Analysis of Oral Microbiota in Elderly Thai Patients with Alzheimer’s Disease and Mild Cognitive Impairment
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. DNA Extraction
2.3. Library Preparation and Sequencing
2.4. Long-Read Sequencing (PacBio) Data Analysis
3. Results
3.1. Participants and Clinical Status
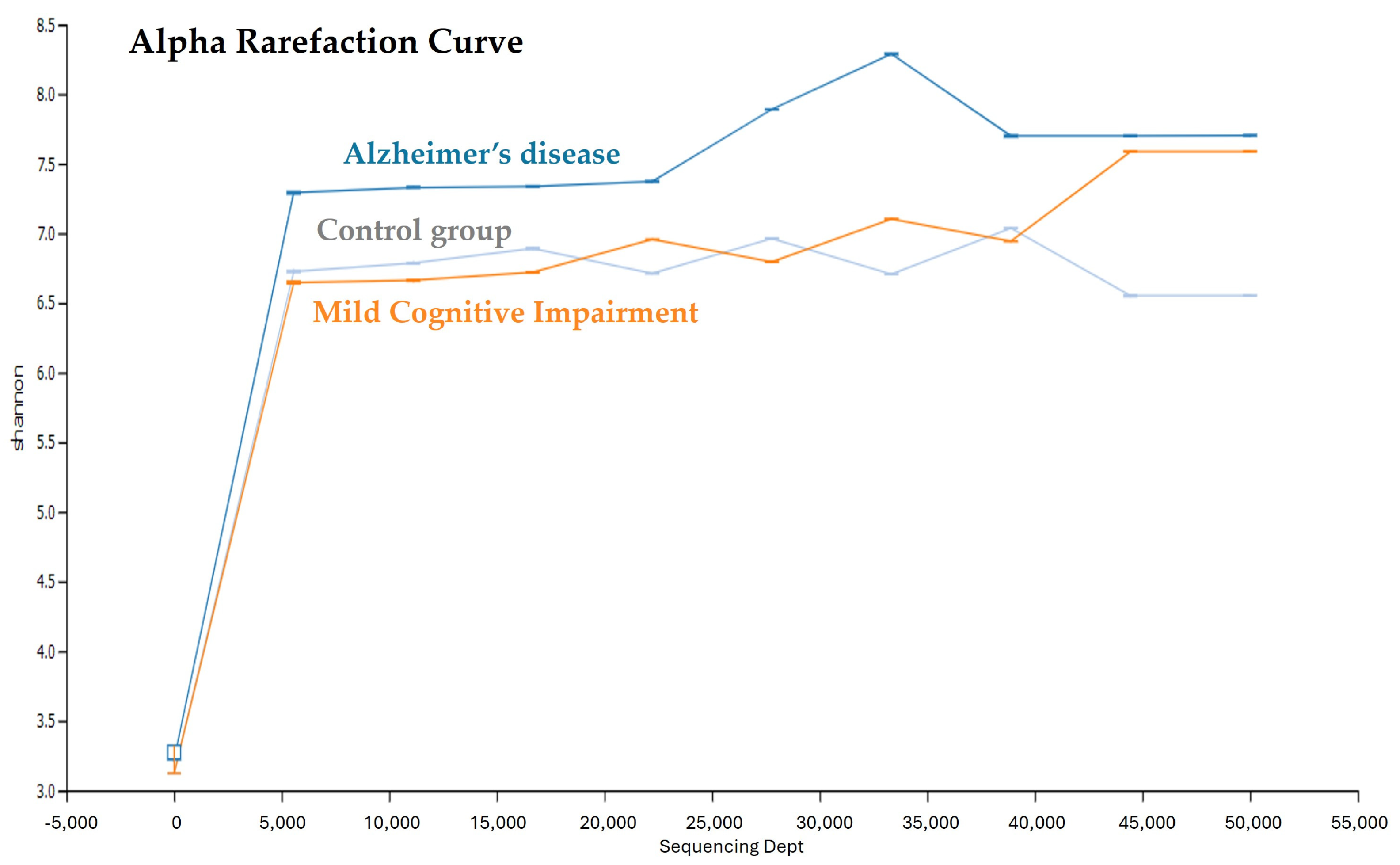
3.2. Saliva Microbial Diversity in the AD, MCI, and Control Groups
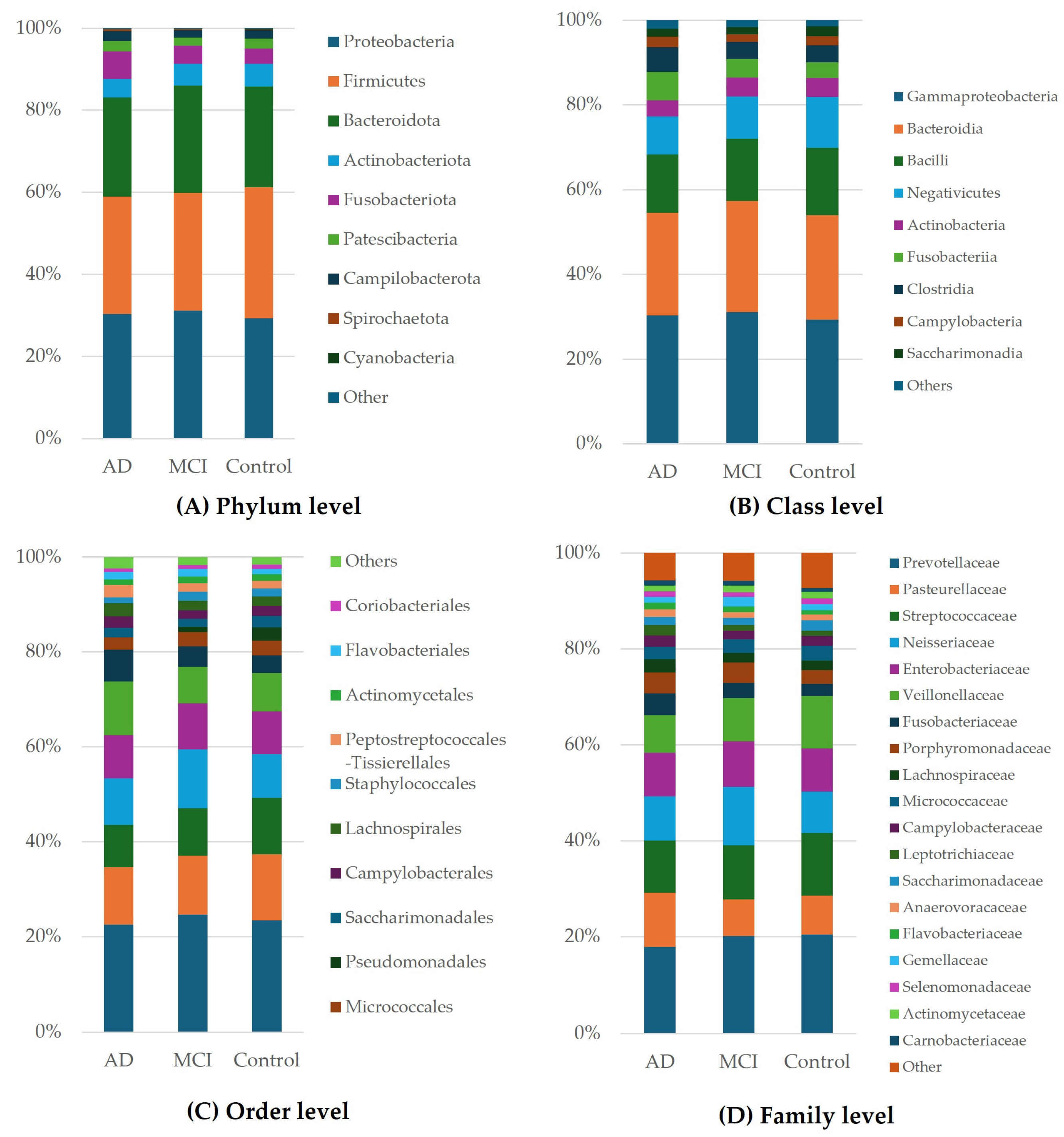
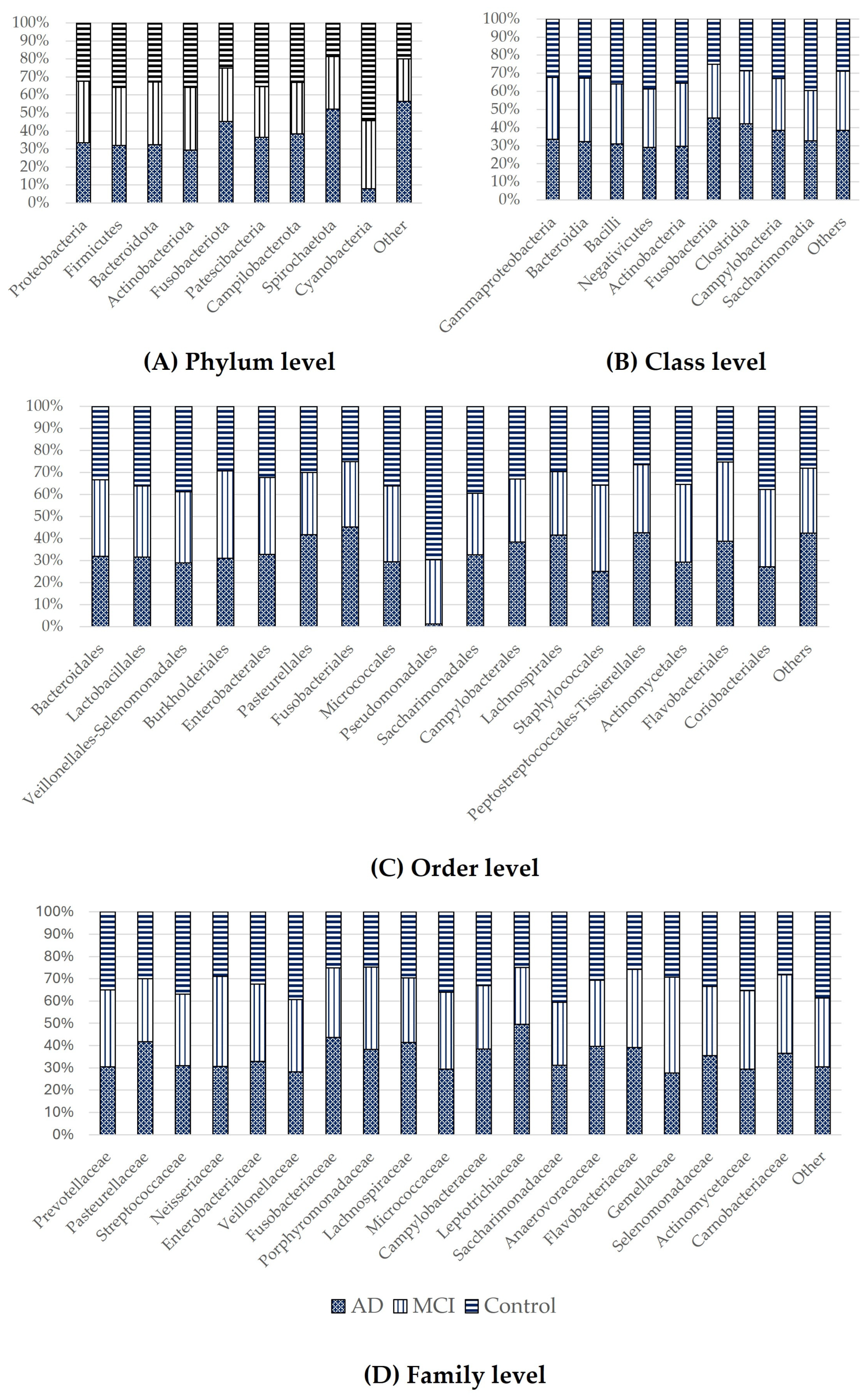
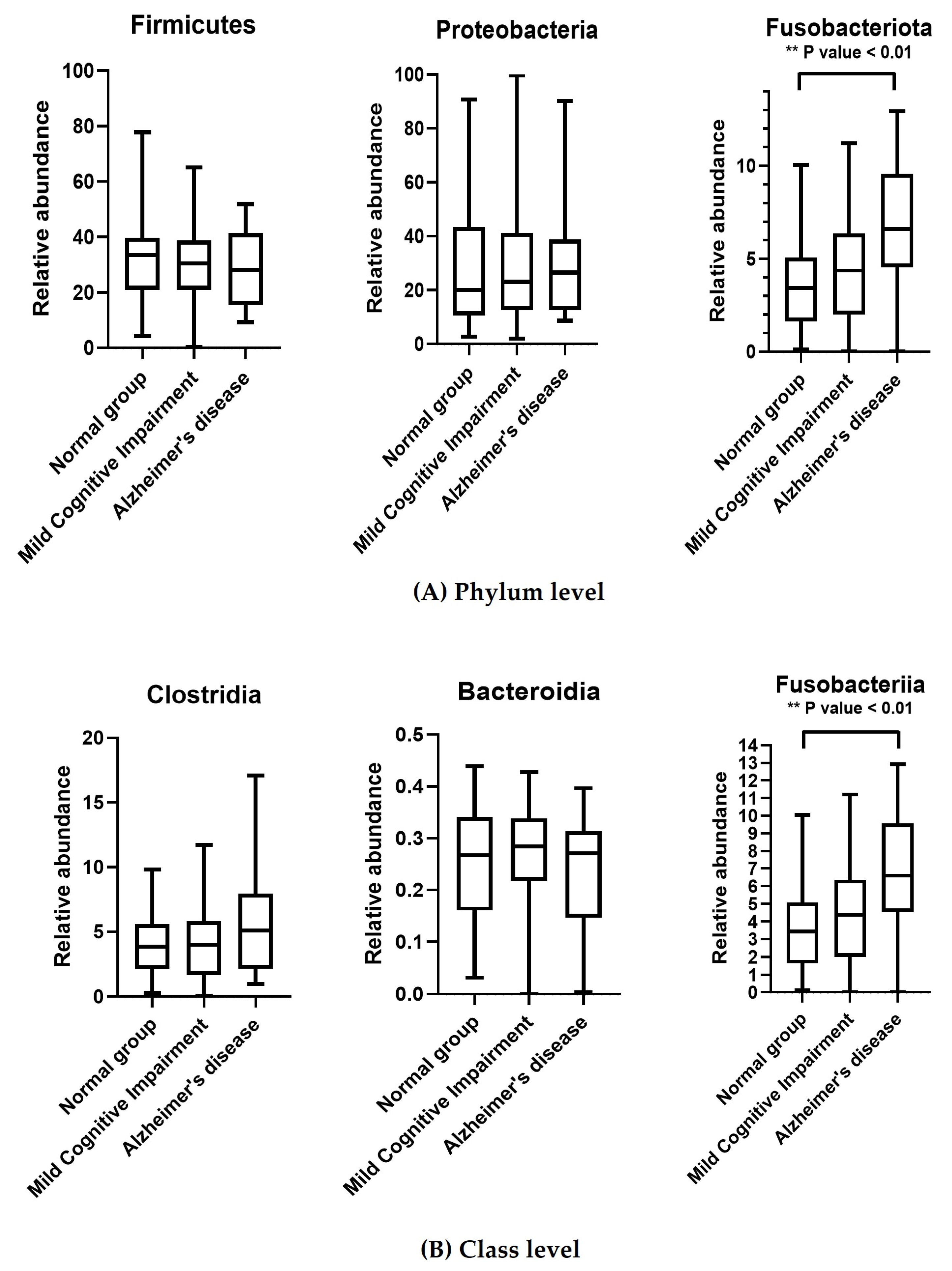
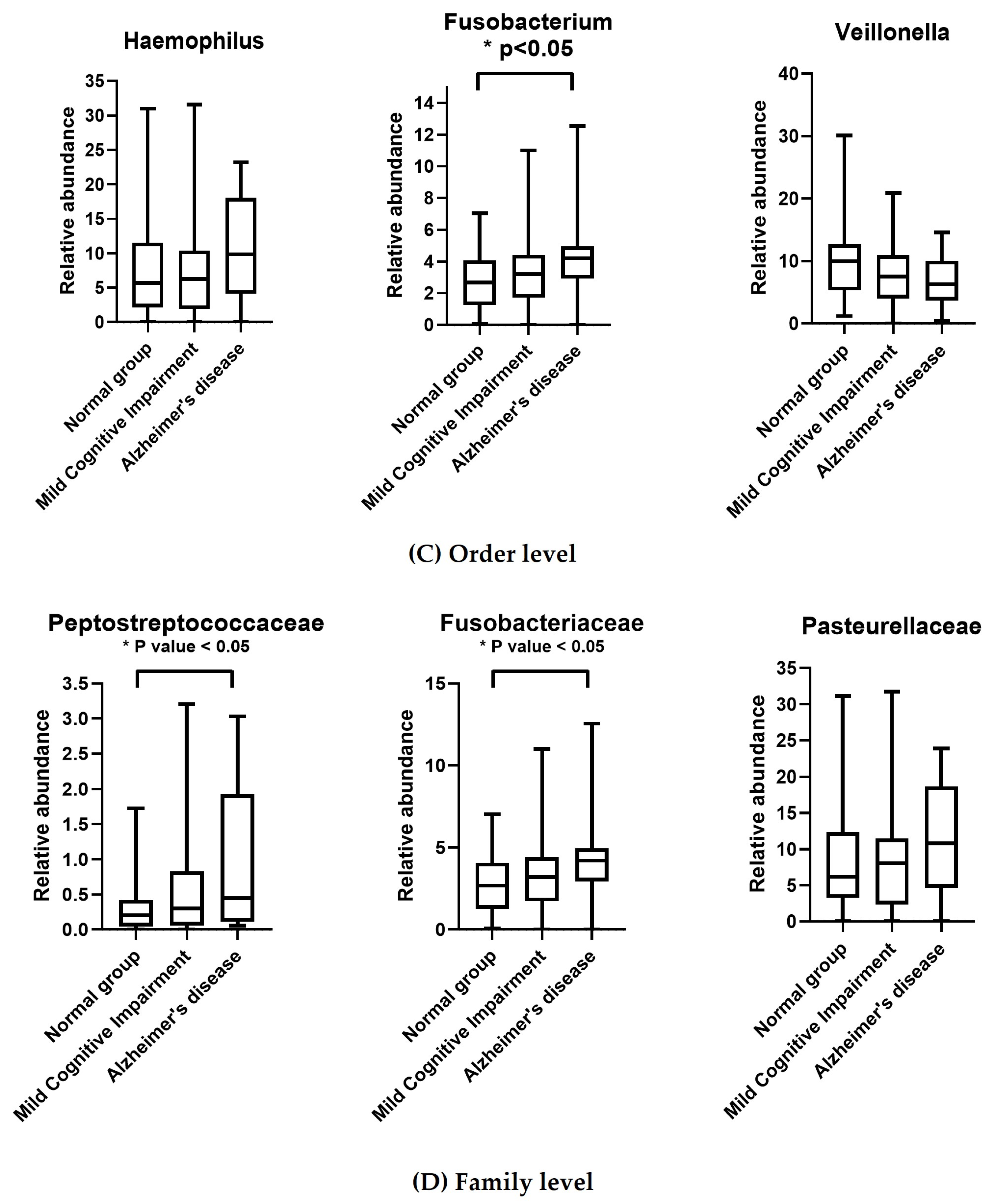
3.3. Taxonomic Classification of OTUs at the Phylum, Order, Family, and Genus Levels
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Selkoe, D.J. Alzheimer’s disease: Genes, proteins, and therapy. Physiol. Rev. 2001, 81, 741–766. [Google Scholar] [CrossRef] [PubMed]
- Prince, M.; Albanese, E.; Guerchet, M.; Prina, M. World Alzheimer Report 2014. Dementia and Risk Reduction: An Analysis of Protective and Modifiable Risk Factors; Alzheimer’s Disease International: London, UK, 2014. [Google Scholar]
- Vasanthakumar, A.; Davis, J.W.; Idler, K.; Waring, J.F.; Asque, E.; Riley-Gillis, B.; Grosskurth, S.; Srivastava, G.; Kim, S.; Nho, K.; et al. Harnessing peripheral DNA methylation differences in the Alzheimer’s Disease Neuroimaging Initiative (ADNI) to reveal novel biomarkers of disease. Clin. Epigenet. 2020, 12, 84. [Google Scholar] [CrossRef] [PubMed]
- Jack, C.R., Jr.; Knopman, D.S.; Jagust, W.J.; Petersen, R.C.; Weiner, M.W.; Aisen, P.S.; Shaw, L.M.; Vemuri, P.; Wiste, H.J.; Weigand, S.D.; et al. Tracking pathophysiological processes in Alzheimer’s disease: An updated hypothetical model of dynamic biomarkers. Lancet Neurol. 2013, 12, 207–216. [Google Scholar] [CrossRef]
- Dujardin, S.; Fernandes, A.; Bannon, R.; Commins, C.; De Los Santos, M.; Kamath, T.V.; Hayashi, M.; Hyman, B.T. Tau propagation is dependent on the genetic background of mouse strains. Brain Commun. 2022, 4, fcac048. [Google Scholar] [CrossRef] [PubMed]
- Oakley, H.; Cole, S.L.; Logan, S.; Maus, E.; Shao, P.; Craft, J.; Guillozet-Bongaarts, A.; Ohno, M.; Disterhoft, J.; Van Eldik, L.; et al. Intraneuronal beta-amyloid aggregates, neurodegeneration, and neuron loss in transgenic mice with five familial Alzheimer’s disease mutations: Potential factors in amyloid plaque formation. J. Neurosci. 2006, 26, 10129–10140. [Google Scholar] [CrossRef]
- Puka, M.; Sundell, K.; Lazarewicz, J.W.; Lehmann, A. Species differences in cerebral taurine concentrations correlate with brain water content. Brain Res. 1991, 548, 267–272. [Google Scholar] [CrossRef]
- Bondi, M.W.; Smith, G.E. Mild cognitive impairment: A concept and diagnostic entity in need of input from neuropsychology. J. Int. Neuropsychol. Soc. 2014, 20, 129–134. [Google Scholar] [CrossRef]
- Vos, S.J.; Verhey, F.; Frolich, L.; Kornhuber, J.; Wiltfang, J.; Maier, W.; Peters, O.; Ruther, E.; Nobili, F.; Morbelli, S.; et al. Prevalence and prognosis of Alzheimer’s disease at the mild cognitive impairment stage. Brain 2015, 138 Pt 5, 1327–1338. [Google Scholar] [CrossRef]
- Goldberg, T.E.; Koppel, J.; Keehlisen, L.; Christen, E.; Dreses-Werringloer, U.; Conejero-Goldberg, C.; Gordon, M.L.; Davies, P. Performance-based measures of everyday function in mild cognitive impairment. Am. J. Psychiatry 2010, 167, 845–853. [Google Scholar] [CrossRef]
- Kamer, A.R.; Craig, R.G.; Pirraglia, E.; Dasanayake, A.P.; Norman, R.G.; Boylan, R.J.; Nehorayoff, A.; Glodzik, L.; Brys, M.; de Leon, M.J. TNF-alpha and antibodies to periodontal bacteria discriminate between Alzheimer’s disease patients and normal subjects. J. Neuroimmunol. 2009, 216, 92–97. [Google Scholar] [CrossRef]
- Paudel, D.; Uehara, O.; Giri, S.; Yoshida, K.; Morikawa, T.; Kitagawa, T.; Matsuoka, H.; Miura, H.; Toyofuku, A.; Kuramitsu, Y.; et al. Effect of psychological stress on the oral-gut microbiota and the potential oral-gut-brain axis. Jpn. Dent. Sci. Rev. 2022, 58, 365–375. [Google Scholar] [CrossRef] [PubMed]
- Murdaca, G.; Colombo, B.M.; Cagnati, P.; Gulli, R.; Spano, F.; Puppo, F. Endothelial dysfunction in rheumatic autoimmune diseases. Atherosclerosis 2012, 224, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Kirjavainen, P.V.; Salminen, S.J.; Isolauri, E. Probiotic bacteria in the management of atopic disease: Underscoring the importance of viability. J. Pediatr. Gastroenterol. Nutr. 2003, 36, 223–227. [Google Scholar] [PubMed]
- Nagpal, R.; Neth, B.J.; Wang, S.; Craft, S.; Yadav, H. Modified Mediterranean-ketogenic diet modulates gut microbiome and short-chain fatty acids in association with Alzheimer’s disease markers in subjects with mild cognitive impairment. EBioMedicine 2019, 47, 529–542. [Google Scholar] [CrossRef]
- David, L.A.; Maurice, C.F.; Carmody, R.N.; Gootenberg, D.B.; Button, J.E.; Wolfe, B.E.; Ling, A.V.; Devlin, A.S.; Varma, Y.; Fischbach, M.A.; et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature 2014, 505, 559–563. [Google Scholar] [CrossRef]
- Cato, L.E.; Mckay, A.K.A.; L’Heureux, J.E.; Vanhatalo, A.; Jones, A.M.; Askew, C.D.; Slater, G.J.; Burke, L.M. Low Carbohydrate, High Fat Diet Alters the Oral Microbiome without Negating the Nitrite Response to Beetroot Juice Supplementation. Nutrients 2023, 15, 5123. [Google Scholar] [CrossRef]
- Shoer, S.; Shilo, S.; Godneva, A.; Ben-Yacov, O.; Rein, M.; Wolf, B.C.; Lotan-Pompan, M.; Bar, N.; Weiss, E.I.; Houri-Haddad, Y.; et al. Impact of dietary interventions on pre-diabetic oral and gut microbiome, metabolites and cytokines. Nat. Commun. 2023, 14, 5384. [Google Scholar] [CrossRef]
- Michels, N.; Zouiouich, S.; Vanderbauwhede, B.; Vanacker, J.; Indave Ruiz, B.I.; Huybrechts, I. Human microbiome and metabolic health: An overview of systematic reviews. Obes. Rev. 2022, 23, e13409. [Google Scholar] [CrossRef]
- Qin, J.; Li, R.; Raes, J.; Arumugam, M.; Burgdorf, K.S.; Manichanh, C.; Nielsen, T.; Pons, N.; Levenez, F.; Yamada, T.; et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature 2010, 464, 59–65. [Google Scholar] [CrossRef]
- Athanasopoulou, K.; Boti, M.A.; Adamopoulos, P.G.; Skourou, P.C.; Scorilas, A. Third-Generation Sequencing: The Spearhead towards the Radical Transformation of Modern Genomics. Life 2021, 12, 30. [Google Scholar] [CrossRef]
- Leblhuber, F.; Ehrlich, D.; Steiner, K.; Geisler, S.; Fuchs, D.; Lanser, L.; Kurz, K. The Immunopathogenesis of Alzheimer’s Disease Is Related to the Composition of Gut Microbiota. Nutrients 2021, 13, 361. [Google Scholar] [CrossRef] [PubMed]
- Jack, C.R., Jr.; Albert, M.S.; Knopman, D.S.; McKhann, G.M.; Sperling, R.A.; Carrillo, M.C.; Thies, B.; Phelps, C.H. Introduction to the recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s Dement. J. Alzheimer’s Assoc. 2011, 7, 257–262. [Google Scholar] [CrossRef]
- PacBio Amplification of Bacterial Full-Length 16S Gene with Barcoded Primers. Available online: https://www.pacb.com/wp-content/uploads/Procedure-checklist-Amplification-of-bacterial-full-length-16S-rRNA-gene-with-barcoded-primers.pdf (accessed on 14 August 2024).
- Zeibich, L.; Koebele, S.V.; Bernaud, V.E.; Ilhan, Z.E.; Dirks, B.; Northup-Smith, S.N.; Neeley, R.; Maldonado, J.; Nirmalkar, K.; Files, J.A.; et al. Surgical Menopause and Estrogen Therapy Modulate the Gut Microbiota, Obesity Markers, and Spatial Memory in Rats. Front. Cell Infect. Microbiol. 2021, 11, 702628. [Google Scholar] [CrossRef] [PubMed]
- Naumova, N.; Alikina, T.; Tupikin, A.; Kalmykova, A.; Soldatova, G.; Vlassov, V.; Kabilov, M. Human Gut Microbiome Response to Short-Term Bifidobacterium-Based Probiotic Treatment. Indian J. Microbiol. 2020, 60, 451–457. [Google Scholar] [CrossRef] [PubMed]
- Larsen, N.; Vogensen, F.K.; van den Berg, F.W.; Nielsen, D.S.; Andreasen, A.S.; Pedersen, B.K.; Al-Soud, W.A.; Sorensen, S.J.; Hansen, L.H.; Jakobsen, M. Gut microbiota in human adults with type 2 diabetes differs from non-diabetic adults. PLoS ONE 2010, 5, e9085. [Google Scholar] [CrossRef]
- Sokol, H.; Pigneur, B.; Watterlot, L.; Lakhdari, O.; Bermudez-Humaran, L.G.; Gratadoux, J.J.; Blugeon, S.; Bridonneau, C.; Furet, J.P.; Corthier, G.; et al. Faecalibacterium prausnitzii is an anti-inflammatory commensal bacterium identified by gut microbiota analysis of Crohn disease patients. Proc. Natl. Acad. Sci. USA 2008, 105, 16731–16736. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Lee, H.Y. Revisiting the Bacterial Phylum Composition in Metabolic Diseases Focused on Host Energy Metabolism. Diabetes Metab. J. 2020, 44, 658–667. [Google Scholar] [CrossRef]
- Yutin, N.; Galperin, M.Y. A genomic update on clostridial phylogeny: Gram-negative spore formers and other misplaced clostridia. Environ. Microbiol. 2013, 15, 2631–2641. [Google Scholar] [CrossRef]
- Ezaki, T. Peptostreptococcaceae fam. nov. In Bergey’s Manual of Systematics of Archaea and Bacteria; John Wiley & Sons, Inc.: Hoboken, NJ, USA; Bergey’s Manual Trust: Gießen, Germany, 2015; pp. 1–5. [Google Scholar]
- Simopoulos, C.M.A.; Ning, Z.; Zhang, X.; Li, L.; Walker, K.; Lavallée-Adam, M.; Figeys, D. pepFunk: A tool for peptide-centric functional analysis of metaproteomic human gut microbiome studies. Bioinformatics 2020, 36, 4171–4179. [Google Scholar] [CrossRef]
- Harris, S.; Croft, J.; O’Flynn, C.; Deusch, O.; Colyer, A.; Allsopp, J.; Milella, L.; Davis, I.J. A Pyrosequencing Investigation of Differences in the Feline Subgingival Microbiota in Health, Gingivitis and Mild Periodontitis. PLoS ONE 2015, 10, e0136986. [Google Scholar] [CrossRef]
- Wells, R.E.; Kerr, C.E.; Wolkin, J.; Dossett, M.; Davis, R.B.; Walsh, J.; Wall, R.B.; Kong, J.; Kaptchuk, T.; Press, D.; et al. Meditation for adults with mild cognitive impairment: A pilot randomized trial. J. Am. Geriatr. Soc. 2013, 61, 642–645. [Google Scholar] [CrossRef] [PubMed]
- Robinson, A.; Wilde, J.; Allen-Vercoe, E. Chapter 6—Fusobacteria: Physiology, form, and function Funding sources: Canadian Cancer Society Research Institute and Cancer Research UK grants to EAV. In Colorectal Neoplasia and the Colorectal Microbiome; Floch, M.H., Ed.; Academic Press: Cambridge, MA, USA, 2020; pp. 95–134. [Google Scholar]
- Park, C.H.; Han, D.S.; Oh, Y.-H.; Lee, A.R.; Lee, Y.-R.; Eun, C.S. Role of Fusobacteria in the serrated pathway of colorectal carcinogenesis. Sci. Rep. 2016, 6, 25271. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Xu, X.; Wu, X.; Cao, H.; Li, X.; Hou, Z.; Wang, B.; Liu, J.; Ji, X.; Zhang, P.; et al. A comparison of the composition and functions of the oral and gut microbiotas in Alzheimer’s patients. Front. Cell Infect. Microbiol. 2022, 12, 942460. [Google Scholar] [CrossRef] [PubMed]
- Moreira de Gouveia, M.I.; Bernalier-Donadille, A.; Jubelin, G. Enterobacteriaceae in the Human Gut: Dynamics and Ecological Roles in Health and Disease. Biology 2024, 13, 142. [Google Scholar] [CrossRef] [PubMed]
- Luca, M.; Chattipakorn, S.C.; Sriwichaiin, S.; Luca, A. Cognitive-Behavioural Correlates of Dysbiosis: A Review. Int. J. Mol. Sci. 2020, 21, 4834. [Google Scholar] [CrossRef]
- Kaur, H.; Bose, C.; Mande, S.S. Tryptophan Metabolism by Gut Microbiome and Gut-Brain-Axis: An in-silico Analysis. Front. Neurosci. 2019, 13, 1365. [Google Scholar] [CrossRef]
- Macke, L.; Schulz, C.; Koletzko, L.; Malfertheiner, P. Systematic review: The effects of proton pump inhibitors on the microbiome of the digestive tract-evidence from next-generation sequencing studies. Aliment. Pharmacol. Ther. 2020, 51, 505–526. [Google Scholar] [CrossRef]
- Karamujić-Čomić, H.; Ahmad, S.; Radjabzadeh, D.; Bonnechere, B.; Kaddurah-Daouk, R.F.; Kraaij, R.; Ikram, M.A.; Amin, N.; van Duijn, C.M. Clostridium shows a higher abundance in less neurovascular and neurodegenerative changes: A microbiome-wide association study. Alzheimer’s Dement. 2020, 16, e044743. [Google Scholar] [CrossRef]
- Yoon, C.H.; Ryu, J.S.; Moon, J.; Kim, M.K. Association between aging-dependent gut microbiome dysbiosis and dry eye severity in C57BL/6 male mouse model: A pilot study. BMC Microbiol. 2021, 21, 106. [Google Scholar] [CrossRef]
- Lu, X.; Xue, Z.; Qian, Y.; Wei, S.; Qiao, Y.; Zhang, W.; Lu, H. Changes in intestinal microflora and its metabolites underlie the cognitive impairment in preterm rats. Front. Cell Infect. Microbiol. 2022, 12, 945851. [Google Scholar] [CrossRef]
- Zhou, P.; Li, X.; Huang, I.H.; Qi, F. Veillonella Catalase Protects the Growth of Fusobacterium nucleatum in Microaerophilic and Streptococcus gordonii-Resident Environments. Appl. Environ. Microbiol. 2017, 83, e01079-17. [Google Scholar] [CrossRef] [PubMed]
- Jungbauer, G.; Stahli, A.; Zhu, X.; Auber Alberi, L.; Sculean, A.; Eick, S. Periodontal microorganisms and Alzheimer disease—A causative relationship? Periodontology 2000 2022, 89, 59–82. [Google Scholar] [CrossRef]
- Maitre, Y.; Micheneau, P.; Delpierre, A.; Mahalli, R.; Guerin, M.; Amador, G.; Denis, F. Did the Brain and Oral Microbiota Talk to Each Other? A Review of the Literature. J. Clin. Med. 2020, 9, 3876. [Google Scholar] [CrossRef]
- Chen, C.K.; Wu, Y.T.; Chang, Y.C. Association between chronic periodontitis and the risk of Alzheimer’s disease: A retrospective, population-based, matched-cohort study. Alzheimers Res. Ther. 2017, 9, 56. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Ishikawa, T.; Sasaki, M.; Chiba, T. Oral and Gut Microbial Dysbiosis and Non-alcoholic Fatty Liver Disease: The Central Role of Porphyromonas gingivalis. Front. Med. 2022, 9, 822190. [Google Scholar] [CrossRef] [PubMed]
- Neculae, E.; Gosav, E.M.; Valasciuc, E.; Dima, N.; Floria, M.; Tanase, D.M. The Oral Microbiota in Valvular Heart Disease: Current Knowledge and Future Directions. Life 2023, 13, 182. [Google Scholar] [CrossRef]
- Li, Y.; Zhu, M.; Liu, Y.; Luo, B.; Cui, J.; Huang, L.; Chen, K.; Liu, Y. The oral microbiota and cardiometabolic health: A comprehensive review and emerging insights. Front. Immunol. 2022, 13, 1010368. [Google Scholar] [CrossRef]
- Zhang, J.; Su, L.; Wang, Y.; Deng, S. Improved High-Throughput Sequencing of the Human Oral Microbiome: From Illumina to PacBio. Can. J. Infect. Dis. Med. Microbiol. 2020, 2020, 6678872. [Google Scholar] [CrossRef] [PubMed]
- Buetas, E.; Jordan-Lopez, M.; Lopez-Roldan, A.; D’Auria, G.; Martinez-Priego, L.; De Marco, G.; Carda-Dieguez, M.; Mira, A. Full-length 16S rRNA gene sequencing by PacBio improves taxonomic resolution in human microbiome samples. BMC Genom. 2024, 25, 310. [Google Scholar] [CrossRef]
- Narayan, N.R.; Mendez-Lagares, G.; Ardeshir, A.; Lu, D.; Van Rompay, K.K.; Hartigan-O’Connor, D.J. Persistent effects of early infant diet and associated microbiota on the juvenile immune system. Gut Microbes 2015, 6, 284–289. [Google Scholar] [CrossRef]
- Bianchi, S.; Fantozzi, G.; Bernardi, S.; Antonouli, S.; Continenza, M.A.; Macchiarelli, G. Commercial oral hygiene products and implant collar surfaces: Scanning electron microscopy observations. Can. J. Dent. Hyg. 2020, 54, 26–31. [Google Scholar] [PubMed]
- Wan, J.; Fan, H. Oral Microbiome and Alzheimer’s Disease. Microorganisms 2023, 11, 2550. [Google Scholar] [CrossRef] [PubMed]
- Pignatelli, P.; Nuccio, F.; Piattelli, A.; Curia, M.C. The Role of Fusobacterium nucleatum in Oral and Colorectal Carcinogenesis. Microorganisms 2023, 11, 2358. [Google Scholar] [CrossRef] [PubMed]
| Basic Profile | AD | MCI | Control |
|---|---|---|---|
| Sample size, N | 10 | 46 | 44 |
| Male:female | 4:6 | 14:32 | 12:32 |
| Age, years | 66.90 ± 7.06 | 68.50 ± 6.35 | 64.73 ± 4.78 |
| CDR score | 1.45 (0.5–2) | 0.41 (0–1) | n/a |
| MoCA score | 13.90 (5–19) | 22.36 (16–27) | 26.91 (20–30) |
| MMSE score | 16.70 (12–21) | 25.89 (20–29) | 28.14 (24–30) |
| Group | AD | MCI | Control |
|---|---|---|---|
| Unique tags | 32,425 ± 17,819 * | 27,309 ± 11,052 | 26,583 ± 6887 |
| No. of OTUs | 31,105 ± 17,441 * | 25,641 ± 10,610 | 25,156.7 ± 7182 |
| Richness index | 60.8 ± 22.56 | 50.67 ± 14.77 | 50.0 ± 11.75 |
| Diversity index (Shannon) | 7.04 ± 1.42 | 6.48 ± 1.22 | 6.54 ± 1.09 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sritana, N.; Phungpinij, A. Analysis of Oral Microbiota in Elderly Thai Patients with Alzheimer’s Disease and Mild Cognitive Impairment. Int. J. Environ. Res. Public Health 2024, 21, 1242. https://doi.org/10.3390/ijerph21091242
Sritana N, Phungpinij A. Analysis of Oral Microbiota in Elderly Thai Patients with Alzheimer’s Disease and Mild Cognitive Impairment. International Journal of Environmental Research and Public Health. 2024; 21(9):1242. https://doi.org/10.3390/ijerph21091242
Chicago/Turabian StyleSritana, Narongrit, and Atitaya Phungpinij. 2024. "Analysis of Oral Microbiota in Elderly Thai Patients with Alzheimer’s Disease and Mild Cognitive Impairment" International Journal of Environmental Research and Public Health 21, no. 9: 1242. https://doi.org/10.3390/ijerph21091242
APA StyleSritana, N., & Phungpinij, A. (2024). Analysis of Oral Microbiota in Elderly Thai Patients with Alzheimer’s Disease and Mild Cognitive Impairment. International Journal of Environmental Research and Public Health, 21(9), 1242. https://doi.org/10.3390/ijerph21091242







