The Impact of Environmental Benzene, Toluene, Ethylbenzene, and Xylene Exposure on Blood-Based DNA Methylation Profiles in Pregnant African American Women from Detroit
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. BTEX Exposure Assignment
2.3. DNA Isolation and Measurement of DNA Methylation
2.4. Methylome-Wide Assessment and Quality Control
2.5. Covariate Assessment
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cassidy-Bushrow, A.E.; Burmeister, C.; Lamerato, L.; Lemke, L.D.; Mathieu, M.; O’Leary, B.F.; Sperone, F.G.; Straughen, J.K.; Reiners, J.J., Jr. Prenatal airshed pollutants and preterm birth in an observational birth cohort study in Detroit, Michigan, USA. Environ. Res. 2020, 189, 109845. [Google Scholar] [CrossRef]
- Miller, C.J.; Runge-Morris, M.; Cassidy-Bushrow, A.E.; Straughen, J.K.; Dittrich, T.M.; Baker, T.R.; Petriello, M.C.; Mor, G.; Ruden, D.M.; O’Leary, B.F.; et al. A review of volatile organic compound contamination in post-industrial urban centers: Reproductive health implications using a Detroit lens. Int. J. Environ. Res. Public Health 2020, 17, 8755. [Google Scholar] [CrossRef]
- Sloto, R.A. Changes in Groundwater Flow and Volatile Organic Compound Concentrations at the Fischer and Porter Superfund Site, Warminster Township, Bucks County, Pennsylvania, 1993–2009 (Scientific Investigations Report 2010–5054); U.S. Department of the Interior, U.S. Geological Survey: Reston, VA, USA, 2010. [Google Scholar]
- Hunt, J.R.; Holden, P.A.; Firestone, M.K. Coupling transport and biodegradation of VOCs in surface and subsurface soils. Environ. Health Perspect. 1995, 103 (Suppl. S5), 75–78. [Google Scholar] [CrossRef] [PubMed]
- Environmental Protection Agency. 40 CFR Part 300: Addition of a subsurface intrusion component to the Hazard Ranking System. Fed. Regist. 2017, 82, 2760–2807. [Google Scholar]
- Fenga, C.; Gangemi, S.; Costa, C. Benzene exposure is associated with epigenetic changes (Review). Mol. Med. Rep. 2016, 13, 3401–3405. [Google Scholar] [CrossRef]
- Blossom, S.J.; Gilbert, K.M. Epigenetic underpinnings of developmental immunotoxicity and autoimmune disease. Curr. Opin. Toxicol. 2018, 10, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Thomas, R.; McHale, C.M.; Lan, Q.; Hubbard, A.E.; Zhang, L.; Vermeulen, R.; Li, G.; Rappaport, S.M.; Yin, S.; Rothman, N.; et al. Global gene expression response of a population exposed to benzene: A pilot study exploring the use of RNA-sequencing technology. Environ. Mol. Mutagen. 2013, 54, 566–573. [Google Scholar] [CrossRef] [PubMed]
- Bollati, V.; Baccarelli, A.; Hou, L.; Bonzini, M.; Fustinoni, S.; Cavallo, D.; Byun, H.M.; Jiang, J.; Marinelli, B.; Pesatori, A.C.; et al. Changes in DNA methylation patterns in subjects exposed to low-dose benzene. Cancer Res. 2007, 67, 876–880. [Google Scholar] [CrossRef]
- Jimenez-Garza, O.; Guo, L.; Byun, H.M.; Carrieri, M.; Bartolucci, G.B.; Barron-Vivanco, B.S.; Baccarelli, A.A. Aberrant promoter methylation in genes related to hematopoietic malignancy in workers exposed to a VOC mixture. Toxicol. Appl. Pharmacol. 2018, 339, 65–72. [Google Scholar] [CrossRef]
- Silvestre, R.T.; Bravo, M.; Santiago, F.; Delmonico, L.; Scherrer, L.; Otero, U.B.; Liehr, T.; Alves, G.; Chantre-Justino, M.; Ornellas, M.H. Hypermethylation in Gene Promoters Are Induced by Chronic Exposure to Benzene, Toluene, Ethylbenzene and Xylenes. Pak. J. Biol. Sci. 2020, 23, 518–525. [Google Scholar] [CrossRef]
- Schiffman, C.; McHale, C.M.; Hubbard, A.E.; Zhang, L.; Thomas, R.; Vermeulen, R.; Li, G.; Shen, M.; Rappaport, S.M.; Yin, S.; et al. Identification of gene expression predictors of occupational benzene exposure. PLoS ONE 2018, 13, e0205427. [Google Scholar] [CrossRef]
- March of Dimes 2018 March of Dimes Premature Birth Report Card United States. Available online: https://www.marchofdimes.org/materials/PrematureBirthReportCard-United%20States-2018.pdf (accessed on 30 November 2023).
- Cassidy-Bushrow, A.E.; Burmeister, C.; Birbeck, J.; Chen, Y.; Lamerato, L.; Lemke, L.D.; Li, J.; Mor, G.; O’Leary, B.F.; Peters, R.M.; et al. Ambient BTEX exposure and mid-pregnancy inflammatory biomarkers in pregnant African American women. J. Reprod. Immunol. 2021, 145, 103305. [Google Scholar] [CrossRef] [PubMed]
- Miller, L.; Lemke, L.D.; Xu, X.H.; Molaroni, S.M.; You, H.Y.; Wheeler, A.J.; Booza, J.; Grgicak-Mannion, A.; Krajenta, R.; Graniero, P.; et al. Intra-urban correlation and spatial variability of air toxics across an international airshed in Detroit, Michigan (USA) and Windsor, Ontario (Canada). Atmos. Environ. 2010, 44, 1162–1174. [Google Scholar] [CrossRef]
- Lemke, L.D.; Lamerato, L.E.; Xu, X.; Booza, J.C.; Reiners, J.; Raymond, D.M.; Villeneuve, P.J.; Lavigne, E.; Larkin, D.; Krouse, H.J. Geospatial relationships of air pollution and asthma morbidity across the Detroit-Windsor international border: Study design and preliminary results. J. Expo. Sci. Environ. Epidemiol. 2014, 24, 346–357. [Google Scholar] [CrossRef] [PubMed]
- O’Leary, B.F.; Lemke, L.D. Modeling spatiotemporal variability of intra-urban air pollutants in Detroit: A pragmatic approach. Atmos. Environ. 2014, 94, 417–427. [Google Scholar] [CrossRef]
- Zhou, W.; Triche, T.J., Jr.; Laird, P.W.; Shen, H. SeSAMe: Reducing artifactual detection of DNA methylation by Infinium BeadChips in genomic deletions. Nucleic Acids Res. 2018, 46, e123. [Google Scholar] [CrossRef] [PubMed]
- Teschendorff, A.E.; Marabita, F.; Lechner, M.; Bartlett, T.; Tegner, J.; Gomez-Cabrero, D.; Beck, S. A beta-mixture quantile normalization method for correcting probe design bias in Illumina Infinium 450 k DNA methylation data. Bioinformatics 2013, 29, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Di Lena, P.; Sala, C.; Prodi, A.; Nardini, C. Missing value estimation methods for DNA methylation data. Bioinformatics 2019, 35, 3786–3793. [Google Scholar] [CrossRef] [PubMed]
- Houseman, E.A.; Kile, M.L.; Christiani, D.C.; Ince, T.A.; Kelsey, K.T.; Marsit, C.J. Reference-free deconvolution of DNA methylation data and mediation by cell composition effects. BMC Bioinform. 2016, 17, 259. [Google Scholar] [CrossRef]
- Aryee, M.J.; Jaffe, A.E.; Corrada-Bravo, H.; Ladd-Acosta, C.; Feinberg, A.P.; Hansen, K.D.; Irizarry, R.A. Minfi: A flexible and comprehensive Bioconductor package for the analysis of Infinium DNA methylation microarrays. Bioinformatics 2014, 30, 1363–1369. [Google Scholar] [CrossRef]
- Pedersen, B.S.; Schwartz, D.A.; Yang, I.V.; Kechris, K.J. Comb-p: Software for combining, analyzing, grouping and correcting spatially correlated p-values. Bioinformatics 2012, 28, 2986–2988. [Google Scholar] [CrossRef]
- Xu, Z.; Niu, L.; Taylor, J.A. The ENmix DNA methylation analysis pipeline for Illumina BeadChip and comparisons with seven other preprocessing pipelines. Clin. Epigenetics 2021, 13, 216. [Google Scholar] [CrossRef]
- Benjamini, Y.; Hochberg, Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B Methodol. 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Yu, G.; Wang, L.G.; He, Q.Y. ChIPseeker: An R/Bioconductor package for ChIP peak annotation, comparison and visualization. Bioinformatics 2015, 31, 2382–2383. [Google Scholar] [CrossRef] [PubMed]
- Kramer, A.; Green, J.; Pollard, J., Jr.; Tugendreich, S. Causal analysis approaches in Ingenuity Pathway Analysis. Bioinformatics 2014, 30, 523–530. [Google Scholar] [CrossRef] [PubMed]
- Jamebozorgi, I.; Majidizadeh, T.; Pouryagoub, G.; Mahjoubi, F. Aberrant DNA Methylation of Two Tumor Suppressor Genes, p14(ARF) and p15(INK4b), after Chronic Occupational Exposure to Low Level of Benzene. Int. J. Occup. Environ. Med. 2018, 9, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Sharpless, N.E. Ink4a/Arf links senescence and aging. Exp. Gerontol. 2004, 39, 1751–1759. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.Y.; Yu, S.Y.; Kim, G.W.; Ahn, J.J.; Kim, Y.; Lim, S.; Son, S.W.; Hwang, S.Y. Identification of time-dependent biomarkers and effects of exposure to volatile organic compounds using high-throughput analysis. Environ. Toxicol. 2016, 31, 1563–1570. [Google Scholar] [CrossRef] [PubMed]
- Phillips, R.V.; Rieswijk, L.; Hubbard, A.E.; Vermeulen, R.; Zhang, J.; Hu, W.; Li, L.; Bassig, B.A.; Wong, J.Y.Y.; Reiss, B.; et al. Human exposure to trichloroethylene is associated with increased variability of blood DNA methylation that is enriched in genes and pathways related to autoimmune disease and cancer. Epigenetics 2019, 14, 1112–1124. [Google Scholar] [CrossRef]
- Uvnas-Moberg, K.; Ekstrom-Bergstrom, A.; Berg, M.; Buckley, S.; Pajalic, Z.; Hadjigeorgiou, E.; Kotlowska, A.; Lengler, L.; Kielbratowska, B.; Leon-Larios, F.; et al. Maternal plasma levels of oxytocin during physiological childbirth—A systematic review with implications for uterine contractions and central actions of oxytocin. BMC Pregnancy Childbirth 2019, 19, 285. [Google Scholar] [CrossRef]
- Chibbar, R.; Miller, F.D.; Mitchell, B.F. Synthesis of oxytocin in amnion, chorion, and decidua may influence the timing of human parturition. J. Clin. Investig. 1993, 91, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Licheri, V.; Talani, G.; Gorule, A.A.; Mostallino, M.C.; Biggio, G.; Sanna, E. Plasticity of GABAA Receptors during Pregnancy and Postpartum Period: From Gene to Function. Neural Plast. 2015, 2015, 170435. [Google Scholar] [CrossRef] [PubMed]
- Win-Shwe, T.T.; Fujimaki, H. Neurotoxicity of toluene. Toxicol. Lett. 2010, 198, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, B.; Baxter, C.S.; Lam, H.M.; Yeramaneni, S.; Levin, L.; Haynes, E.; Ho, S.M. Hypomethylation of dual specificity phosphatase 22 promoter correlates with duration of service in firefighters and is inducible by low-dose benzo[a]pyrene. J. Occup. Environ. Med. 2012, 54, 774–780. [Google Scholar] [CrossRef]
- Martin, J.A.; Hamilton, B.E.; Osterman, M.J.; Driscoll, A.K.; Mathews, T.J. Births: Final Data for 2015. Natl. Vital Stat. Rep. 2017, 66, 1. [Google Scholar]
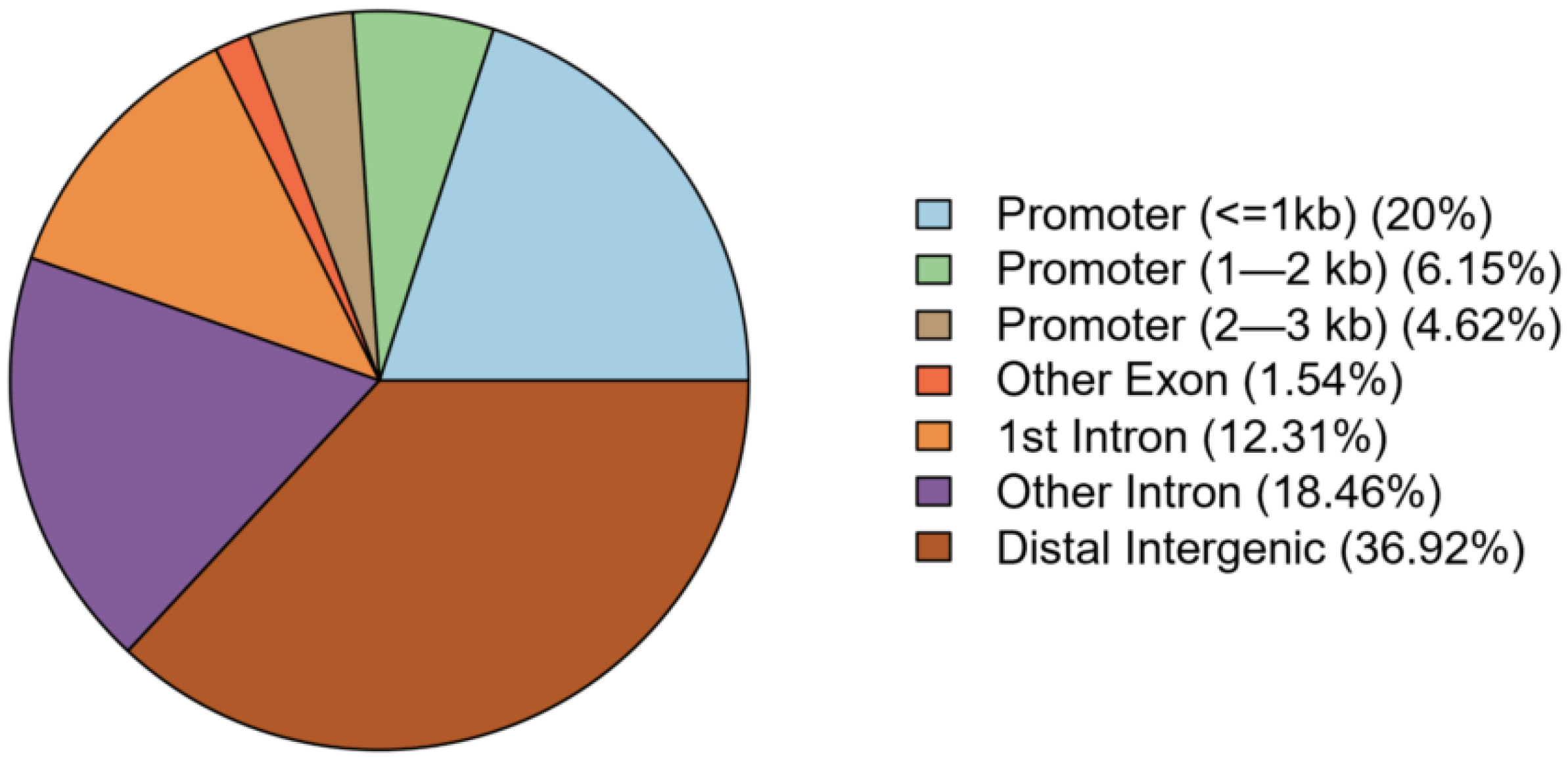
| Combined Population (N = 64) | Low BTEX (N = 32) | High BTEX (N = 32) | p-Value | |
|---|---|---|---|---|
| Maternal education (≥ college), n (%) | 20 (36.4) | 7 (28.0) | 13 (43.3) | 0.370 |
| Maternal age at blood draw, mean (SD) | 25.84 (5.89) | 27.72 (6.49) | 23.95 (4.59) | 0.009 |
| Pre-pregnancy BMI (kg/m2), mean (SD) | 28.13 (6.66) | 29.77 (6.24) | 26.48 (6.76) | 0.047 |
| Married, n (%) | 12 (18.8) | 8 (25.0) | 4(12.5) | 0.337 |
| Prenatal smoking, n (%) | 7 (10.9) | 3 (9.4) | 4 (12.5) | 1.000 |
| ETS exposed, n (%) | 21 (70.0) | 13(76.5) | 8 (61.5) | 0.630 |
| Parity, mean (SD) | 1.05 (1.43) | 1.09 (1.12) | 1.00 (1.70) | 0.796 |
| Methylation-based predicted immune cell %, mean (SD) | ||||
| CD8+ T-cell | 8.97 (3.77) | 8.39 (2.90) | 9.55 (4.44) | 0.219 |
| CD4+ T-cell | 7.11 (3.38) | 7.68 (3.35) | 6.54 (3.36) | 0.181 |
| NK-cell | 1.69 (2.37) | 1.57 (1.46) | 1.82 (3.05) | 0.681 |
| B-cell | 3.10 (1.69) | 3.19 (1.84) | 3.02 (1.54) | 0.696 |
| Monocytes | 9.64 (2.27) | 9.59 (2.12) | 9.69 (2.45) | 0.870 |
| Granulocytes | 69.48 (7.00) | 69.59 (6.13) | 69.38 (7.87) | 0.909 |
| Infant sex (male), n (%) | 33 (52.4) | 18 (56.2) | 15 (48.4) | 0.710 |
| Birthweight (grams), mean (SD) | 3151.29 (610.96) | 3182.22 (605.63) | 3119.35 (624.76) | 0.687 |
| Gestational age at delivery (weeks), mean (SD) | 38.73 (2.47) | 38.81 (1.99) | 38.64 (2.90) | 0.780 |
| CpG Sites * | ||||||||
|---|---|---|---|---|---|---|---|---|
| Chromosome | BP Start | BP Width | Functional Annotation | Gene Symbol | FDR | Total | Hyper | Hypo |
| 4 | 184,908,253 | 766 | Distal intergenic | LINC01093 | 1.67 × 10−9 | 11 | 0 | 10 |
| 6 | 291,686 | 911 | Promoter (≤1 kb) | DUSP22 | 1.76 × 10−9 | 8 | 0 | 8 |
| 11 | 6,291,624 | 888 | Distal intergenic | CCKBR | 1.76 × 10−9 | 7 | 0 | 7 |
| 17 | 40,274,523 | 289 | Promoter (≤1 kb) | WIPF2 | 1.02 × 10−7 | 7 | 0 | 6 |
| 1 | 42,384,283 | 365 | Intron | RIMKLA | 2.23 × 10−7 | 8 | 0 | 8 |
| 7 | 69,064,092 | 87 | Distal intergenic | CT66 | 3.26 × 10−7 | 4 | 0 | 4 |
| 2 | 48,132,739 | 315 | Intron | FOXN2 | 3.26 × 10−7 | 7 | 0 | 7 |
| 17 | 80,408,535 | 394 | Intron | RNF213-AS1 | 3.30 × 10−7 | 8 | 0 | 6 |
| 21 | 47,532,059 | 275 | Distal intergenic | PRMT2 | 6.89 × 10−7 | 5 | 0 | 4 |
| 12 | 123,319,893 | 165 | Exon | SBNO1 | 8.02 × 10−7 | 5 | 0 | 4 |
| 14 | 93,698,773 | 172 | Intron | UNC79 | 8.02 × 10−7 | 3 | 0 | 3 |
| 11 | 123,430,574 | 376 | Promoter (≤1 kb) | GRAMD1B | 8.67 × 10−7 | 6 | 0 | 6 |
| 1 | 161,008,461 | 366 | Intron | F11R | 8.90 × 10−7 | 8 | 3 | 3 |
| 12 | 123,750,781 | 84 | Promoter (≤1 kb) | ATP6V0A2 | 9.13 × 10−7 | 3 | 0 | 3 |
| 5 | 170,814,528 | 309 | Distal intergenic | GABRP | 9.13 × 10−7 | 8 | 0 | 8 |
| 19 | 2,163,592 | 241 | Promoter (≤1 kb) | DOT1L | 1.01 × 10−6 | 4 | 0 | 4 |
| 11 | 9,697,192 | 238 | Intron | SWAP70 | 1.03 × 10−6 | 2 | 1 | 1 |
| 17 | 53,828,262 | 254 | Intron | KIF2B | 1.03 × 10−6 | 6 | 0 | 4 |
| 19 | 35,645,555 | 158 | Promoter (1–2 kb) | ETV2 | 1.03 × 10−6 | 8 | 0 | 6 |
| 16 | 56,228,384 | 361 | Promoter (2–3 kb) | GNAO1 | 1.87 × 10−6 | 9 | 0 | 7 |
| 3 | 9,932,179 | 145 | Promoter (1–2 kb) | IL17RC | 1.93 × 10−6 | 6 | 0 | 4 |
| 2 | 48,844,762 | 307 | Intron | STON1-GTF2A1L | 2.31 × 10−6 | 6 | 0 | 5 |
| 22 | 44,422,011 | 189 | Distal intergenic | LINC01656 | 2.46 × 10−6 | 5 | 3 | 2 |
| 16 | 10,837,596 | 110 | Distal intergenic | TVP23A | 2.71 × 10−6 | 8 | 0 | 6 |
| 6 | 85,823,948 | 269 | Distal intergenic | SNHG5 | 4.21 × 10−6 | 4 | 0 | 4 |
| 11 | 117,352,729 | 211 | Promoter (≤1 kb) | CEP164 | 5.14 × 10−6 | 4 | 0 | 4 |
| 14 | 105,287,325 | 103 | Intron | BRF1 | 5.46 × 10−6 | 3 | 0 | 3 |
| 19 | 37,825,319 | 254 | Promoter (2–3 kb) | LOC644554 | 6.11 × 10−6 | 6 | 0 | 6 |
| 10 | 133,938,603 | 291 | Distal intergenic | FRG2B | 6.18 × 10−6 | 5 | 0 | 3 |
| 3 | 15,469,026 | 292 | Intron | COLQ | 8.95 × 10−6 | 6 | 0 | 4 |
| 19 | 49,222,966 | 288 | Distal intergenic | TRPM4 | 1.16 × 10−5 | 4 | 0 | 3 |
| 11 | 116,658,839 | 232 | Distal intergenic | LINC02702 | 3.16 × 10−5 | 5 | 0 | 5 |
| 7 | 95,064,396 | 79 | Intron | PPP1R9A | 3.97 × 10−5 | 5 | 0 | 4 |
| 16 | 1,199,498 | 27 | Intron | CACNA1H | 4.70 × 10−5 | 2 | 0 | 2 |
| 9 | 130,533,824 | 27 | Distal intergenic | FUBP3 | 6.41 × 10−5 | 4 | 0 | 2 |
| 16 | 1,031,442 | 17 | Distal intergenic | SSTR5-AS1 | 7.07 × 10−5 | 2 | 0 | 2 |
| 16 | 66,638,395 | 205 | Promoter (1–2 kb) | CMTM4 | 1.27 × 10−4 | 6 | 0 | 4 |
| 16 | 12,070,415 | 274 | Intron | SNX29 | 1.42 × 10−4 | 5 | 0 | 4 |
| 19 | 44,259,187 | 10 | Promoter (≤1 kb) | ZNF233 | 1.44 × 10−4 | 2 | 0 | 2 |
| 10 | 101,282,815 | 69 | Distal intergenic | LINC02681 | 2.29 × 10−4 | 2 | 2 | 0 |
| 12 | 111,126,997 | 143 | Intron | CUX2 | 6.16 × 10−4 | 4 | 1 | 1 |
| 1 | 166,958,580 | 4 | Intron | ILDR2 | 0.001 | 2 | 0 | 2 |
| 3 | 88,198,600 | 152 | Distal intergenic | C3orf38 | 0.002 | 4 | 0 | 1 |
| 11 | 368,564 | 75 | Promoter (≤1 kb) | B4GALNT4 | 0.003 | 5 | 0 | 2 |
| 2 | 8,597,158 | 31 | Distal intergenic | LINC01814 | 0.003 | 3 | 0 | 2 |
| 2 | 1846836 | 18 | Intron | MYT1L | 0.004 | 2 | 0 | 2 |
| Ingenuity Canonical Pathways | p-Value | Proportion | Genes |
|---|---|---|---|
| GABA Receptor Signaling | 0.00219 | 0.023 | CACNA1H, GABRP, GNAO1 |
| Oxytocin In Brain Signaling Pathway | 0.00646 | 0.015 | CACNA1H, GNAO1, NLRP5 |
| Gustation Pathway | 0.00708 | 0.015 | CACNA1H, GABRP, TRPM4 |
| Assembly of RNA Polymerase III Complex | 0.02510 | 0.077 | BRF1 |
| Role of Macrophages, Fibroblasts and Endothelial Cells in Rheumatoid Arthritis | 0.02570 | 0.009 | GNAO1, IL17RC, RIPK1 |
| G Beta Gamma Signaling | 0.02570 | 0.016 | CACNA1H, GNAO1 |
| Role of IL-17A in Psoriasis | 0.02690 | 0.071 | IL17RC |
| Endocannabinoid Neuronal Synapse Pathway | 0.03390 | 0.013 | CACNA1H, GNAO1 |
| Corticotropin Releasing Hormone Signaling | 0.03470 | 0.013 | CACNA1H, GNAO1 |
| Androgen Signaling | 0.04270 | 0.012 | CACNA1H, GNAO1 |
| IL-17A Signaling in Gastric Cells | 0.04900 | 0.039 | IL17RC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Straughen, J.K.; Loveless, I.; Chen, Y.; Burmeister, C.; Lamerato, L.; Lemke, L.D.; O’Leary, B.F.; Reiners, J.J.; Sperone, F.G.; Levin, A.M.; et al. The Impact of Environmental Benzene, Toluene, Ethylbenzene, and Xylene Exposure on Blood-Based DNA Methylation Profiles in Pregnant African American Women from Detroit. Int. J. Environ. Res. Public Health 2024, 21, 256. https://doi.org/10.3390/ijerph21030256
Straughen JK, Loveless I, Chen Y, Burmeister C, Lamerato L, Lemke LD, O’Leary BF, Reiners JJ, Sperone FG, Levin AM, et al. The Impact of Environmental Benzene, Toluene, Ethylbenzene, and Xylene Exposure on Blood-Based DNA Methylation Profiles in Pregnant African American Women from Detroit. International Journal of Environmental Research and Public Health. 2024; 21(3):256. https://doi.org/10.3390/ijerph21030256
Chicago/Turabian StyleStraughen, Jennifer K., Ian Loveless, Yalei Chen, Charlotte Burmeister, Lois Lamerato, Lawrence D. Lemke, Brendan F. O’Leary, John J. Reiners, F. Gianluca Sperone, Albert M. Levin, and et al. 2024. "The Impact of Environmental Benzene, Toluene, Ethylbenzene, and Xylene Exposure on Blood-Based DNA Methylation Profiles in Pregnant African American Women from Detroit" International Journal of Environmental Research and Public Health 21, no. 3: 256. https://doi.org/10.3390/ijerph21030256
APA StyleStraughen, J. K., Loveless, I., Chen, Y., Burmeister, C., Lamerato, L., Lemke, L. D., O’Leary, B. F., Reiners, J. J., Sperone, F. G., Levin, A. M., & Cassidy-Bushrow, A. E. (2024). The Impact of Environmental Benzene, Toluene, Ethylbenzene, and Xylene Exposure on Blood-Based DNA Methylation Profiles in Pregnant African American Women from Detroit. International Journal of Environmental Research and Public Health, 21(3), 256. https://doi.org/10.3390/ijerph21030256





