Management of Patients with Colorectal Cancer through Fast-Track Surgery
Abstract
1. Introduction
2. Materials and Methods
2.1. Define
2.2. Measure
- Age;
- Gender;
- Type of admission;
- Ward of admission;
- Type of tumor;
- Diabetes;
- Hypertension;
- Abdominal Adherences;
- Cardiological disorders;
- Respiratory disorders;
- Gallstones;
- Peritonitis;
- Other tumors;
- Complicated procedure;
- Mode of discharge.
- Surgical procedures:
- ▪ 4571 multiple segmental resection of the large intestine;
- ▪ 4572 cecum resection;
- ▪ 4573 right hemicolectomy;
- ▪ 4574 transverse colon resection;
- ▪ 4575 left hemicolectomy;
- ▪ 4576 sigmoidectomy;
- ▪ 4579 other partial resection of the large intestine;
- ▪ 458 total intra-abdominal colectomy;
- ▪ 485 rectum resection by the abdominoperineal route;
- ▪ 4861 transaxillary rectosigmoidectomy;
- ▪ 4862 anterior resection of the rectum with concomitant colostomy;
- ▪ 4863 other anterior resection of the rectum;
- ▪ 4864 posterior resection of the rectum;
- ▪ 4865 resection of the rectum according to Duhamel;
- ▪ 4869 other resection of the rectum.
- A main or secondary diagnosis of the following:
- ▪ 1530 malignant tumors of the hepatic flexure;
- ▪ 1531 malignant tumors of the transverse colon;
- ▪ 1532 malignant tumors of the descending colon;
- ▪ 1533 malignant tumors of the sigma;
- ▪ 1534 malignant tumors of the cecum;
- ▪ 1535 malignant tumors of the appendix;
- ▪ 1536 malignant tumors of the ascending colon;
- ▪ 1537 malignant tumors of the splenic flexure;
- ▪ 1538 malignant tumors of other (specified) sites of the large intestine;
- ▪ 1540 malignant tumors of the rectosigmoid junction;
- ▪ 1541 malignant tumors of the rectum;
- ▪ 1542 malignant tumors of the anal canal;
- ▪ 1543 malignant tumors of the anus, unspecified;
- ▪ 1548 other malignant tumors of the rectum, rectosigmoid junction, and anus.
2.3. Analyze
2.4. Improve
2.5. Control
3. Results
4. Discussion
5. Conclusions
6. Authors’ Opinion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- IARC Globocan 2012. Cancer Fact Sheet: Colorectal Cancer Estimated Incidence, Mortality and Prevalence Worldwide in 2012. Available online: http://globocan.iarc.fr/Pages/fact_sheets_cancer.aspx (accessed on 23 December 2023).
- Rossi, P.G.; Vicentini, M.; Sacchettini, C.; Di Felice, E.; Caroli, S.; Ferrari, F.; Mangone, L.; Pezzarossi, A.; Roncaglia, F.; Campari, C.; et al. Impact of screening program on incidence of colorectal cancer: A cohort study in Italy. Off. J. Am. Coll. Gastroenterol. ACG 2015, 110, 1359–1366. [Google Scholar] [CrossRef] [PubMed]
- Mandel, J.S.; Bond, J.H.; Church, T.R.; Snover, D.C.; Bradley, G.M.; Schuman, L.M.; Ederer, F. Reducing mortality from colorectal cancer by screening for faecal occult blood. N. Engl. J. Med. 1993, 328, 1365–1371. [Google Scholar] [CrossRef] [PubMed]
- Hardcastle, J.D.; Chamberlain, J.O.; Robinson, M.H.; Moss, S.M.; Amar, S.S.; Balfour, T.W.; James, P.D.; Mangham, C.M. Randomised controlled trial of faecal occult blood screening for colorectal cancer. Lancet 1996, 348, 1472–1477. [Google Scholar] [CrossRef] [PubMed]
- Kewenter, J.; Brevinge, H.; Engaras, B.; Haglind, E.; Ährén, C. Results of screening, rescreening, and follow-up in a prospective, randomized study for the detection of colorectal cancer by fecal occult blood testing. Results for 68308 subjects. Scand. J. Gastroenterol. 1994, 29, 468–473. [Google Scholar] [CrossRef] [PubMed]
- Kronborg, O.; Fenger, C.; Olsen, J.; Jørgensen, O.D.; Søndergaard, O. Randomised study of screening for colorectal cancer with faecal occult blood test. Lancet 1996, 348, 1467–1471. [Google Scholar] [CrossRef]
- Hewitson, P.; Glasziou, P.P.; Irwig, L.; Towler, B.; Watson, E. Screening for colorectal cancer using the faecal occult blood test, Hemoccult. Cochrane Database Syst. Rev. 2007, 24, CD001216. [Google Scholar] [CrossRef]
- Allaix, M.E.; Furnee, E.J.; Mistrangelo, M.; Arezzo, A.; Morino, M. Conversion of laparoscopic colorectal resection for cancer: What is the impact on short-term outcomes and survival? World J. Gastroenterol. 2016, 22, 8304–8313. [Google Scholar] [CrossRef]
- Ni, X.; Jia, D.; Chen, Y.; Wang, L.; Suo, J. Is the enhanced recovery after surgery (ERAS) program effective and safe in laparoscopic colorectal cancer surgery? A meta-analysis of randomized controlled trials. J. Gastrointest. Surg. 2019, 23, 1502–1512. [Google Scholar] [CrossRef]
- Jacobs, M.; Verdeja, J.C.; Goldstein, H.S. Minimally invasive colon resection (laparoscopic colectomy). Surg. Laparosc. Endosc. 1991, 1, 144–150. [Google Scholar]
- Noel, J.K.; Fahrbach, K.; Estok, R.; Cella, C.; Frame, D.; Linz, H.; Cima, R.R.; Dozois, E.J.; Senagore, A.J. Minimally invasive colorectal resection outcomes: Short-term comparison with open procedures. J. Am. Coll. Surg. 2007, 204, 291–307. [Google Scholar] [CrossRef]
- Kaltoft, B.; Gogenur, I.; Rosenberg, J. Reduced length of stay and convalescence in laparoscopic vs open sigmoid resection with traditional care: A double blinded randomized clinical trial. Color. Dis. Off. J. Assoc. Coloproctology Great Br. Irel. 2011, 13, e123–e130. [Google Scholar] [CrossRef] [PubMed]
- Mueck, K.M.; Putnam, L.R.; Kao, L.S. Improving the quality of quality improvement reporting standards for quality improvement reporting excellence (SQUIRE) 2.0 guidelines. JAMA Surg. 2016, 151, 311–312. [Google Scholar] [CrossRef]
- Castorina, S.; Guglielmino, C.; Castrogiovanni, P.; Szychlinska, M.A.; Ioppolo, F.; Massimino, P.; Musumeci, G. Clinical evidence of traditional vs fast track recovery methodologies after total arthroplasty for osteoarthritic knee treatment. A retrospective observational study. Muscles Ligaments Tendons J. 2017, 7, 504–513. [Google Scholar] [CrossRef] [PubMed]
- Aditya, J.N.; Prabhakar, S. Fast-track surgery: Toward comprehensive peri-operative care. Anesth. Essays Res. 2014, 8, 127–133. [Google Scholar]
- Husted, H. Fast-track hip and knee arthroplasty: Clinical and organizational aspects. Acta Orthop. Suppl. 2012, 83, 1–39. [Google Scholar] [CrossRef]
- Kehlet, H.; Slim, K. The future of fast–track surgery. Br. J. Surg. 2012, 99, 1025–1026. [Google Scholar] [CrossRef]
- Kehlet, H. Fast-track hip and knee arthroplasty. Lancet 2013, 381, 1600–1602. [Google Scholar] [CrossRef] [PubMed]
- Robinson, S.; Radnor, Z.J.; Burgess, N.; Worthington, C. SimLean: Utilising Simulation in the Implementation of Lean in Healthcare. Eur. J. Oper. Res. 2012, 219, 188–197. [Google Scholar] [CrossRef]
- Scala, A.; Trunfio, T.A.; Improta, G. Classification and regression model to manage the hospitalization for laparoscopic cholecystectomy. Sci. Rep. 2023, 13, 14700. [Google Scholar] [CrossRef]
- Mosadeghrad, A.M. Developing and Validating a Total Quality Management Model for Healthcare Organisations. TQM J. 2015, 27, 544–564. [Google Scholar] [CrossRef]
- Improta, G.; Ponsiglione, A.M.; Parente, G.; Romano, M.; Cesarelli, G.; Rea, T.; Russo, M.; Triassi, M. Evaluation of Medical Training Courses Satisfaction: Qualitative Analysis and Analytic Hierarchy Process. In Proceedings of the 8th European Medical and Biological Engineering Conference, Portorož, Slovenia, 29 November–3 December 2020; Jarm, T., Cvetkoska, A., Mahnič-Kalamiza, S., Miklavcic, D., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 518–526. [Google Scholar]
- Bevan, H. Lean Six Sigma: Some Basic Concepts; NHS Institute for Innovation and Improvement: Coventry, UK, 2006. [Google Scholar]
- Sorrentino, A.; Scala, A.; Fiorillo, A.; Latessa, I.; Abbate, V.; Dell’Aversana Orabona, G. Six Sigma Approach for a First Evaluation of a Pharmacological Therapy in Tongue Cancer. In Proceedings of the 8th European Medical and Biological Engineering Conference, Portorož, Slovenia, 29 November–3 December 2020; Jarm, T., Cvetkoska, A., Mahnič-Kalamiza, S., Miklavcic, D., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 1028–1037. [Google Scholar]
- Joosten, T.; Bongers, I.; Janssen, R. Application of Lean Thinking to Health Care: Issues and Observations. Int. J. Qual. Health Care 2009, 21, 341–347. [Google Scholar] [CrossRef] [PubMed]
- van Lent, W.A.; Goedbloed, N.; van Harten, W. Improving the efficiency of a chemotherapy day unit: Applying a business approach to oncology. Eur. J. Cancer. 1990, 45, 800–806. [Google Scholar] [CrossRef] [PubMed]
- Bisgaard, S.; Does, R.J.M.M. Quality Quandaries: Healthcare quality: Reducing the length of stay at a hospital. Qual. Eng. 2008, 21, 117–131. [Google Scholar] [CrossRef]
- Fiorillo, A.; Sorrentino, A.; Scala, A.; Abbate, V.; Dell’aversana Orabona, G. Improving performance of the hospitalization process by applying the principles of Lean Thinking. TQM J. 2021, 33, 253–271. [Google Scholar] [CrossRef]
- Mahesh, B.P.; Soragaon, B.; Annigeri, A.R. Reduction of patient wait time at a multi-specialty hospital using DMAIC methodology and factor Analysis. Int. J. Eng. Technol 2018, 7, 309–312. [Google Scholar]
- Scala, A.; Ponsiglione, A.M.; Loperto, I.; Della Vecchia, A.; Borrelli, A.; Russo, G.; Triassi, M.; Improta, G. Lean six sigma approach for reducing length of hospital stay for patients with femur fracture in a university hospital. Int. J. Environ. Res. Public Health 2021, 18, 2843. [Google Scholar] [CrossRef]
- Martens, L.; Goode, G.; Wold, J.F.; Beck, L.; Martin, G.; Perings, C.; Stolt, P.; Baggerman, L. Structured syncope care pathways based on lean six sigma methodology optimises resource use with shorter time to diagnosis and increased diagnostic yield. PLoS ONE 2014, 9, e100208. [Google Scholar] [CrossRef] [PubMed]
- Trunfio, T.A.; Marino, M.R.; Giglio, C.; Majolo, M.; Longo, G.; Basso, M.A.; Rossi, G.; Borrelli, A.; Triassi, M. Impact of COVID-19 in a Surgery Department: Comparison Between Two Italian Hospitals. In Proceedings of the International Symposium on Biomedical and Computational Biology, Virtual Event, 13–15 August 2022; Springer International Publishing: Cham, Switzerland; pp. 537–544. [Google Scholar]
- Granziera, E.; Guglieri, I.; Del Bianco, P.; Capovilla, E.; Ciccarese, A.A.; Kilmartin, D.; Manfredi, V.; De Salvo, G.L. A multidisciplinary approach to improve preoperative understanding and reduce anxiety: A randomised study. Eur. J. Anaesthesiol. EJA 2013, 30, 734–742. [Google Scholar] [CrossRef]
- Wongkietkachorn, A.; Wongkietkachorn, N.; Rhunsiri, P. Preoperative needs-based education to reduce anxiety, increase satisfaction, and decrease time spent in day surgery: A randomized controlled trial. World J. Surg. 2018, 42, 666–674. [Google Scholar] [CrossRef]
- Pressoir, M.; Desné, S.; Berchery, D.; Rossignol, G.; Poiree, B.; Meslier, M.; Traversier, S.; Vittot, M.; Simon, M.; Gekiere, J.P.; et al. Prevalence, risk factors and clinical implications of malnutrition in French Comprehensive Cancer Centres. Br. J. Cancer 2010, 102, 966–971. [Google Scholar] [CrossRef]
- Jie, B.; Jiang, Z.M.; Nolan, M.T.; Zhu, S.N.; Yu, K.; Kondrup, J. Impact of preoperative nutritional support on clinical outcome in abdominal surgical patients at nutritional risk. Nutrition 2012, 28, 1022–1027. [Google Scholar] [CrossRef] [PubMed]
- Walsh, H. Reducing Postoperative Length of Stay for Colorectal Cancer stoma Creation Patients. Ph.D. Thesis, Royal College of Surgeons in Ireland, Dublin, Ireland, 2023. [Google Scholar]
- Zhao, J.H.; Sun, J.X.; Gao, P.; Chen, X.W.; Song, Y.X.; Huang, X.Z.; Xu, H.M.; Wang, Z.N. Fast-track surgery versus traditional perioperative care in laparoscopic colorectal cancer surgery: A meta-analysis. BMC Cancer 2014, 14, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Kehlet, H. Fast-track colorectal surgery. Lancet 2008, 371, 791–793. [Google Scholar] [CrossRef] [PubMed]
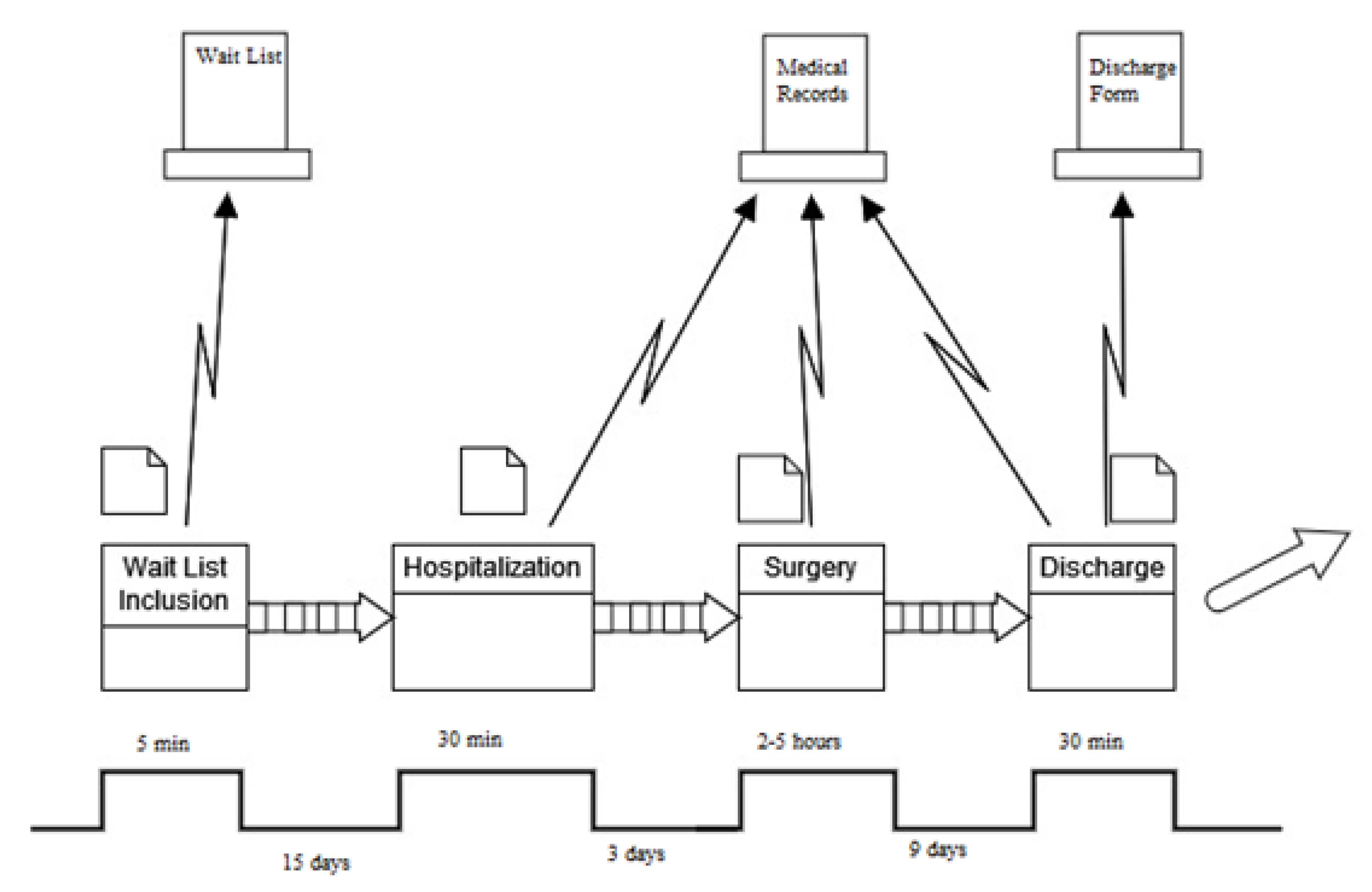


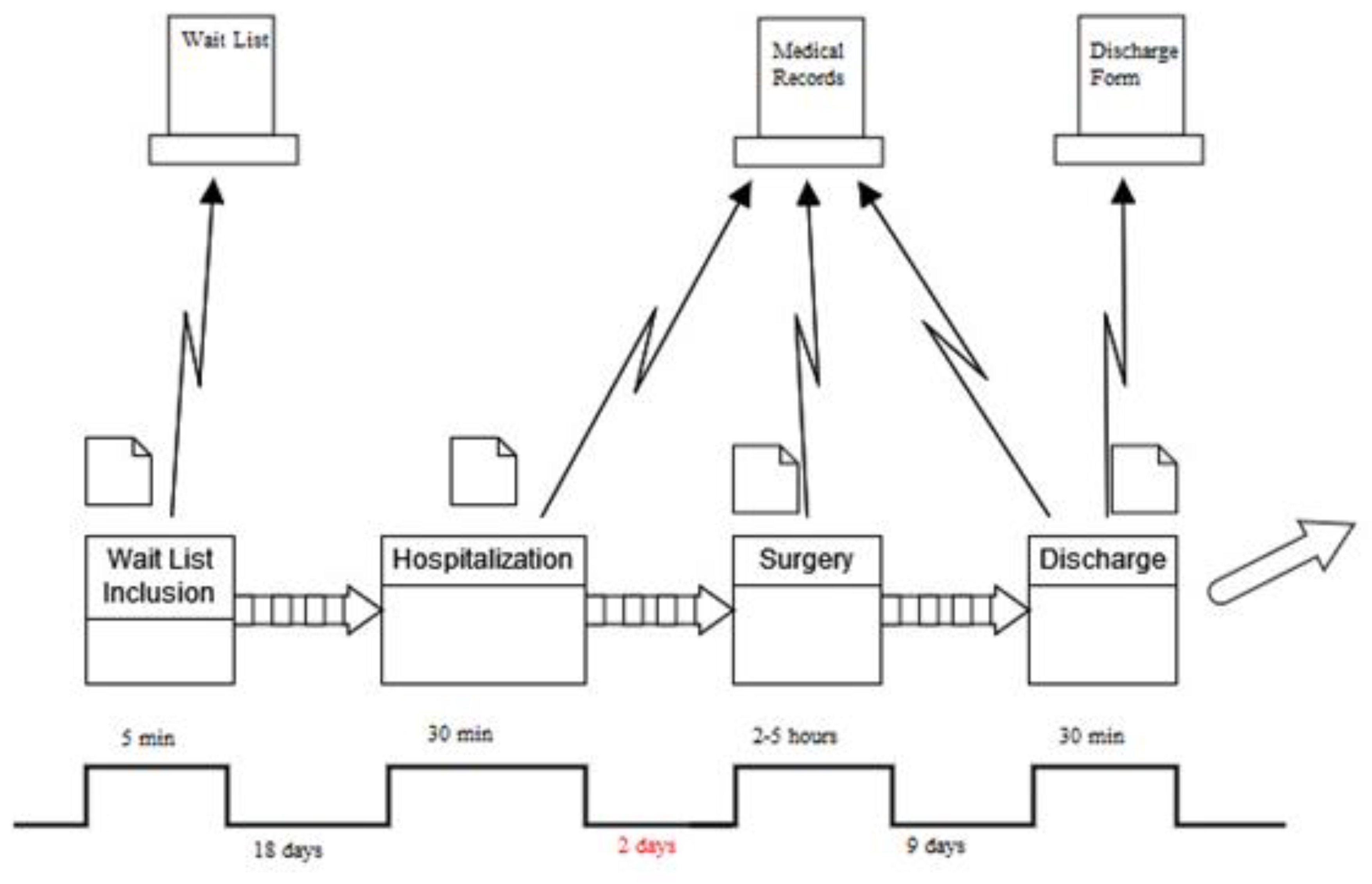

| Project Title Fast-Track Surgery for Colorectal Cancer | |
|---|---|
| Problem statement An inappropriate prolongation of the length of hospital stay for patients undergoing surgery for colorectal cancer | Objective statement Introduce a clinical pathway that can solve the presented problem |
| Critical to quality The CTQ is the duration of the length of hospital stay | Target Realize a corrective measure to reduce the CTQ |
| Project leader: Names will be added if the paper is accepted Project champion: Team members: | |
| Timeline Define → May–August 2018 Measure → September–October 2018 Analyze → November 2018–April 2019 Improve → May–June 2019 Control → January 2020–December 2022 | |
In scope
| Out of scope
|
| Supplier | Input | Process | Output | Customer |
|---|---|---|---|---|
| Complex Operative Unit of General Surgery | Surgical and medical services. | Care process:
|
|
|
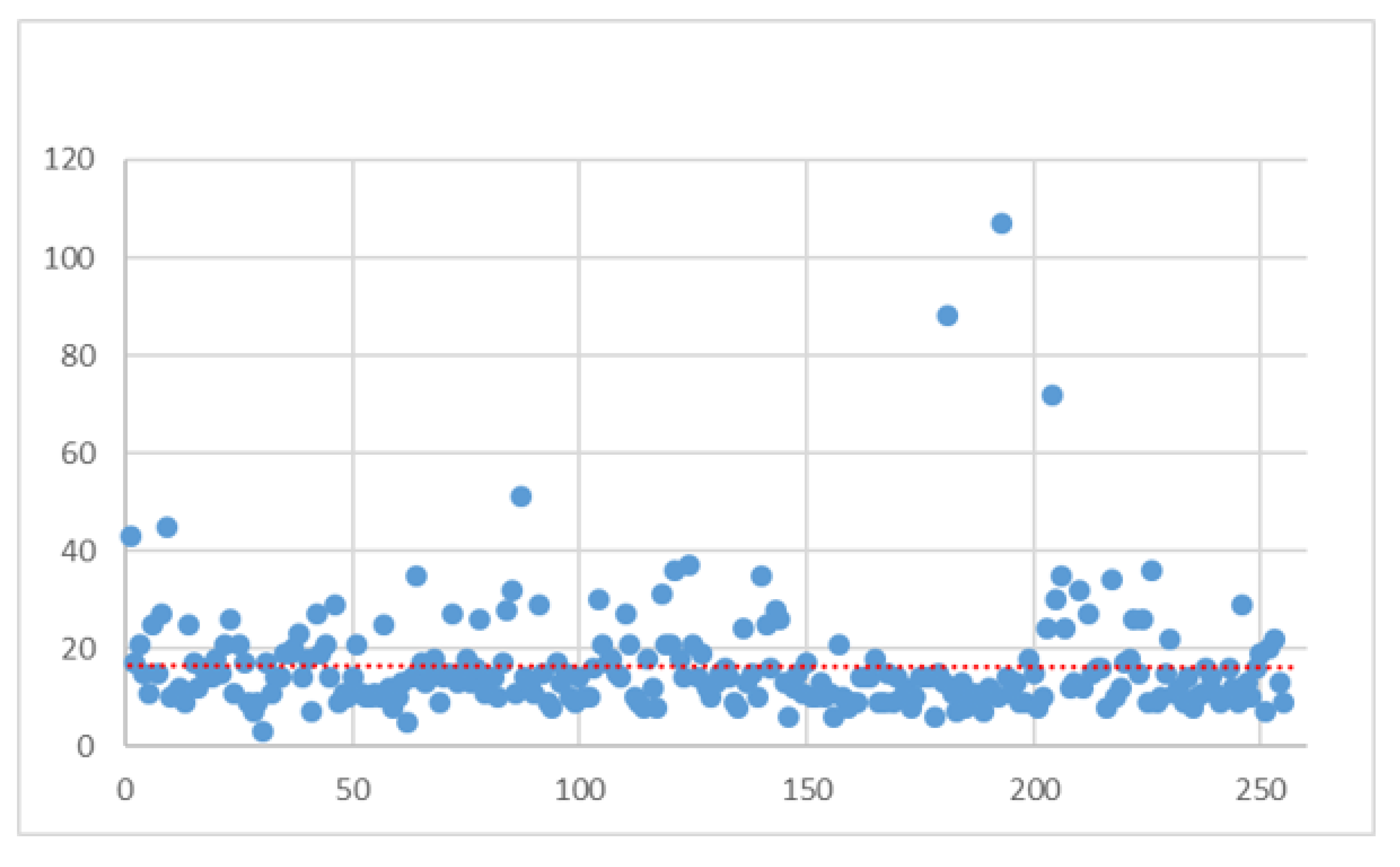
| Variable | Value | N | Standard Deviation | Min | Max | Median |
|---|---|---|---|---|---|---|
| All | All | 255 | 11.01 | 3 | 107 | 14 |
| Age | Under 50 | 18 | 8.13 | 6 | 32 | 13 |
| 50 ≤ Age ≤ 65 | 76 | 10.85 | 7 | 88 | 12 | |
| 66 ≤ Age ≤ 75 | 90 | 10.19 | 6 | 72 | 14 | |
| Over 75 | 71 | 12.75 | 3 | 107 | 14 | |
| Gender | Male | 140 | 11.20 | 3 | 88 | 14 |
| Female | 115 | 10.79 | 5 | 107 | 14 | |
| Type of Admission | Scheduled | 63 | 13.47 | 8 | 88 | 14 |
| Scheduled with pre-hospitalization | 192 | 10.02 | 3 | 107 | 14 | |
| Ward of Admission | Other surgeries | 234 | 10.70 | 3 | 107 | 14 |
| Specialized ward | 21 | 14.31 | 7 | 72 | 13 | |
| Type of Tumor | Colon | 107 | 8.97 | 3 | 72 | 14 |
| Rectum–sigma | 130 | 12.71 | 6 | 107 | 14 | |
| Abdominal organs | 4 | 7.05 | 10 | 27 | 20.5 | |
| Other abdominal tumors | 14 | 8.81 | 5 | 36 | 10.5 | |
| Diabetes | No | 243 | 11.21 | 3 | 107 | 14 |
| Yes | 12 | 6.05 | 8 | 30 | 15 | |
| Hypertension | No | 229 | 11.48 | 3 | 107 | 14 |
| Yes | 26 | 5.18 | 7 | 27 | 14 | |
| Abdominal Adherences | No | 244 | 9.45 | 3 | 88 | 14 |
| Yes | 11 | 29.06 | 8 | 107 | 11 | |
| Cardiological Disorders | No | 204 | 9.76 | 5 | 88 | 13 |
| Yes | 51 | 14.61 | 3 | 107 | 16 | |
| Respiratory Disorders | No | 227 | 9.53 | 5 | 88 | 13 |
| Yes | 28 | 18.12 | 3 | 107 | 21 | |
| Gallstones | No | 248 | 11.12 | 3 | 107 | 14 |
| Yes | 7 | 6.32 | 9 | 27 | 13 | |
| Peritonitis | No | 234 | 8.21 | 3 | 88 | 14 |
| Yes | 21 | 23.82 | 8 | 107 | 24 | |
| Other Tumors | No | 234 | 11.37 | 3 | 107 | 14 |
| Yes | 21 | 5.63 | 7 | 27 | 14 | |
| Complicated Procedure | No | 138 | 11.99 | 5 | 107 | 12 |
| Yes | 117 | 9.38 | 3 | 72 | 16 | |
| Mode of Discharge | Dead | 9 | 12.98 | 3 | 45 | 24 |
| To home | 239 | 7.97 | 5 | 72 | 14 | |
| Voluntary | 5 | 33.24 | 6 | 88 | 19 | |
| Transferred to another institution | 0 | 0.00 | 0 | 0 | 0 | |
| Transferred to another regime | 2 | 64.35 | 16 | 107 | 61.5 | |
| Transferred to rehabilitation institution | 0 | 0.00 | 0 | 0 | 0 |
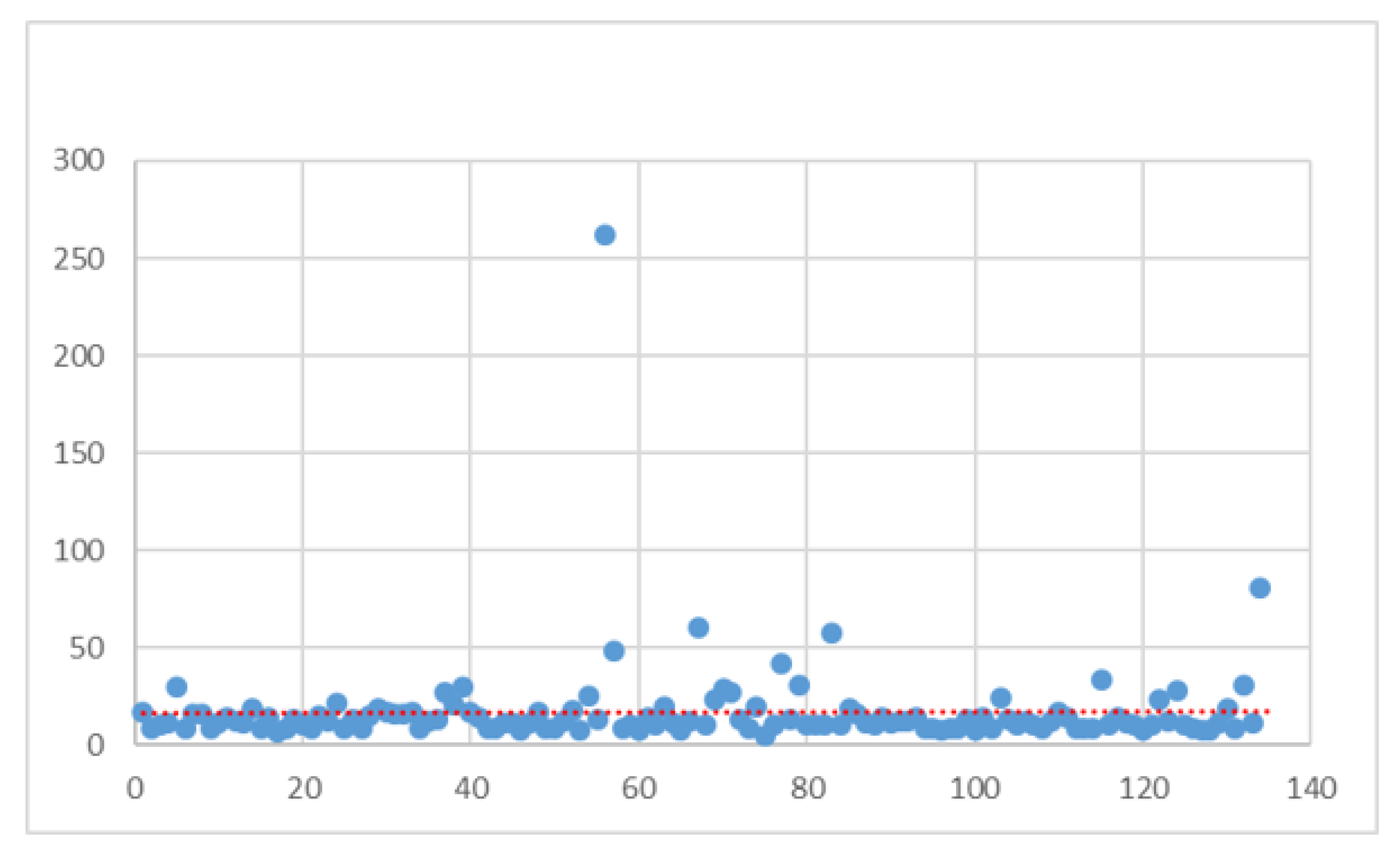
| Variable | Value | N | Standard Deviation | Min | Max | Median |
|---|---|---|---|---|---|---|
| All | All | 134 | 23.80 | 5 | 262 | 12 |
| Age | Under 50 | 7 | 9.69 | 9 | 34 | 17 |
| 50 ≤ Age ≤ 65 | 42 | 9.95 | 8 | 58 | 11.5 | |
| 66 ≤ Age ≤ 75 | 52 | 11.61 | 5 | 81 | 11 | |
| Over 75 | 33 | 44.10 | 8 | 262 | 13 | |
| Gender | Male | 87 | 28.77 | 5 | 262 | 11 |
| Female | 47 | 9.39 | 8 | 58 | 13 | |
| Type of Admission | Scheduled | 24 | 12.42 | 8 | 58 | 16 |
| Scheduled with pre-hospitalization | 110 | 25.64 | 5 | 262 | 11 | |
| Ward of Admission | Other surgeries | 124 | 10.84 | 5 | 81 | 11 |
| Specialized ward | 10 | 77.86 | 9 | 262 | 15.5 | |
| Type of Tumor | Colon | 64 | 31.68 | 7 | 262 | 12 |
| Rectum–sigma | 60 | 13.84 | 8 | 81 | 11 | |
| Abdominal organs | 1 | 0.00 | 25 | 25 | 25 | |
| Other abdominal tumors | 9 | 8.48 | 5 | 34 | 10 | |
| Diabetes | No | 128 | 24.32 | 5 | 262 | 12 |
| Yes | 6 | 1.60 | 9 | 13 | 11 | |
| Hypertension | No | 123 | 24.73 | 5 | 262 | 12 |
| Yes | 11 | 8.20 | 8 | 31 | 10 | |
| Abdominal Adherences | No | 124 | 24.60 | 5 | 262 | 11.5 |
| Yes | 10 | 10.05 | 9 | 42 | 12.5 | |
| Cardiological Disorders | No | 116 | 25.31 | 5 | 262 | 12 |
| Yes | 18 | 9.94 | 9 | 42 | 11.5 | |
| Respiratory Disorders | No | 126 | 8.49 | 5 | 60 | 12 |
| Yes | 8 | 86.35 | 9 | 262 | 21 | |
| Gallstones | No | 129 | 24.23 | 5 | 262 | 11 |
| Yes | 5 | 7.05 | 9 | 28 | 16 | |
| Peritonitis | No | 123 | 24.69 | 5 | 262 | 12 |
| Yes | 11 | 9.71 | 9 | 42 | 12 | |
| Other Tumors | No | 129 | 24.24 | 5 | 262 | 11 |
| Yes | 5 | 5.52 | 11 | 25 | 16 | |
| Complicated Procedure | No | 91 | 27.69 | 7 | 262 | 11 |
| Yes | 43 | 12.29 | 5 | 60 | 12 | |
| Mode of Discharge | Dead | 2 | 0.00 | 10 | 10 | 10 |
| To home | 129 | 8.91 | 5 | 60 | 12 | |
| Voluntary | 0 | 0.00 | 0 | 0 | 0 | |
| Transferred to another institution | 1 | 0.00 | 31 | 31 | 31 | |
| Transferred to another regime | 0 | 0.00 | 0 | 0 | 0 | |
| Transferred to rehabilitation institution | 2 | 127.99 | 81 | 262 | 171.5 |
| Variable | Value | Pre-Improvement | Post-Improvement | p-Value | ||
|---|---|---|---|---|---|---|
| N | Median | N | Median | |||
| All | All | 255 | 14 | 134 | 12 | 0.014 |
| Age | Under 50 | 18 | 13 | 7 | 17 | 0.627 |
| 50 ≤ Age ≤ 65 | 76 | 12 | 42 | 11.5 | 0.273 | |
| 66 ≤ Age ≤ 75 | 90 | 14 | 52 | 11 | 0.012 | |
| Over 75 | 71 | 14 | 33 | 13 | 0.453 | |
| Gender | Male | 140 | 14 | 87 | 11 | 0.003 |
| Female | 115 | 14 | 47 | 13 | 0.907 | |
| Type of Admission | Scheduled | 63 | 14 | 24 | 16 | 0.693 |
| Scheduled with pre-hospitalization | 192 | 14 | 110 | 11 | 0.007 | |
| Ward of Admission | Other surgeries | 234 | 14 | 124 | 11 | 0.004 |
| Specialized ward | 21 | 13 | 10 | 15.5 | 0.385 | |
| Type of Tumor | Colon | 107 | 14 | 64 | 12 | 0.211 |
| Rectum–sigma | 130 | 14 | 60 | 11 | 0.050 | |
| Abdominal organs | 4 | 20.5 | 1 | 25 | 0.800 | |
| Other abdominal tumors | 14 | 10.5 | 9 | 10 | 0.611 | |
| Diabetes | No | 243 | 14 | 128 | 12 | 0.035 |
| Yes | 12 | 15 | 6 | 11 | 0.020 | |
| Hypertension | No | 229 | 14 | 123 | 12 | 0.021 |
| Yes | 26 | 14 | 11 | 10 | 0.443 | |
| Abdominal Adherences | No | 244 | 14 | 124 | 11.5 | 0.010 |
| Yes | 11 | 11 | 10 | 12.5 | 0.750 | |
| Cardiological Disorders | No | 204 | 13 | 116 | 12 | 0.102 |
| Yes | 51 | 16 | 18 | 11.5 | 0.077 | |
| Respiratory Disorders | No | 227 | 13 | 126 | 12 | 0.044 |
| Yes | 28 | 21 | 8 | 21 | 0.909 | |
| Gallstones | No | 248 | 14 | 129 | 11 | 0.007 |
| Yes | 7 | 13 | 5 | 16 | 0.318 | |
| Peritonitis | No | 234 | 14 | 123 | 12 | 0.042 |
| Yes | 21 | 24 | 11 | 12 | 0.099 | |
| Other Tumors | No | 234 | 14 | 129 | 11 | 0.009 |
| Yes | 21 | 14 | 5 | 16 | 0.696 | |
| Complicated Procedure | No | 138 | 12 | 91 | 11 | 0.524 |
| Yes | 117 | 16 | 43 | 12 | 0.016 | |
| Mode of Discharge | Dead | 9 | 24 | 2 | 10 | 0.145 |
| To home | 239 | 14 | 129 | 12 | 0.016 | |
| Voluntary | 5 | 19 | 0 | 0 | - | |
| Transferred to another institution | 0 | 0 | 1 | 31 | - | |
| Transferred to another regime | 2 | 61.5 | 0 | 0 | - | |
| Transferred to rehabilitation institution | 0 | 0 | 2 | 171.5 | - | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scala, A.; D’Amore, A.; Mannelli, M.P.; Mensorio, M.; Improta, G. Management of Patients with Colorectal Cancer through Fast-Track Surgery. Int. J. Environ. Res. Public Health 2024, 21, 1226. https://doi.org/10.3390/ijerph21091226
Scala A, D’Amore A, Mannelli MP, Mensorio M, Improta G. Management of Patients with Colorectal Cancer through Fast-Track Surgery. International Journal of Environmental Research and Public Health. 2024; 21(9):1226. https://doi.org/10.3390/ijerph21091226
Chicago/Turabian StyleScala, Arianna, Antonio D’Amore, Maria Pia Mannelli, Mario Mensorio, and Giovanni Improta. 2024. "Management of Patients with Colorectal Cancer through Fast-Track Surgery" International Journal of Environmental Research and Public Health 21, no. 9: 1226. https://doi.org/10.3390/ijerph21091226
APA StyleScala, A., D’Amore, A., Mannelli, M. P., Mensorio, M., & Improta, G. (2024). Management of Patients with Colorectal Cancer through Fast-Track Surgery. International Journal of Environmental Research and Public Health, 21(9), 1226. https://doi.org/10.3390/ijerph21091226








