRef] [PubMed]
- Boutrin, M.C.; Gately, Z.; McLarty, C.; Andersen, M. Socio-economic determinants of mortality among children aged 0–10 years in Béré, Chad. Discov. Soc. Sci. Health 2025, 5, 52. [Google Scholar] [CrossRef]
- UNICEF. Chad (TCD) Demographics. Chad (TCD)—Demographics, Health & Infant Mortality—UNICEF DATA. 2024. Available online: https://data.unicef.org/country/tcd/ (accessed on 13 December 2024).
- He, Z.; Ghose, B.; Cheng, Z. Diarrhea as a Disease of Poverty Among Under-Five Children in Sub-Saharan Africa: A Cross-Sectional Study. Inq. J. Health Care Organ. Provis. Financ. 2023, 60, 469580231202988. [Google Scholar] [CrossRef]
- Shube, H.; Azagegn, T.; Kebede, S. Rn isotope as a tool for monitoring functionality of water wells. J. Environ. Radioact. 2024, 280, 107529. [Google Scholar] [CrossRef]
- Hamit, M.A.; Tidjani, M.T.; Bilong Bilong, C.F. Recent data on the prevalence of intestinal parasites in N’Djamena, Chad Republic. Afr. J. Environ. Sci. Technol. 2008, 2, 407–411. [Google Scholar]
- Fejfar, D.; Tracy, W.; Kelly, E.; Moffa, M.; Bain, R.; Bartram, J.; Anderson, D.; Cronk, R. Identifying predictors of E. coli in rural household water in sub-Saharan Africa using elimination regression. Environ. Sci. Water Res. Technol. 2024, 10, 1147–1159. [Google Scholar] [CrossRef]
- Diouf, K.; Tabatabai, P.; Rudolph, J.; Marx, M. Diarrhoea prevalence in children under five years of age in rural Burundi: An assessment of social and behavioural factors at the household level. Glob. Health Action 2014, 7, 24895. [Google Scholar] [CrossRef] [PubMed]
- Geuther, N.; Mbarushimana, D.; Habarugira, F.; Buregeya, J.D.; Kollatzsch, M.; Pfüller, R.; Mugabowindekwe, M.; Ndoli, J.; Mockenhaupt, F.P. ESBL-producing Enterobacteriaceae in a rural Rwandan community: Carriage among community members, livestock, farm products and environment. Trop. Med. Int. Health 2023, 28, 855–863. [Google Scholar] [CrossRef] [PubMed]
- Opara, A.A. Water supplies in some rural communities around Calabar, Cross River State, Nigeria: Impact on water-related diseases. Southeast Asian J. Trop. Med. Public Health 2005, 36, 1028–1031. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Solomon, E.T.; Robele, S.; Kloos, H.; Mengistie, B. Effect of household water treatment with chlorine on diarrhea among children under the age of five years in rural areas of Dire Dawa, eastern Ethiopia: A cluster randomized controlled trial. Infect. Dis. Poverty 2020, 9, 64. [Google Scholar] [CrossRef]
- La Con, G.; Schilling, K.; Harris, J.; Person, B.; Owuor, M.; Ogange, K.; Faith, S.; Quick, R. Evaluation of Student Handwashing Practices During a School-Based Hygiene Program in Rural Western Kenya, 2007. Int. Q. Community Health Educ. 2017, 37, 121–128. [Google Scholar] [CrossRef]
- Opryszko, M.C.; Guo, Y.; MacDonald, L.; Kiihl, S.; Schwab, K.J. Impact of water-vending kiosks and hygiene education on household drinking water quality in rural Ghana. Am. J. Trop. Med. Hyg. 2013, 88, 651–660. [Google Scholar] [CrossRef]
- Ejemot-Nwadiaro, R.I.; Ehiri, J.; Arikpo, D.; Meremikwu, M.M.; Critchley, J.A. Hand-washing promotion for preventing diarrhoea. Cochrane Database Syst. Rev. 2021, 12, CD004265. [Google Scholar] [CrossRef]
- Günther, I.; Schipper, Y. Pumps, germs and storage: The impact of improved water containers on water quality and health. Health Econ. 2013, 22, 757–774. [Google Scholar] [CrossRef]
- Murphy, J.L.; Ayers, T.L.; Knee, J.; Oremo, J.; Odhiambo, A.; Faith, S.H.; Nyagol, R.O.; Stauber, C.E.; Lantagne, D.S.; Quick, R.E. Evaluating four measures of water quality in clay pots and plastic safe storage containers in Kenya. Water Res. 2016, 104, 312–319. [Google Scholar] [CrossRef]
- Helton, J. TheBorgenProject: 6 Facts About Sanitation in Chad. 2020. Available online: https://borgenproject.org/6-facts-about-sanitation-in-chad/ (accessed on 19 July 2025).
- Baker, K.K.; O’Reilly, C.E.; Levine, M.M.; Kotloff, K.L.; Nataro, J.P.; Ayers, T.L.; Farag, T.H.; Nasrin, D.; Blackwelder, W.C.; Wu, Y. Sanitation and Hygiene-Specific Risk Factors for Moderate-to-Severe Diarrhea in Young Children in the Global Enteric Multicenter Study, 2007–2011: Case-Control Study. PLoS Med. 2016, 13, e1002010. [Google Scholar] [CrossRef]
- Heijnen, M.; Cumming, O.; Peletz, R.; Chan, G.K.-S.; Brown, J.; Baker, K.; Clasen, T. Shared Sanitation Versus Individual Household Latrines in Urban Slums: A Cross-Sectional Study in Orissa, India. Am. J. Trop. Med. Hyg. 2015, 93, 263–268. [Google Scholar] [CrossRef] [PubMed]
- Heijnen, M.; Rosa, G.; Fuller, J.; Eisenberg, J.N.; Clasen, T. The geographic and demographic scope of shared sanitation: An analysis of national survey data from low- and middle-income countries. Trop. Med. Int. Health 2014, 19, 1334–1345. [Google Scholar] [CrossRef] [PubMed]
- Biran, A.; Jenkins, M.W.; Dabrase, P.; Bhagwat, I. Patterns and determinants of communal latrine usage in urban poverty pockets in Bhopal, India. Trop. Med. Int. Health 2011, 16, 854–862. [Google Scholar] [CrossRef]
- Nwokoro, U.U.; Ugwa, O.; Onwuliri, C.D.; Obi, I.F.; Ngozi, M.O.; Agunwa, C. Water, sanitation and hygiene risk factors associated with diarrhoea morbidity in a rural community of Enugu, South East Nigeria. Pan Afr. Med. J. 2020, 37, 115. [Google Scholar] [CrossRef]
- Mernie, G.; Kloos, H.; Adane, M. Prevalence of and factors associated with acute diarrhea among children under five in rural areas in Ethiopia with and without implementation of community-led total sanitation and hygiene. BMC Pediatr. 2022, 22, 148. [Google Scholar] [CrossRef]
- Seidu, A.A.; Ahinkorah, B.O.; Kissah-Korsah, K.; Agbaglo, E.; Dadzie, L.K.; Ameyaw, E.K.; Budu, E.; Hagan, J.E. A multilevel analysis of individual and contextual factors associated with the practice of safe disposal of children’s faeces in sub-Saharan Africa. PLoS ONE 2021, 16, e0254774. [Google Scholar] [CrossRef] [PubMed]
- Asmally, R.; Imam, A.A.; Eissa, A.; Saeed, A.; Mohamed, A.; Abdalla, E.; Esmaeel, M.A.M.; Elbashir, M.; Elbadawi, M.H.; Omer, M. Water, Sanitation and Hygiene in a Conflict Area: A Cross-Sectional Study in South Kordofan, Sudan. J. Epidemiol. Glob. Health 2025, 15, 4. [Google Scholar] [CrossRef]
- Belay, D.G.; Kibret, A.A.; Diress, M.; Gela, Y.Y.; Sinamaw, D.; Simegn, W.; Andualem, A.A.; Sei, A.M.; Bitew, D.A.; Seid, M.A. Deworming among preschool age children in sub-Saharan Africa: Pooled prevalence and multi-level analysis. Trop. Med. Health 2022, 50, 74. [Google Scholar] [CrossRef]
- Okosa, C.; Ukpai, O.M.; Lawrence, Q.O. Community burden of intestinal parasites and its public health concerns in Obizi, Amakama Olokoro, Umuahia South, Abia State, Nigeria. J. Parasit. Dis. 2023, 47, 118–123. [Google Scholar] [CrossRef]
- Nikolay, B.; Mwansawiro, C.S.; Kihara, J.H.; Okoyo, C.; Cano, J.; Mwanje, M.T.; Sultani, H.; Alusala, D.; Turner, H.C.; Teti, C. Understanding Heterogeneity in the Impact of National Neglected Tropical Disease Control Programmes: Evidence from School-Based Deworming in Kenya. PLoS Negl. Trop. Dis. 2015, 9, e0004108. [Google Scholar] [CrossRef]
- Edward, A.; Jung, Y.; Chhorvann, C.; Ghee, A.E.; Chege, J. Association of mother’s handwashing practices and pediatric diarrhea: Evidence from a multi-country study on community oriented interventions. J. Prev. Med. Hyg. 2019, 60, E93–E102. [Google Scholar] [PubMed]
- Alareqi, M.M.; Alshoaibi, L.H.; Liu, Y.; Dhital, S.; Zhang, B. The Role of Water, Sanitation and Hygiene (WaSH) Interventions on Health and Behavioral Outcomes during Humanitarian Crisis: A Systematic Review and Meta-Analysis. Iran. J. Public Health 2024, 53, 335–347. [Google Scholar] [CrossRef]
- Strunz, E.C.; Addiss, D.G.; Stocks, M.E.; Ogden, S.; Utzinger, J.; Freeman, M.C. Water, sanitation, hygiene, and soil-transmitted helminth infection: A systematic review and meta-analysis. PLoS Med. 2014, 11, e1001620. [Google Scholar] [CrossRef] [PubMed]
- Yates, T.; Lantagne, D.; Mintz, E.; Quick, R. The impact of water, sanitation, and hygiene interventions on the health and well-being of people living with HIV: A systematic review. JAIDS J. Acquir. Immune Defic. Syndr. 2015, 68 (Suppl. S3), S318–S330. [Google Scholar] [CrossRef] [PubMed]
- Hutton, G.; Chase, C. The Knowledge Base for Achieving the Sustainable Development Goal Targets on Water Supply, Sanitation and Hygiene. Int. J. Environ. Res. Public Health 2016, 13, 536. [Google Scholar] [CrossRef]
- Lo Vecchio, A.; Basile, F.W.; Bruzzese, D.; di Dato, F.; Aol, P.; Smarrazzo, A.; Guarino, A. Diarrhea in Children with Plasmodium falciparum Malaria: A Case-Control Study on the Prevalence and Response to Antimalarial Treatment. Am. J. Trop. Med. Hyg. 2020, 104, 659–665. [Google Scholar] [CrossRef]
- Matte, M.; Ntaro, M.; Kenney, J.; Wesuta, A.; Kawungezi, P.C.; Bwambale, S.; Ayebare, D.; Baguma, S.; Bagenda, F.; Stone, G. Assessment of pre-referral treatment for malaria, diarrhea, and pneumonia by rural community health workers in Southwestern Uganda: A cross-sectional study. BMC Health Serv. Res. 2024, 24, 95. [Google Scholar] [CrossRef]
- Pongponratn, E.; Riganti, M.; Punpoowong, B.; Aikawa, M. Microvascular sequestration of parasitized erythrocytes in human falciparum malaria: A pathological study. Am. J. Trop. Med. Hyg. 1991, 44, 168–175. [Google Scholar] [CrossRef]
- Church, J.; Maitland, K. Invasive bacterial co-infection in African children with Plasmodium falciparum malaria: A systematic review. BMC Med. 2014, 12, 31. [Google Scholar] [CrossRef] [PubMed]
- Olupot-Olupot, P.; Urban, B.C.; Jemutai, J.; Nteziyaremye, J.; Fanjo, H.M.; Karanja, H.; Karisa, J.; Ongodia, P.; Bwonyo, P.; Gitau, E.N. Endotoxaemia is common in children with Plasmodium falciparum malaria. BMC Infect. Dis. 2013, 13, 117. [Google Scholar] [CrossRef] [PubMed]
- Church, J.A.; Nyamako, L.; Oluput-Oluput, P.; Maitland, K.; Urban, B.C. Increased adhesion of Plasmodium falciparum infected erythrocytes to ICAM-1 in children with acute intestinal injury. Malar. J. 2016, 15, 54. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | Item | Count | Percentage (%) |
|---|---|---|---|
| Respondents (n = 484); Respondents reported household adults (n = 1407); Respondents’ reported household children (n = 2084) | |||
| Household type | Nuclear family | 379 | 78.3 |
| Multi-family | 105 | 21.7 | |
| Religion | Seventh Day Adventist | 20 | 4.1 |
| Evangelical | 304 | 62.8 | |
| Muslim | 9 | 1.9 | |
| Catholic | 121 | 25.0 | |
| Animist | 11 | 2.3 | |
| Other | 7 | 1.4 | |
| No religion | 12 | 2.5 | |
| Ethnicity | Ngambay | 48 | 9.9 |
| Nangtchéré | 421 | 87.0 | |
| Fulani | 2 | 0.4 | |
| Arabic | 7 | 1.4 | |
| Other | 6 | 1.2 | |
| House standard | Low | 85 | 17.6 |
| Medium | 271 | 56.0 | |
| High | 128 | 26.4 | |
| Age group | <20 years old | 3 | 0.6 |
| 20–29 years old | 74 | 15.3 | |
| 30–39 years old | 161 | 33.3 | |
| 40–59 years old | 207 | 42.8 | |
| 60+ years old | 39 | 8.1 | |
| Education level | None | 97 | 20.0 |
| Primary | 141 | 29.1 | |
| Secondary | 180 | 37.2 | |
| Baccalauréat | 36 | 7.4 | |
| Baccalauréat + | 30 | 6.2 | |
| Education period | Number of years | mean = 8.1 | (SD = 5.6) |
| Household head gender | Male | 424 | 87.6 |
| Female | 60 | 12.4 | |
| Household size | Number of members | mean = 6.7 | (SD = 3.4) |
| Received health advice | From CHW/TBA | ||
| Yes | 442 | 91.3 | |
| No | 42 | 8.7 | |
| From CHW only | |||
| Yes | 374 | 77.3 | |
| No | 110 | 22.7 | |
| From TBA only | |||
| Yes | 306 | 63.2 | |
| No | 178 | 36.8 | |
| Water sources | Primary water source—Dry season | ||
| Rainwater | 50 | 10.3 | |
| Water vendor | 6 | 1.2 | |
| Bottle/Bag of water | 1 | 0.2 | |
| Pumped well | 45 | 9.3 | |
| Pit well | 382 | 78.9 | |
| Spring | 0 | 0 | |
| Primary water source—Rainy season | |||
| Rainwater | 240 | 49.6 | |
| Water vendor | 2 | 0.4 | |
| Bottle/Bag of water | 0 | 0 | |
| Pumped well | 33 | 6.8 | |
| Pit well | 208 | 43.0 | |
| Spring | 1 | 0.2 | |
| Type of pit well—Dry season | |||
| Covered | 124 | 27.2 | |
| Uncovered | 332 | 72.8 | |
| Type of pit well—Rainy season | |||
| Covered | 126 | 27.9 | |
| Uncovered | 325 | 72.1 | |
| Minutes fetching water—Dry season | mean = 12.4 | (SD = 11.2) | |
| Minutes fetching water—Rainy season | mean = 11.7 | (SD = 13.2) | |
| Times per day obtaining drinking water—Dry season | mean = 7.8 | (SD = 5.3) | |
| Times per day obtaining drinking water—Rainy season | mean = 5.99 | (SD = 4.3) | |
| Age of drinking water female carrier (years) | mean = 25.6 | (SD = 11.9) | |
| Malaria | Household members who experienced the following symptoms in last 7 days | ||
| Fever | |||
| Adults | 326 | 23.2 a | |
| Children | 645 | 31.0 a | |
| Headache | |||
| Adults | 129 | 9.2 a | |
| Children | 395 | 19.0 a | |
| Appetite loss | |||
| Adults | 112 | 8.0 a | |
| Children | 213 | 10.2 a | |
| Vomiting | |||
| Adults | 79 | 5.6 a | |
| Children | 265 | 12.7 a | |
| Convulsions | |||
| Adults | 14 | 1.0 a | |
| Children | 23 | 1.1 a | |
| Diarrhea | |||
| Adults | 172 | 12.2 a | |
| Children | 586 | 28.1 a | |
| Joint pain | |||
| Adults | 92 | 6.5 a | |
| Children | 8 | 0.4 a | |
| Sweating/Shivering | |||
| Adults | 16 | 1.1 a | |
| Children | 39 | 1.9 a | |
| Last time mosquito nets were treated with insecticide | |||
| Less than 6 months | 67 | 13.8 | |
| 6 months to 2 years | 110 | 22.7 | |
| More than 2 years | 22 | 4.5 | |
| Never | 285 | 58.9 | |
| Number of times malaria experienced in household in last 12 months | 5.2 | (SD = 5.8) | |
| Number of times malaria was treated in household in last 12 months | 4.4 | (SD = 4.6) | |
| Source of malaria treatment: | |||
| Government hospital/Clinic | |||
| Yes | 251 | 51.9 | |
| No | 233 | 48.1 | |
| Adventist hospital | |||
| Yes | 271 | 56.0 | |
| No | 213 | 44.0 | |
| Market | |||
| Yes | 90 | 18.6 | |
| No | 394 | 81.4 | |
| No treatment | |||
| Yes | 22 | 4.5 | |
| No | 462 | 95.5 | |
| Things done to reduce malaria risk in last 12 months: | |||
| Nothing | |||
| Yes | 162 | 33.5 | |
| No | 322 | 66.5 | |
| Changed clothing | |||
| Yes | 74 | 15.3 | |
| No | 410 | 84.7 | |
| Used insect repellent on body | |||
| Yes | 238 | 49.2 | |
| No | 246 | 50.8 | |
| Used insecticide around the house | |||
| Yes | 33 | 6.8 | |
| No | 451 | 93.2 | |
| Belief malaria is a health threat | |||
| Yes | 478 | 98.8 | |
| No | 6 | 1.2 | |
| Diarrhea | Household members who experienced diarrhea in last 12 months | ||
| Adults | 456 | 32.4 a | |
| mean = 0.9 | (SD = 0.9) | ||
| Children | 1083 | 52.0 a | |
| mean = 2.2 | (SD = 2.1) | ||
| Household members who received diarrhea treatment in last 12 months | |||
| Adults | 426 | 30.3 a | |
| mean = 0.9 | (SD = 0.9) | ||
| Children | 1052 | 50.5 a | |
| mean = 2.2 | (SD = 2.1) | ||
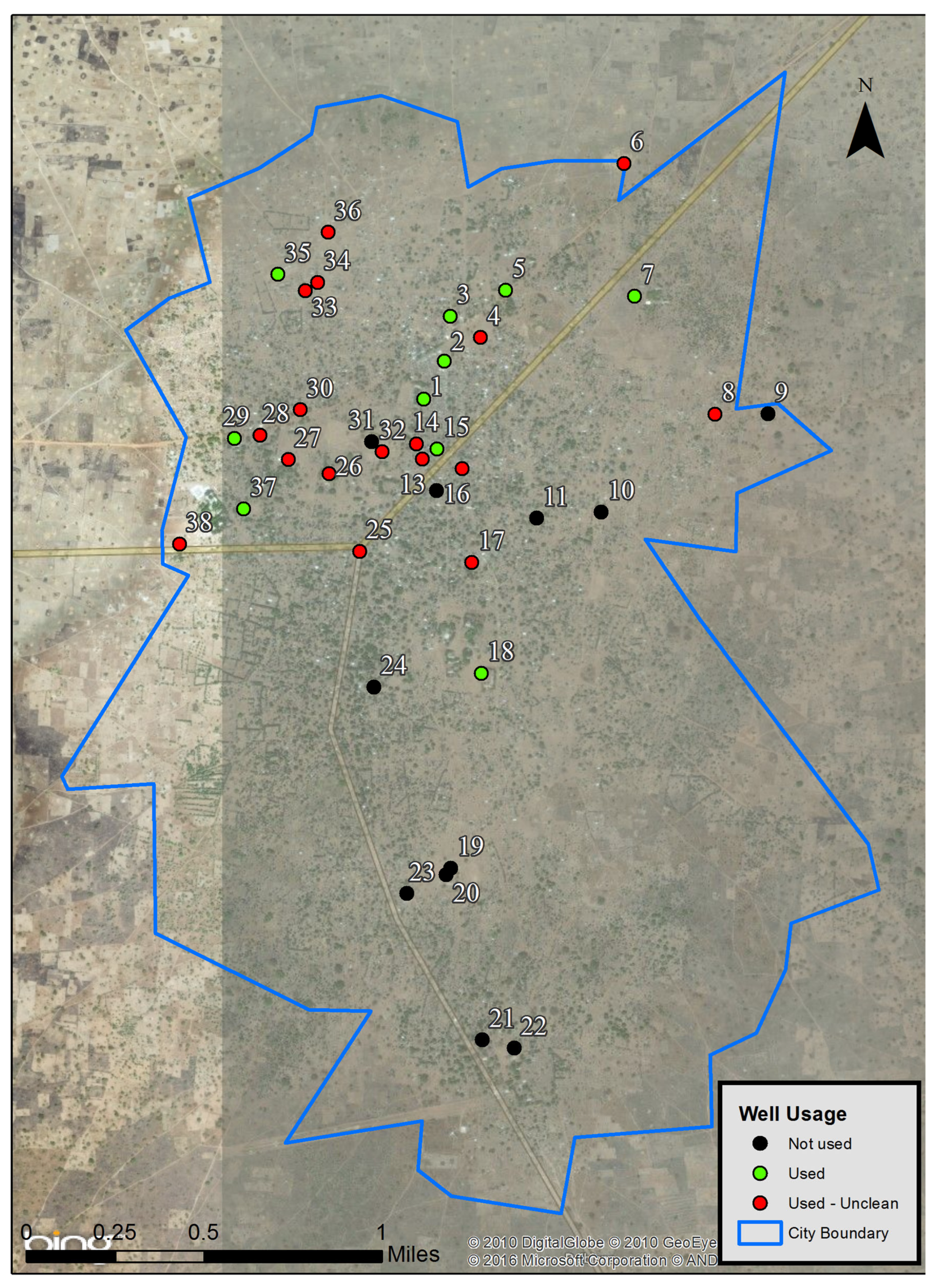
| Map ID | Name | Usage | Description |
|---|---|---|---|
| 1 | Singuir | Used | Works well. |
| 2 | Borno | Used | Works well. |
| 4 | Tcha-Asse Église No. 2 | Used | Works well. Water is red when the well is not used. |
| 3 | Béré Mission A | Used | Works well. |
| 5 | Béré Mission B | Used | Works well. |
| 6 | Béré Mission B 2 | Used | Works well. Water is red. Remote location. |
| 7 | Tcha-Asse Lycée | Used | Works well. |
| 8 | Tchirou Yendei | Used | Works well. Water is yellow and red in the morning. |
| 9 | Tchirou Yendei 2 | Not used | Has not worked for 1 year. |
| 10 | Béré Yendei Église No. 7 | Not used | Has not worked for nearly 1 year. Water was red. |
| 12 | Béré Borno | Used | Works well. Water is red; tastes bad in the morning. |
| 13 | Béré Borno 2 | Used | Works well. Water is red; tastes bad in the morning. |
| 14 | Béré Borno ABA-Gana | Used | Works well. Water is red, oily. For washing only. |
| 15 | Béré Borno ABA-Gana 2 | Used | Works well. |
| 16 | Yendei | Not used | Water is always red. |
| 11 | Yendei 2 | Not used | Has not worked for 5 years. Pump not working. |
| 17 | Église No. 3 | Used | Works well. Water is red in the morning. |
| 18 | École des Filles | Used | Works well. At a girls’ school. |
| 19 | Nergue-Goujiba École | Not used | Pump is not working. |
| 20 | Nergue-Goujiba École 2 | Not used | Pump is not working. |
| 21 | Nergue-Goujiba | Not used | Has not worked for 7–8 years. |
| 22 | Nergue-Goujiba Église | Not used | Has not worked for 7 years. |
| 23 | Tchamangue | Not used | Has not worked for 2 years. Water was good. |
| 24 | Église Mission Bangar No. 4 | Not used | Has not worked for 2 years. Water was red. |
| 25 | Esthar Singuir | Used | Works well. Water is red in the morning. |
| 26 | Singuir Prefecture | Used | Works well. Water is red in the evening. |
| 27 | District Santé | Used | Works well. Water is always yellow. For cooking only. |
| 28 | Singuir Mere | Used | Works well. Water is red in the morning. |
| 29 | Dàma Béré Poste | Used | Works well. Water is red in the morning. |
| 30 | Mission Béré Bolo | Used | Works well. Water is always red, oily taste. Not for drinking. |
| 31 | Sous-préfecture de Béré | Not used | Has not worked for 2 years. Water was red. |
| 32 | Sous-préfecture de Singuir | Used | Works well. Water is always red. |
| 33 | Béré Bolo Kadamou | Used | Works well. Water is yellow in the morning; looks normal after. |
| 34 | Béré Bolo Kadamou 2 | Used | Works well. Water is red in the morning. |
| 35 | Béré Bolo Kadamou 3 | Used | Works well. Pumping takes a long time. |
| 36 | Béré Mission A Guedetague | Used | Works well. Water is red in the morning but looks normal after |
| 37 | Béré Posté Papa Samidi | Used | Works well. Water looks normal. Not true pumped well. |
| 38 | Béré Posté École | Used | Pumping takes time. Water does not look normal. Not for drinking. |
| Practices | Item | Count | Percentage (%) |
|---|---|---|---|
| Respondents (n = 484); Respondents reported household adults (n = 1407); Respondents’ reported household children (n = 2084) | |||
| Water storage | Container type | ||
| Plastic container | 12 | 2.5 | |
| Terra cotta/Clay-like container | 471 | 97.3 | |
| Other | 1 | 0.2 | |
| Container cleaned | |||
| Yes | 482 | 99.6 | |
| No | 2 | 0.4 | |
| Cleaning frequency with water | |||
| No | 104 | 21.5 | |
| Every week | 372 | 76.9 | |
| Every month | 8 | 1.7 | |
| Water treatment | Water treated | ||
| Yes | 418 | 86.4 | |
| No | 66 | 13.6 | |
| Treatment—Boiled | |||
| Yes | 12 | 2.5 | |
| No | 472 | 97.5 | |
| Treatment—Bleach | |||
| Yes | 310 | 64.0 | |
| No | 174 | 36.0 | |
| Treatment—Solar disinfection | |||
| Yes | 2 | 0.4 | |
| No | 482 | 99.6 | |
| Sanitation | Type of toilet facility used by household | ||
| Improved facilities | |||
| Flush to septic tank | |||
| Adults | 11 | 0.8 a | |
| Children | 8 | 0.4 a | |
| Flush to pit latrine | |||
| Adults | 34 | 2.4 a | |
| Children | 44 | 2.1 a | |
| Flush elsewhere | |||
| Adults | 3 | 0.2 a | |
| Children | 14 | 0.7 a | |
| Flush to unknown place | |||
| Adults | 1 | 0.1 a | |
| Children | 0 | 0.0 a | |
| Ventilated pit latrine | |||
| Adults | 14 | 1.0 a | |
| Children | 27 | 1.3 a | |
| Pit latrine with slab | |||
| Adults | 146 | 10.4 a | |
| Children | 256 | 12.3 a | |
| Composting toilet | |||
| Adult | 2 | 0.1 a | |
| Children | 4 | 0.2 a | |
| Unimproved facilities | |||
| Bush/Field/No facilities | |||
| Adults | 65 | 4.6 a | |
| Children | 110 | 5.3 a | |
| Pit latrine without slab | |||
| Adults | 772 | 54.9 a | |
| Children | 1435 | 68.9 a | |
| Toilet facility shared with other households | |||
| Yes | 308 | 63.6 | |
| No | 176 | 36.4 | |
| Number of households sharing toilet facility | mean = 2.0 | (SD = 2.5) | |
| Public has access to use toilet | |||
| Yes | 267 | 55.2 | |
| No | 217 | 44.8 | |
| Disposal method of youngest child stools | |||
| Child used toilet/latrine | 63 | 13.0 | |
| Put/Rinsed stools into toilet/latrine | 97 | 20.0 | |
| Put/Rinsed stools in drain/ditch | 1 | 0.2 | |
| Stools thrown into garbage | 115 | 23.8 | |
| Stools buried | 136 | 28.1 | |
| Stools left in open | 44 | 9.1 | |
| Other | 28 | 5.8 | |
| Hand washing | Handwashing practiced in household | ||
| Yes | 444 | 98.4 | |
| No | 7 | 1.6 | |
| After using toilet—With water | |||
| Always | 160 | 33.1 | |
| Sometimes | 171 | 35.3 | |
| No | 153 | 31.6 | |
| After using toilet—With water and soap | |||
| Always | 181 | 37.4 | |
| Sometimes | 80 | 16.5 | |
| No | 223 | 46.1 | |
| Before eating—With water | |||
| Always | 245 | 50.6 | |
| Sometimes | 23 | 4.8 | |
| No | 216 | 44.6 | |
| Before eating—With water and soap | |||
| Always | 186 | 38.4 | |
| Sometimes | 170 | 35.1 | |
| No | 128 | 26.4 | |
| Deworming | Household members participated in deworming program in last 12 months | ||
| Yes | 397 | 82.0 | |
| No | 87 | 18.0 | |
| Household members who participated in deworming programs in last 12 months | |||
| Adults | 504 | 35.8 a | |
| Children | 1269 | 60.9 a | |
| Number of times members participated in deworming | |||
| Adults | mean = 1.2 | (SD = 1.3) | |
| Children | mean = 1.7 | (SD = 1.4) | |
| Factors | Unstandardized B | 95% CI | p |
|---|---|---|---|
| Adults | |||
| Times per day obtaining drinking water—Dry season | −0.037 | −0.068–(−0.007) | 0.017 |
| Times per day obtaining drinking water—Rainy season | 0.038 | 0.001–0.075 | 0.044 |
| Minutes fetching drinking water—Rainy season | 0.011 | 0.001–0.021 | 0.037 |
| Drinking water treated | −0.630 | −0.922–(−0.337) | <0.001 |
| Drinking water treatment—Bleach | −0.458 | −0.672–(−0.244) | <0.001 |
| Drinking water treatment—Solar disinfection | −1.318 | −2.555–(−0.082) | 0.037 |
| Drinking water storage cleaned | 1.280 | 0.029–2.531 | 0.045 |
| Fever (malaria)—Adults | 0.392 | 0.291–0.492 | <0.001 |
| Headache (malaria)—Adults | 0.177 | 0.028–0.325 | 0.020 |
| Vomiting (malaria)—Adults | 0.241 | 0.036–0.447 | 0.021 |
| Vomiting (malaria)—Children | −0.107 | −0.200–(−0.013) | 0.026 |
| Diarrhea (malaria)—Adults | 0.203 | 0.059–0.347 | 0.006 |
| Number of times malaria experienced in household in last 12 months | −0.033 | −0.059–(−0.007) | 0.006 |
| Number of times malaria treated in household in last 12 months | 0.040 | 0.006–0.073 | 0.012 |
| Action to lower malaria risk—Changed clothes | 0.315 | 0.085–0.546 | 0.008 |
| Action to lower malaria risk—Used house insect repellent | 0.353 | 0.010–0.696 | 0.044 |
| Used toilet facility with a septic tank—Adults | −2.260 | −4.228–(−0.292) | 0.025 |
| Used toilet facility with a septic tank—Children | 3.250 | 0.508–5.993 | 0.020 |
| Used pit latrine—Adults | 0.283 | 0.025–0.542 | 0.032 |
| Used ventilated pit latrine—Adults | 0.771 | 0.022–1.520 | 0.044 |
| Used pit latrine with no slab—Adults | 0.134 | 0.046–0.221 | 0.003 |
| Used pit latrine with no slab—Children | 0.036 | 0.005–0.067 | 0.024 |
| Used bush or field/No toilet facility—Adults | 0.477 | 0.205–0.750 | <0.001 |
| Number of households that share the toilet facility | 0.057 | 0.018–0.097 | 0.004 |
| Toilet facility can be used by the public | −0.394 | −0.677–(−0.112) | 0.006 |
| Experienced diarrhea in last 12 months—Children | 0.285 | 0.190–0.380 | <0.001 |
| Received diarrhea treatment in last 12 months—Adults | 0.953 | 0.915–0.990 | <0.001 |
| Received diarrhea treatment in last 12 months—Children | −0.285 | −0.382–(−0.188) | <0.001 |
| Children | |||
| Minutes fetching drinking water—Dry season | 0.028 | 0.001–0.054 | 0.045 |
| Drinking water treated | −2.054 | −2.709–(−1.400) | <0.001 |
| Drinking water treatment—Bleach | −1.427 | −1.906–(−0.948) | <0.001 |
| Fever (malaria)—Adults | 0.186 | 0.028–0.344 | 0.021 |
| Fever (malaria)—Children | 0.618 | 0.531–0.705 | <0.001 |
| Headache (malaria)—Children | 0.237 | 0.126–0.348 | <0.001 |
| Appetite loss (malaria)—Children | 0.426 | 0.267–0.584 | <0.001 |
| Diarrhea (malaria)—Children | 0.363 | 0.266–0.460 | <0.001 |
| Joint pain (malaria)—Children | 0.881 | 0.024–1.738 | 0.044 |
| Source of malaria treatment—Market | 0.428 | 0.082–0.775 | 0.016 |
| Action to lower malaria risk—Changed clothes | 0.422 | 0.060–0.783 | 0.022 |
| Action to lower malaria risk—Used house insect repellent | 0.881 | 0.344–1.418 | 0.001 |
| CHW gave health advice | 1.017 | 0.471–1.564 | <0.001 |
| TBA gave health advice | 0.703 | 0.280–1.126 | 0.001 |
| Used pit latrine—Children | 0.344 | 0.025–0.664 | 0.035 |
| Used pit latrine with slab—Children | 0.320 | 0.185–0.455 | <0.001 |
| Used pit latrine with no slab—Adults | −0.334 | −0.483–(−0.186) | <0.001 |
| Used pit latrine with no slab—Children | 0.491 | 0.438–0.544 | <0.001 |
| Used bush or field/No toilet facility—Children | 0.403 | 0.190–0.616 | <0.001 |
| Number of households that share the toilet facility | 0.085 | 0.018–0.152 | 0.013 |
| Toilet facility can be used by the public | −1.008 | −1.488–(−0.529) | <0.001 |
| Experienced diarrhea—Adults | 0.240 | 0.160–0.321 | <0.001 |
| Received diarrhea treatment in last 12 months—Adults | −0.252 | −0.335–(−0.168) | <0.001 |
| Received diarrhea treatment in last 12 months—Children | 1.000 | 0.981–1.018 | <0.001 |
| Factors | Odd Ratios | 95% CI | p |
|---|---|---|---|
| Adults | |||
| Drinking water from pit well—Rainy season | 0.108 | 0.064–0.183 | <0.001 |
| Drinking water from rainwater—Rainy season | 1 (reference) | - | |
| Cleaning of drinking water container with water—Every week | 17.561 | 2.938–104.955 | 0.002 |
| No cleaning of drinking water container with water | 1 (reference) | - | |
| Cleaning of drinking water container with soap—Every week | 0.320 | 0.180–0.570 | <0.001 |
| Cleaning of drinking water container with soap—Every month | 0.016 | 0.001–0.324 | 0.007 |
| No cleaning of drinking water container with soap | 1 (reference) | - | |
| Last time mosquito nets were treated with insecticide—Less than 6 months ago | 2.286 | 1.228–4.257 | 0.009 |
| Last time mosquito nets were treated with insecticide—More than 2 years ago | 0.287 | 0.110–0.750 | 0.011 |
| Last time mosquito nets were treated with insecticide—Never | 1 (reference) | - | |
| Children stools disposal—Thrown in garbage | 3.927 | 1.770–8.714 | <0.001 |
| Children stools disposal—Toilet/Latrine | 1 (reference) | - | |
| Hand washing with water after using toilet—Sometimes | 4.055 | 1.822–9.027 | <0.001 |
| Hand washing with water after using toilet—None | 1 (reference) | - | |
| Children | |||
| Storage of drinking water—Terra cotta/Clay-like container | 4.772 | 1.298–17.548 | 0.019 |
| Storage of drinking water—Plastic container | 1 (reference) | - | - |
| Drinking water from pit well—Rainy season | 0.467 | 0.272–0.802 | 0.009 |
| Drinking water from rainwater—Rainy season | 1 (reference) | - | - |
| Last time mosquito nets were treated with insecticide—Less than 6 months ago | 2.700 | 1.163–6.266 | 0.021 |
| Last time mosquito nets were treated with insecticide—Never | 1 (reference) | - | |
| Hand washing with water after using toilet—Always | 3.709 | 1.781–7.724 | <0.001 |
| Hand washing with water after using toilet—Sometimes | 16.060 | 5.591–46.135 | <0.001 |
| Hand washing with water after using toilet—None | 1 (reference) | - | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boutrin, M.-C.; Andersen, M.; Gately, Z.; McLarty, C.; Santos, E. Association of Diarrhea Outcomes with Drinking Water Factors, Sanitation, Hygiene, and Malaria Practices in the Population of Béré, Chad. Int. J. Environ. Res. Public Health 2025, 22, 1497. https://doi.org/10.3390/ijerph22101497
Boutrin M-C, Andersen M, Gately Z, McLarty C, Santos E. Association of Diarrhea Outcomes with Drinking Water Factors, Sanitation, Hygiene, and Malaria Practices in the Population of Béré, Chad. International Journal of Environmental Research and Public Health. 2025; 22(10):1497. https://doi.org/10.3390/ijerph22101497
Chicago/Turabian StyleBoutrin, Marie-Claire, Marci Andersen, Zach Gately, Charis McLarty, and Edirlei Santos. 2025. "Association of Diarrhea Outcomes with Drinking Water Factors, Sanitation, Hygiene, and Malaria Practices in the Population of Béré, Chad" International Journal of Environmental Research and Public Health 22, no. 10: 1497. https://doi.org/10.3390/ijerph22101497
APA StyleBoutrin, M.-C., Andersen, M., Gately, Z., McLarty, C., & Santos, E. (2025). Association of Diarrhea Outcomes with Drinking Water Factors, Sanitation, Hygiene, and Malaria Practices in the Population of Béré, Chad. International Journal of Environmental Research and Public Health, 22(10), 1497. https://doi.org/10.3390/ijerph22101497






