Predictors of Patient Engagement in Telehealth-Delivered Tobacco Cessation Treatment during the COVID-19 Pandemic
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Patient Characteristics
3.2. Predictors of Patient Engagement
3.3. Predictors of Treatment Preferences among Patients Who Engaged
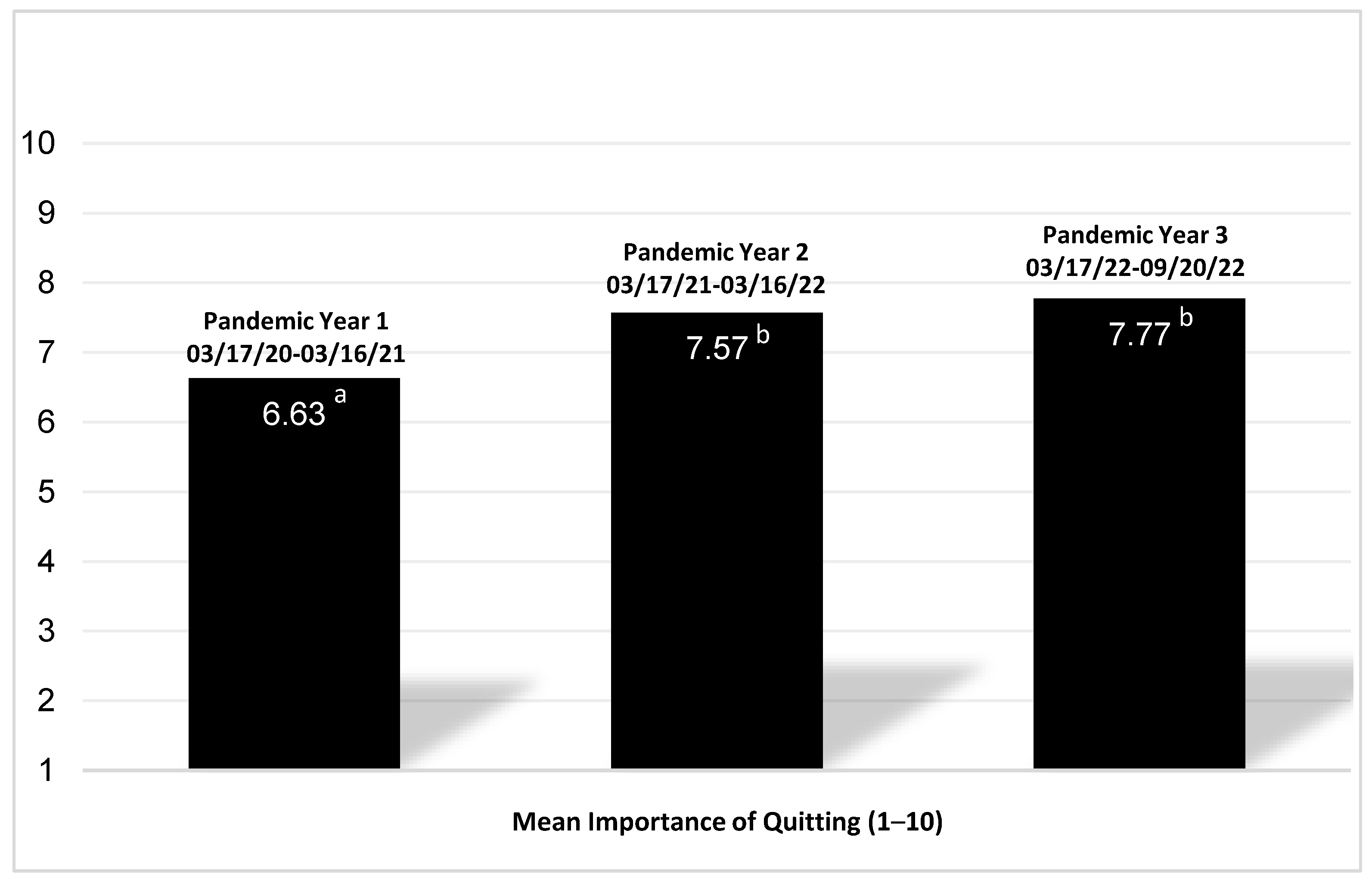
3.4. Perceived Importance of Quitting Tobacco
4. Discussion
5. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- A Clinical Practice Guideline for Treating Tobacco Use and Dependence: 2008 Update. Am. J. Prev. Med. 2008, 35, 158–176. [CrossRef]
- CDCTobaccoFree. 2014 Surgeon General’s Report: The Health Consequences of Smoking—50 Years of Progress. Centers for Disease Control and Prevention. Available online: https://www.cdc.gov/tobacco/sgr/50th-anniversary/index.htm (accessed on 11 June 2023).
- Gajdos, C.; Hawn, M.T.; Campagna, E.J.; Henderson, W.G.; Singh, J.A.; Houston, T. Adverse Effects of Smoking on Postoperative Outcomes in Cancer Patients. Ann. Surg. Oncol. 2012, 19, 1430–1438. [Google Scholar] [CrossRef]
- Peppone, L.J.; Mustian, K.M.; Morrow, G.R.; Dozier, A.M.; Ossip, D.J.; Janelsins, M.C.; Sprod, L.K.; McIntosh, S. The Effect of Cigarette Smoking on Cancer Treatment–Related Side Effects. Oncologist 2011, 16, 1784–1792. [Google Scholar] [CrossRef] [PubMed]
- Jassem, J. Tobacco Smoking after Diagnosis of Cancer: Clinical Aspects. Transl. Lung Cancer Res. 2019, 8 (Suppl. S1), S50–S58. [Google Scholar] [CrossRef] [PubMed]
- WHO Coronavirus (COVID-19) Dashboard. Available online: https://covid19.who.int (accessed on 11 June 2023).
- Patanavanich, R.; Glantz, S.A. Smoking Is Associated With COVID-19 Progression: A Meta-Analysis. Nicotine Tob. Res. 2020, 22, 1653–1656. [Google Scholar] [CrossRef] [PubMed]
- Reddy, R.K.; Charles, W.N.; Sklavounos, A.; Dutt, A.; Seed, P.T.; Khajuria, A. The Effect of Smoking on COVID-19 Severity: A Systematic Review and Meta-analysis. J. Med. Virol. 2021, 93, 1045–1056. [Google Scholar] [CrossRef]
- Shastri, M.D.; Shukla, S.D.; Chong, W.C.; Kc, R.; Dua, K.; Patel, R.P.; Peterson, G.M.; O’Toole, R.F. Smoking and COVID-19: What We Know so Far. Respir. Med. 2021, 176, 106237. [Google Scholar] [CrossRef] [PubMed]
- Yingst, J.M.; Krebs, N.M.; Bordner, C.R.; Hobkirk, A.L.; Allen, S.I.; Foulds, J. Tobacco Use Changes and Perceived Health Risks among Current Tobacco Users during the COVID-19 Pandemic. Int. J. Environ. Res. Public Health 2021, 18, 1795. [Google Scholar] [CrossRef] [PubMed]
- Kalkhoran, S.M.; Levy, D.E.; Rigotti, N.A. Smoking and E-Cigarette Use Among U.S. Adults During the COVID-19 Pandemic. Am. J. Prev. Med. 2022, 62, 341–349. [Google Scholar] [CrossRef]
- Koopmann, A.; Georgiadou, E.; Reinhard, I.; Müller, A.; Lemenager, T.; Kiefer, F.; Hillemacher, T. The Effects of the Lockdown during the COVID-19 Pandemic on Alcohol and Tobacco Consumption Behavior in Germany. Eur. Addict. Res. 2021, 27, 242–256. [Google Scholar] [CrossRef]
- Gendall, P.; Hoek, J.; Stanley, J.; Jenkins, M.; Every-Palmer, S. Changes in Tobacco Use During the 2020 COVID-19 Lockdown in New Zealand. Nicotine Tob. Res. 2021, 23, 866–871. [Google Scholar] [CrossRef]
- White, A.M.; Li, D.; Snell, L.M.; O’Connor, R.; Hoetger, C.; Croft, D.; Lester, R.C.; McIntosh, S.; Underwood, M.; Schneller, L.; et al. Perceptions of Tobacco Product-Specific COVID-19 Risk and Changes in Tobacco Use Behaviors Among Smokers, E-Cigarette Users, and Dual Users. Nicotine Tob. Res. 2021, 23, 1617–1622. [Google Scholar] [CrossRef]
- Smoking Cessation—The Role of Healthcare Professionals and Health Systems. Available online: https://www.cdc.gov/tobacco/sgr/2020-smoking-cessation/fact-sheets/healthcare-professionals-health-systems/index.html (accessed on 15 June 2023).
- Rodgers-Melnick, S.N.; Zanotti, K.; Lee, R.T.; Webb Hooper, M. Demographic and Clinical Predictors of Engaging in Tobacco Cessation Counseling at a Comprehensive Cancer Center. JCO Oncol. Pract. 2022, 18, e721–e730. [Google Scholar] [CrossRef]
- Audrain-McGovern, J.; Hughes Halbert, C.; Rodriguez, D.; Epstein, L.H.; Tercyak, K.P. Predictors of Participation in a Smoking Cessation Program among Young Adult Smokers. Cancer Epidemiol. Biomark. Prev. 2007, 16, 617–619. [Google Scholar] [CrossRef][Green Version]
- Sirody, J.; Munday Stryffeler, M.; Webb Hooper, M. Predictors of Participant ‘No-Shows’ for Intensive Behavioral Tobacco Cessation Treatment: Recruitment, Demographics, and Distance. J. Smok. Cessat. 2020, 15, 109–112. [Google Scholar] [CrossRef]
- Messer, K.; Trinidad, D.R.; Al-Delaimy, W.K.; Pierce, J.P. Smoking Cessation Rates in the United States: A Comparison of Young Adult and Older Smokers. Am. J. Public Health 2008, 98, 317–322. [Google Scholar] [CrossRef] [PubMed]
- CDC. Current Cigarette Smoking Among Adults in the United States. Centers for Disease Control and Prevention. Available online: https://www.cdc.gov/tobacco/data_statistics/fact_sheets/adult_data/cig_smoking/index.htm (accessed on 9 December 2023).
- D’Angelo, H.; Webb Hooper, M.; Burris, J.L.; Rolland, B.; Adsit, R.; Pauk, D.; Rosenblum, M.; Fiore, M.C.; Baker, T.B. Achieving Equity in the Reach of Smoking Cessation Services Within the NCI Cancer Moonshot-Funded Cancer Center Cessation Initiative. Health Equity 2021, 5, 424–430. [Google Scholar] [CrossRef]
- Gritz, E.R.; Carr, C.R.; Rapkin, D.; Abemayor, E.; Chang, L.J.; Wong, W.K.; Belin, T.R.; Calcaterra, T.; Robbins, K.T.; Chonkich, G. Predictors of Long-Term Smoking Cessation in Head and Neck Cancer Patients. Cancer Epidemiol. Biomark. Prev. 1993, 2, 261–270. [Google Scholar]
- Schnoll, R.A.; Rothman, R.L.; Lerman, C.; Miller, S.M.; Newman, H.; Movsas, B.; Sherman, E.; Ridge, J.A.; Unger, M.; Langer, C.; et al. Comparing Cancer Patients Who Enroll in a Smoking Cessation Program at a Comprehensive Cancer Center with Those Who Decline Enrollment. Head Neck 2004, 26, 278–286. [Google Scholar] [CrossRef] [PubMed]
- Klemperer, E.M.; Mermelstein, R.; Baker, T.B.; Hughes, J.R.; Fiore, M.C.; Piper, M.E.; Schlam, T.R.; Jorenby, D.E.; Collins, L.M.; Cook, J.W. Predictors of Smoking Cessation Attempts and Success Following Motivation-Phase Interventions Among People Initially Unwilling to Quit Smoking. Nicotine Tob. Res. 2020, 22, 1446–1452. [Google Scholar] [CrossRef]
- Gali, K.; Pike, B.; Kendra, M.S.; Tran, C.; Fielding-Singh, P.; Jimenez, K.; Mirkin, R.; Prochaska, J.J. Integration of Tobacco Treatment Services into Cancer Care at Stanford. Int. J. Environ. Res. Public Health 2020, 17, 2101. [Google Scholar] [CrossRef]
- Richter, K.P.; Ellerbeck, E.F. It’s Time to Change the Default for Tobacco Treatment: Changing the Default. Addiction 2015, 110, 381–386. [Google Scholar] [CrossRef]
- Richter, K.P.; Catley, D.; Gajewski, B.J.; Faseru, B.; Shireman, T.I.; Zhang, C.; Scheuermann, T.S.; Mussulman, L.M.; Nazir, N.; Hutcheson, T.; et al. The Effects of Opt-out vs Opt-in Tobacco Treatment on Engagement, Cessation, and Costs: A Randomized Clinical Trial. JAMA Intern. Med. 2023, 183, 331. [Google Scholar] [CrossRef]
- Bates-Pappas, G.E.; Ostroff, J.; Lui, M.; Chichester, L.E.; Kosten, C.; Carter-Bawa, L.M.; Whitlock, C.; O’Brien, M.G. An Opt-Out Referral Strategy Mitigates Racial and Ethnic Disparities in Referral and Engagement of Cancer Patients in Tobacco Treatment Services. Presented at the Scientific Forum for Presentation and Respectful Discussion of Cutting-Edge Nicotine and Tobacco Research, San Antonio, TX, USA, 1–4 March 2023. p. 28. Available online: https://cdn.ymaws.com/www.srnt.org/resource/resmgr/conferences/2023_annual_meeting/documents/SRNT23_Abstracts022423.pdf (accessed on 11 June 2023).
- McCuistian, C.; Le, T.; Delucchi, K.; Pagano, A.; Hosakote, S.; Guydish, J. Racial/Ethnic Differences in Tobacco Use and Cessation Services among Individuals in Substance Use Treatment. J. Psychoact. Drugs 2021, 53, 483–490. [Google Scholar] [CrossRef] [PubMed]
- Salloum, R.G.; Fleisher, L.; Hohl, S.D.; Clark, S.V.; Klass, E.; Dahl, N.; Pike, B.; Lenhoff, K.L.; Presant, C.A.; Shoenbill, K.A.; et al. Sustainability of tobacco treatment programs in the cancer center cessation initiative. J. Natl. Compr. Cancer Netw. 2021, 19, S16–S20. [Google Scholar] [CrossRef]
- Salloum, R.G.; D’Angelo, H.; Theis, R.P.; Rolland, B.; Hohl, S.; Pauk, D.; LeLaurin, J.H.; Asvat, Y.; Chen, L.-S.; Day, A.T.; et al. Mixed-Methods Economic Evaluation of the Implementation of Tobacco Treatment Programs in National Cancer Institute-Designated Cancer Centers. Implement. Sci. Commun. 2021, 2, 41. [Google Scholar] [CrossRef] [PubMed]
- Gali, K.; Prochaska, J.J. Treating tobacco use in cancer survivors. In Essentials of Cancer Survivorship: A Guide for Medical Professionals; Schapira, L., Ed.; CRC Press: Abingdon, UK, 2022; pp. 141–157. [Google Scholar]
- Cancer MoonshotSM—NCI. Available online: https://www.cancer.gov/research/key-initiatives/moonshot-cancer-initiative (accessed on 11 September 2023).
- Croyle, R.T.; Morgan, G.D.; Fiore, M.C. Addressing a Core Gap in Cancer Care—The NCI Moonshot Program to Help Oncology Patients Stop Smoking. N. Engl. J. Med. 2019, 380, 512–515. [Google Scholar] [CrossRef] [PubMed]
- D’Angelo, H.; Rolland, B.; Adsit, R.; Baker, T.B.; Rosenblum, M.; Pauk, D.; Morgan, G.D.; Fiore, M.C. Tobacco Treatment Program Implementation at NCI Cancer Centers: Progress of the NCI Cancer Moonshot-Funded Cancer Center Cessation Initiative. Cancer Prev. Res. 2019, 12, 735–740. [Google Scholar] [CrossRef] [PubMed]
- Karam-Hage, M.; Oughli, H.A.; Rabius, V.; Beneventi, D.; Wippold, R.C.; Blalock, J.A.; Cinciripini, P.M. Tobacco Cessation Treatment Pathways for Patients With Cancer: 10 Years in the Making. J. Natl. Compr. Cancer Netw. 2016, 14, 1469–1477. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Abdelmutti, N.; Brual, J.; Papadakos, J.; Fathima, S.; Goldstein, D.; Eng, L.; Papadakos, T.; Liu, G.; Jones, J.; Giuliani, M. Implementation of a Comprehensive Smoking Cessation Program in Cancer Care. Curr. Oncol. 2019, 26, 361–368. [Google Scholar] [CrossRef]
- Nyenhuis, S.M.; Greiwe, J.; Zeiger, J.S.; Nanda, A.; Cooke, A. Exercise and Fitness in the Age of Social Distancing During the COVID-19 Pandemic. J. Allergy Clin. Immunol. Pract. 2020, 8, 2152–2155. [Google Scholar] [CrossRef]
- Riera, R.; Bagattini, Â.M.; Pacheco, R.L.; Pachito, D.V.; Roitberg, F.; Ilbawi, A. Delays and Disruptions in Cancer Health Care Due to COVID-19 Pandemic: Systematic Review. JCO Glob. Oncol. 2021, 7, 311–323. [Google Scholar] [CrossRef]
- Are There Gender Differences in Tobacco Smoking? National Institutes of Health. 12 April 2021. Available online: https://nida.nih.gov/publications/research-reports/tobacco-nicotine-e-cigarettes/are-there-gender-differences-in-tobacco-smoking (accessed on 15 June 2023).
- Centers for Disease Control and Prevention. 2019. Available online: https://www.cdc.gov/nchs/products/databriefs/db380.htm (accessed on 23 September 2023).
- Suls, J.M.; Luger, T.M.; Curry, S.J.; Mermelstein, R.J.; Sporer, A.K.; An, L.C. Efficacy of Smoking-Cessation Interventions for Young Adults. Am. J. Prev. Med. 2012, 42, 655–662. [Google Scholar] [CrossRef] [PubMed]
- Jordan, H.; Hidajat, M.; Payne, N.; Adams, J.; White, M.; Ben-Shlomo, Y. What Are Older Smokers’ Attitudes to Quitting and How Are They Managed in Primary Care? An Analysis of the Cross-Sectional English Smoking Toolkit Study. BMJ Open 2017, 7, e018150. [Google Scholar] [CrossRef] [PubMed]
- Lang, I.A.; Rice, N.E.; Wallace, R.B.; Guralnik, J.M.; Melzer, D. Smoking Cessation and Transition into Retirement: Analyses from the English Longitudinal Study of Ageing. Age Ageing 2007, 36, 638–643. [Google Scholar] [CrossRef] [PubMed]
- Zantinge, E.M.; Van Den Berg, M.; Smit, H.A.; Picavet, H.S.J. Retirement and a Healthy Lifestyle: Opportunity or Pitfall? A Narrative Review of the Literature. Eur. J. Public Health 2014, 24, 433–439. [Google Scholar] [CrossRef] [PubMed]
- Kerr, S.; Watson, H.; Tolson, D.; Lough, M.; Brown, M. Smoking after the Age of 65 Years: A Qualitative Exploration of Older Current and Former Smokers’ Views on Smoking, Stopping Smoking, and Smoking Cessation Resources and Services: Smoking after the Age of 65 Years. Health Soc. Care Community 2006, 14, 572–582. [Google Scholar] [CrossRef]
- Hawley, C.E.; Genovese, N.; Owsiany, M.T.; Triantafylidis, L.K.; Moo, L.R.; Linsky, A.M.; Sullivan, J.L.; Paik, J.M. Rapid Integration of Home Telehealth Visits Amidst COVID-19: What Do Older Adults Need to Succeed? J. Am. Geriatr. Soc. 2020, 68, 2431–2439. [Google Scholar] [CrossRef] [PubMed]
- Babb, S.; Malarcher, A.; Asman, K.; Johns, M.; Caraballo, R.; VanFrank, B.; Garrett, B. Disparities in Cessation Behaviors Between Hispanic and Non-Hispanic White Adult Cigarette Smokers in the United States, 2000–2015. Prev. Chronic Dis. 2020, 17, 190279. [Google Scholar] [CrossRef]
- Stead, L.F.; Koilpillai, P.; Fanshawe, T.R.; Lancaster, T. Combined Pharmacotherapy and Behavioural Interventions for Smoking Cessation. Cochrane Database Syst. Rev. 2016, 2016, CD008286. [Google Scholar] [CrossRef]
- Cabassa, L.J.; Zayas, L.H.; Hansen, M.C. Latino Adults’ Access to Mental Health Care: A Review of Epidemiological Studies. Adm. Policy Ment. Health 2006, 33, 316–330. [Google Scholar] [CrossRef] [PubMed]

| All Eligible Patients (n = 1313) | Enrolled Patients (n = 448) | |||
|---|---|---|---|---|
| Patient Demographics | n (%) | Mean (SD) | n (%) | Mean (SD) |
| Age (years) | 59.4 (14.4) | 58.8 (12.5) | ||
| Sex | ||||
| Male | 787 (59.9%) | 232 (51.8%) | ||
| Female | 526 (40.1%) | 216 (48.2%) | ||
| Race | ||||
| White | 812 (62.8%) | 283 (63.2%) | ||
| Black | 97 (7.5%) | 29 (6.5%) | ||
| Asian/Pacific Islander | 141 (10.9%) | 41 (9.2%) | ||
| Missing/Other | 263 (18.7%) | 95 (21.2%) | ||
| Ethnicity | ||||
| Non-Hispanic/Latinx | 1085 (82.6%) | 360 (80.4%) | ||
| Hispanic/Latinx | 170 (12.9%) | 71 (15.8%) | ||
| Missing | 58 (4.4%) | 17 (3.8%) | ||
| Variable | % Engaged | β | SE | Wald’s χ2 | df | p | Exp (B) |
|---|---|---|---|---|---|---|---|
| Age | 28.27 | 5 | <0.001 | ||||
| Ages 18–35 (Ref) | 18% | ||||||
| Ages 36–45 | 39% | 1.072 | 0.332 | 10.43 | 1 | 0.001 | 2.92 |
| Ages 46–55 | 43% | 1.214 | 0.318 | 14.60 | 1 | <0.001 | 3.37 |
| Ages 56–65 | 37% | 0.966 | 0.306 | 9.98 | 1 | 0.002 | 2.63 |
| Ages 66–75 | 33% | 0.801 | 0.307 | 6.82 | 1 | 0.009 | 2.23 |
| Ages 76+ | 21% | 0.184 | 0.352 | 0.27 | 1 | 0.601 | 1.20 |
| Sex | |||||||
| Male (Ref) | 30% | ||||||
| Female | 41% | 0.471 | 0.124 | 14.40 | 1 | <0.001 | 1.60 |
| Race | 2.33 | 3 | 0.507 | ||||
| White (Ref) | 35% | ||||||
| Black | 30% | −0.361 | 0.244 | 2.20 | 1 | 0.138 | 0.70 |
| Asian American | 29% | −0.111 | 0.211 | 0.28 | 1 | 0.599 | 0.90 |
| Missing/Other | 36% | −0.044 | 0.185 | 0.06 | 1 | 0.813 | 0.96 |
| Ethnicity | 5.37 | 2 | 0.068 | ||||
| Non-Hispanic (Ref). | 33% | ||||||
| Hispanic | 42% | 0.405 | 0.205 | 3.89 | 1 | 0.049 | 1.50 |
| Missing | 29% | −0.233 | 0.325 | 0.51 | 1 | 0.474 | 0.79 |
| Pandemic Year | 38.99 | 2 | <0.001 | ||||
| Year 1 (Ref) | 42% | ||||||
| Year 2 | 28% | −0.648 | 0.133 | 23.75 | 1 | <0.001 | 0.52 |
| Year 3 | 19% | −1.1092 | 0.226 | 23.42 | 1 | <0.001 | 0.34 |
| Constant | −1.367 | 0.302 | 20.53 | 1 | <0.001 | 0.26 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jagielo, A.D.; Chieng, A.; Tran, C.; Pirkl, A.; Cao-Nasalga, A.; Bragg, A.; Mirkin, R.; Prochaska, J.J. Predictors of Patient Engagement in Telehealth-Delivered Tobacco Cessation Treatment during the COVID-19 Pandemic. Int. J. Environ. Res. Public Health 2024, 21, 131. https://doi.org/10.3390/ijerph21020131
Jagielo AD, Chieng A, Tran C, Pirkl A, Cao-Nasalga A, Bragg A, Mirkin R, Prochaska JJ. Predictors of Patient Engagement in Telehealth-Delivered Tobacco Cessation Treatment during the COVID-19 Pandemic. International Journal of Environmental Research and Public Health. 2024; 21(2):131. https://doi.org/10.3390/ijerph21020131
Chicago/Turabian StyleJagielo, Annemarie D., Amy Chieng, Cindy Tran, Amy Pirkl, Ann Cao-Nasalga, Ashley Bragg, Rachelle Mirkin, and Judith J. Prochaska. 2024. "Predictors of Patient Engagement in Telehealth-Delivered Tobacco Cessation Treatment during the COVID-19 Pandemic" International Journal of Environmental Research and Public Health 21, no. 2: 131. https://doi.org/10.3390/ijerph21020131
APA StyleJagielo, A. D., Chieng, A., Tran, C., Pirkl, A., Cao-Nasalga, A., Bragg, A., Mirkin, R., & Prochaska, J. J. (2024). Predictors of Patient Engagement in Telehealth-Delivered Tobacco Cessation Treatment during the COVID-19 Pandemic. International Journal of Environmental Research and Public Health, 21(2), 131. https://doi.org/10.3390/ijerph21020131







