An Assessment of Multipollutant Exposures Using Silicone Wristbands Among Bangladeshi Youth
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Covariate Data Collection
2.3. Exposure Data Collection
2.4. Statistical Analysis
3. Results
3.1. Descriptive Statistics
3.2. Exposure Assessment
3.3. Single Chemical Linear Regression
3.4. Analysis by Chemical Classification
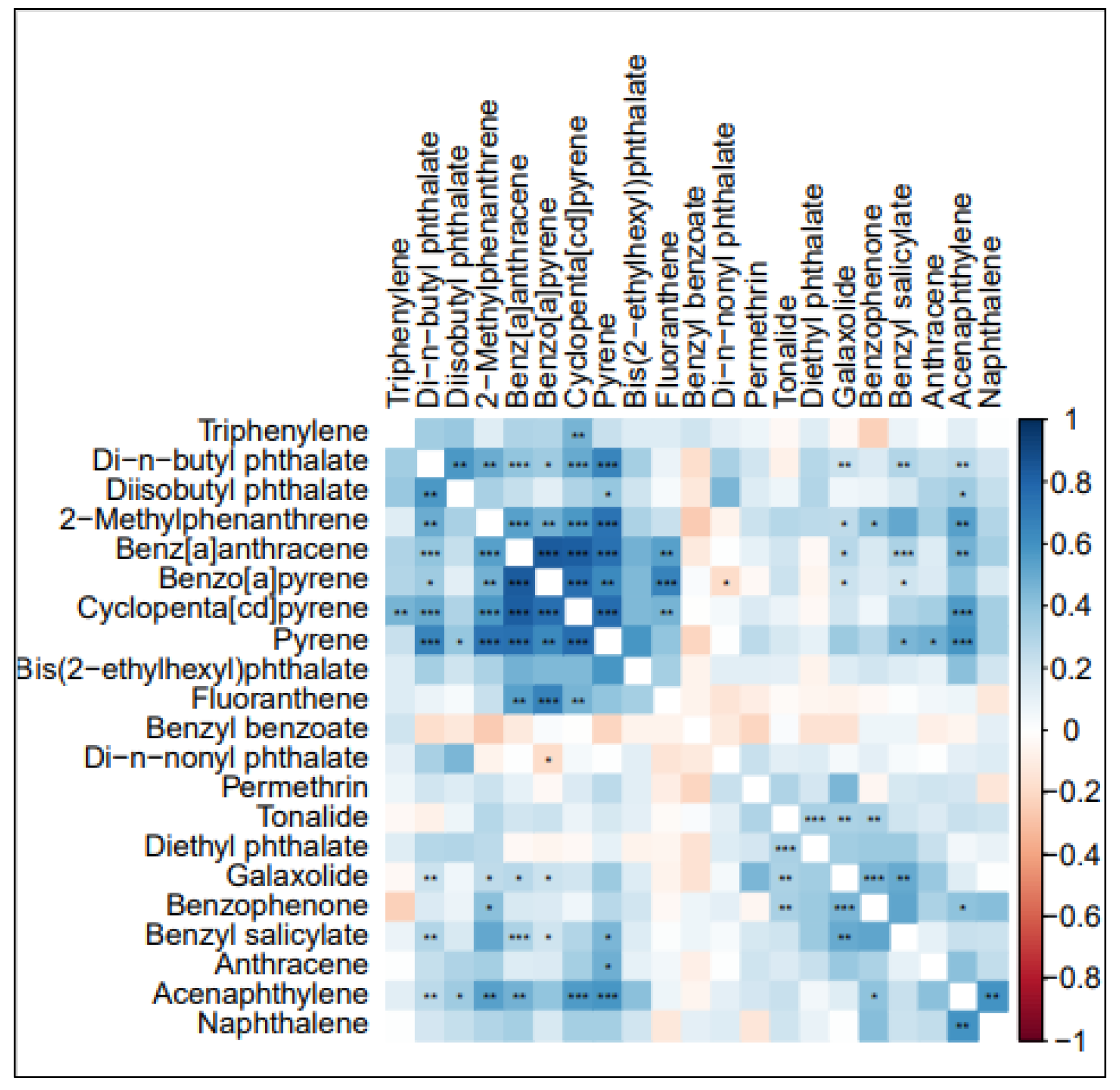
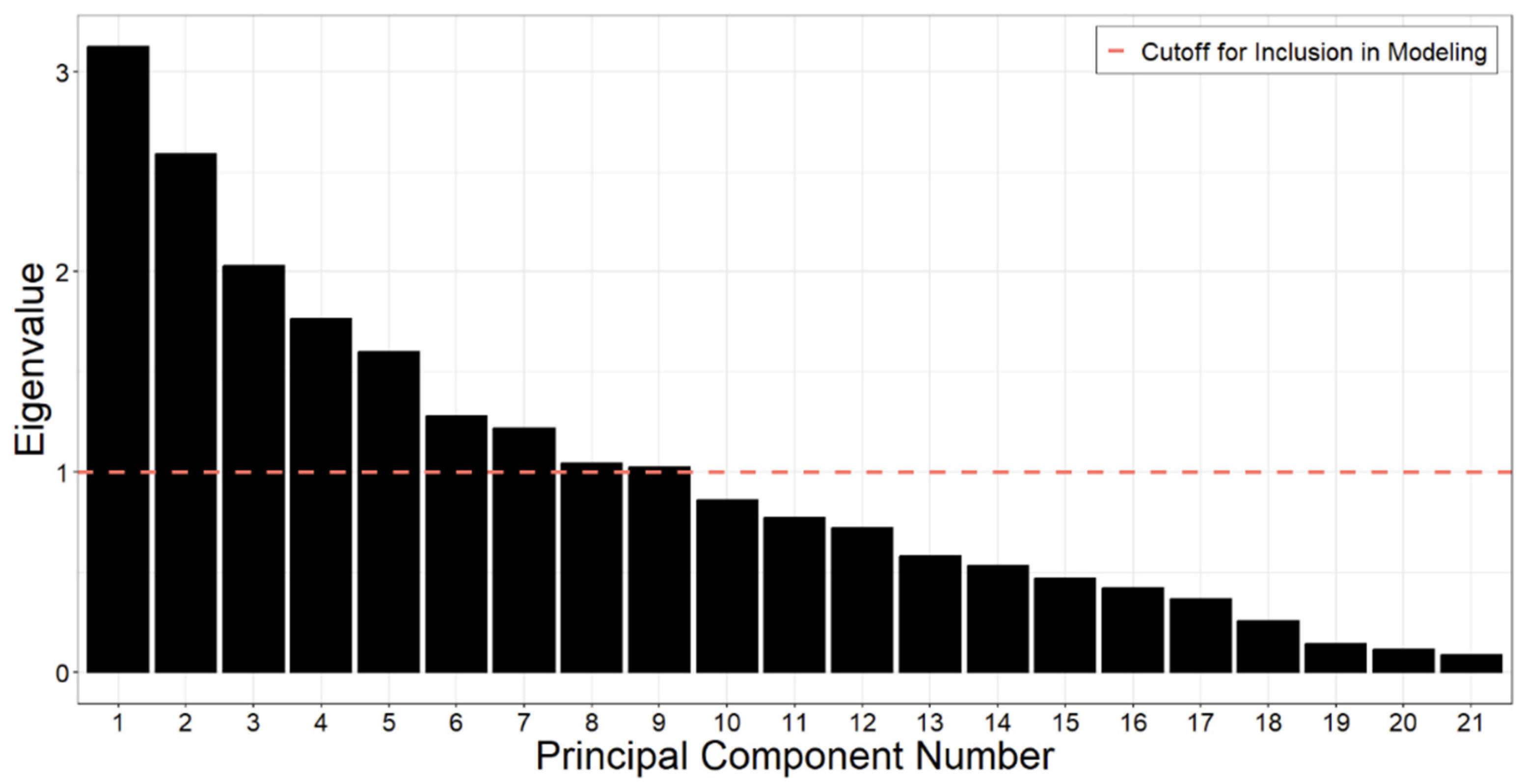
3.5. PCA Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Argos, M.; Kalra, T.; Rathouz, P.J.; Chen, Y.; Pierce, B.; Parvez, F.; Islam, T.; Ahmed, A.; Rakibuz-Zaman, M.; Hasan, R.; et al. Arsenic exposure from drinking water, and all-cause and chronic-disease mortalities in Bangladesh (HEALS): A prospective cohort study. Lancet 2010, 376, 252–258. [Google Scholar] [CrossRef] [PubMed]
- Akter, T.; Jhohura, F.T.; Akter, F.; Chowdhury, T.R.; Mistry, S.K.; Dey, D.; Barua, M.K.; Islam, A.; Rahman, M. Water Quality Index for measuring drinking water quality in rural Bangladesh: A cross-sectional study. J. Health Popul. Nutr. 2016, 35, 4. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, R.; Khan, K.M.; Dibaba, D.T.; Khan, A.; Ahmed, A.; Islam, M.Z. Health Implications of Drinking Water Salinity in Coastal Areas of Bangladesh. Int. J. Environ. Res. Public Health 2019, 16, 3746. [Google Scholar] [CrossRef]
- Chen, Y.; Graziano, J.H.; Parvez, F.; Liu, M.; Slavkovich, V.; Kalra, T.; Argos, M.; Islam, T.; Ahmed, A.; Rakibuz-Zaman, M.; et al. Arsenic exposure from drinking water and mortality from cardiovascular disease in Bangladesh: Prospective cohort study. BMJ 2011, 342, d2431. [Google Scholar] [CrossRef] [PubMed]
- Raqib, R.; Akhtar, E.; Haq, A.; Ahmed, S.; Haque, F.; Chowdhury, M.A.H.; Shahriar, M.H.; Begum, B.A.; Eunus, M.; Sarwar, G.; et al. Reduction of household air pollution through clean fuel intervention and recovery of cellular immune balance. Environ. Int. 2023, 179, 108137. [Google Scholar] [CrossRef] [PubMed]
- Kamal, A.; Cincinelli, A.; Martellini, T.; Malik, R.N. A review of PAH exposure from the combustion of biomass fuel and their less surveyed effect on the blood parameters. Environ. Sci. Pollut. Res. Int. 2015, 22, 4076–4098. [Google Scholar] [CrossRef]
- Zhu, K.; Kawyn, M.N.; Kordas, K.; Mu, L.; Yoo, E.-H.; Seibert, R.; Smith, L.E. Assessing exposure to household air pollution in children under five: A scoping review. Environ. Pollut. 2022, 311, 119917. [Google Scholar] [CrossRef]
- Prasad, R.; Abhijeet, S.; Garg, R.; Hosmane, G.B. Biomass fuel exposure and respiratory diseases in India. Biosci. Trends 2012, 6, 219–228. [Google Scholar] [CrossRef]
- Sheam, M.M.; Biswas, S.K.; Ahmed, K.R.; Syed, S.B.; Hossain, M.S.; Khan, M.S.A.; Hasan, M.R.; Zohra, F.T.; Rahman, M.M. Mycoremediation of reactive red HE7B dye by Aspergillus salinarus isolated from textile effluents. Curr. Res. Microb. Sci. 2021, 2, 100056. [Google Scholar] [CrossRef]
- Hussam, A.; Alauddin, M.; Khan, A.H.; Chowdhury, D.; Bibi, H.; Bhattacharjee, M.; Sultana, S. Solid phase microextraction: Measurement of volatile organic compounds (VOCs) in Dhaka City air pollution. J. Environ. Sci. Health A Tox. Hazard Subst. Environ. Eng. 2002, 37, 1223–1239. [Google Scholar] [CrossRef]
- Wang, Y.; Peris, A.; Rifat, M.R.; Ahmed, S.I.; Aich, N.; Nguyen, L.V.; Urík, J.; Eljarrat, E.; Vrana, B.; Jantunen, L.M.; et al. Measuring exposure of e-waste dismantlers in Dhaka Bangladesh to organophosphate esters and halogenated flame retardants using silicone wristbands and T-shirts. Sci. Total Environ. 2020, 720, 137480. [Google Scholar] [CrossRef] [PubMed]
- Doherty, B.T.; Pearce, J.L.; Anderson, K.A.; Karagas, M.R.; Romano, M.E. Assessment of Multipollutant Exposures During Pregnancy Using Silicone Wristbands. Front. Public Health 2020, 8, 547239. [Google Scholar] [CrossRef]
- McLarnan, S.M.; Bramer, L.M.; Dixon, H.M.; Scott, R.P.; Calero, L.; Holmes, D.; Gibson, E.A.; Cavalier, H.M.; Rohlman, D.; Miller, R.L.; et al. Predicting personal PAH exposure using high dimensional questionnaire and wristband data. J. Expo. Sci. Environ. Epidemiol. 2024, 34, 679–687. [Google Scholar] [CrossRef] [PubMed]
- Anderson, K.A.; Points, G.L.; Donald, C.E.; Dixon, H.M.; Scott, R.P.; Wilson, G.; Tidwell, L.G.; Hoffman, P.D.; Herbstman, J.B.; O’Connell, S.G. Preparation and performance features of wristband samplers and considerations for chemical exposure assessment. J. Expo. Sci. Environ. Epidemiol. 2017, 27, 551–559. [Google Scholar] [CrossRef] [PubMed]
- MyExposome. Full Screen (Quantitative): List of Tested Compounds for Quantitative (1500+) Screen (Version 4). myexposome.com: MyExposome Inc. Available online: https://www.myexposome.com/fullscreen (accessed on 2 February 2024).
- Bergmann, A.J.; Points, G.L.; Scott, R.P.; Wilson, G.; Anderson, K.A. Development of quantitative screen for 1550 chemicals with GC-MS. Anal. Bioanal. Chem. 2018, 410, 3101–3110. [Google Scholar] [CrossRef] [PubMed]
- Samon, S.M.; Hammel, S.C.; Stapleton, H.M.; Anderson, K.A. Silicone wristbands as personal passive sampling devices: Current knowledge, recommendations for use, and future directions. Environ. Int. 2022, 169, 107339. [Google Scholar] [CrossRef]
- Wacławik, M.; Rodzaj, W.; Wielgomas, B. Silicone Wristbands in Exposure Assessment: Analytical Considerations and Comparison with Other Approaches. Int. J. Environ. Res. Public Health 2022, 19, 1935. [Google Scholar] [CrossRef]
- Paterson, C.; Sharpe, R.; Taylor, T.; Morrissey, K. Indoor PM2.5, VOCs and asthma outcomes: A systematic review in adults and their home environments. Environ. Res. 2021, 202, 111631. [Google Scholar] [CrossRef]
- Das, I.; Jagger, P.; Yeatts, K. Biomass Cooking Fuels and Health Outcomes for Women in Malawi. Ecohealth 2017, 14, 7–19. [Google Scholar] [CrossRef]
- Sultana, R.; Ferdous, K.J.; Hossain, M.; Zahid, M.S.H.; Islam, L.N. Immune functions of the garment workers. Int. J. Occup. Environ. Med. 2012, 3, 195–200. [Google Scholar]
- Jarhyan, P.; Hutchinson, A.; Khaw, D.; Prabhakaran, D.; Mohan, S. Prevalence of chronic obstructive pulmonary disease and chronic bronchitis in eight countries: A systematic review and meta-analysis. Bull. World Health Organ. 2022, 100, 216–230. [Google Scholar] [CrossRef] [PubMed]
- Bleich, S.N.; Koehlmoos, T.L.; Rashid, M.; Peters, D.H.; Anderson, G. Noncommunicable chronic disease in Bangladesh: Overview of existing p.rograms and priorities going forward. Health Policy 2011, 100, 282–289. [Google Scholar] [CrossRef] [PubMed]
- Banik, S.; Ghosh, A. Prevalence of chronic kidney disease in Bangladesh: A systematic review and meta-analysis. Int. Urol. Nephrol. 2021, 53, 713–718. [Google Scholar] [CrossRef] [PubMed]
- Ahsan, H.; Chen, Y.; Parvez, F.; Argos, M.; Hussain, A.I.; Momotaj, H.; Levy, D.; van Geen, A.; Howe, G.; Graziano, J. Health Effects of Arsenic Longitudinal Study (HEALS): Description of a multidisciplinary epidemiologic investigation. J. Expo. Sci. Environ. Epidemiol. 2006, 16, 191–205. [Google Scholar] [CrossRef]
- Farzan, S.F.; Shahriar, M.; Kibriya, M.G.; Jasmine, F.; Sarwar, G.; Slavkovic, V.; Graziano, J.H.; Ahsan, H.; Argos, M. Urinary arsenic and relative telomere length in 5–7 year old children in Bangladesh. Environ. Int. 2021, 156, 106765. [Google Scholar] [CrossRef]
- Kile, M.L.; Scott, R.P.; O’connell, S.G.; Lipscomb, S.; MacDonald, M.; McClelland, M.; Anderson, K.A. Using silicone wristbands to evaluate preschool children’s exposure to flame retardants. Environ. Res. 2016, 147, 365–372. [Google Scholar] [CrossRef]
- O’Connell, S.G.; Kincl, L.D.; Anderson, K.A. Silicone wristbands as personal passive samplers. Environ. Sci. Technol. 2014, 48, 3327–3335. [Google Scholar] [CrossRef]
- Kim, S.; Chen, J.; Cheng, T.; Gindulyte, A.; He, J.; He, S.; Li, Q.; Shoemaker, B.A.; Thiessen, P.A.; Yu, B.; et al. PubChem 2023 update. Nucleic Acids Res. 2023, 51, D1373–D1380. [Google Scholar] [CrossRef]
- Mendoza-Sanchez, I.; Uwak, I.; Myatt, L.; Van Cleve, A.; Pulczinski, J.C.; Rychlik, K.A.; Sweet, S.; Ramani, T.; Zietsman, J.; Zamora, M.L.; et al. Maternal exposure to polycyclic aromatic hydrocarbons in South Texas, evaluation of silicone wristbands as personal passive samplers. J. Expo. Sci. Environ. Epidemiol. 2022, 32, 280–288. [Google Scholar] [CrossRef]
- Dixon, H.M.; Scott, R.P.; Holmes, D.; Calero, L.; Kincl, L.D.; Waters, K.M.; Camann, D.E.; Calafat, A.M.; Herbstman, J.B.; Anderson, K.A. Silicone wristbands compared with traditional polycyclic aromatic hydrocarbon exposure assessment methods. Anal. Bioanal. Chem. 2018, 410, 3059–3071. [Google Scholar] [CrossRef]
- Samburova, V.; Connolly, J.; Gyawali, M.; Yatavelli, R.L.; Watts, A.C.; Chakrabarty, R.K.; Zielinska, B.; Moosmüller, H.; Khlystov, A. Polycyclic aromatic hydrocarbons in biomass-burning emissions and their contribution to light absorption and aerosol toxicity. Sci. Total Environ. 2016, 568, 391–401. [Google Scholar] [CrossRef]
- Running, L.S.; Olson, J.R.; Aga, D.S.; Travis, S.C.; Daleiro, M.; Queirolo, E.I.; Kordas, K. Polycyclic aromatic hydrocarbons in silicone wristbands of Uruguayan children: Measurement and exposure source exploration. Environ. Sci. Adv. 2024, 3, 751–762. [Google Scholar] [CrossRef]
- Travis, S.C.; Aga, D.S.; Queirolo, E.I.; Olson, J.R.; Daleiro, M.; Kordas, K. Catching flame retardants and pesticides in silicone wristbands: Evidence of exposure to current and legacy pollutants in Uruguayan children. Sci. Total Environ. 2020, 740, 140136. [Google Scholar] [CrossRef] [PubMed]
- Gibson, E.A.; Stapleton, H.M.; Calero, L.; Holmes, D.; Burke, K.; Martinez, R.; Cortes, B.; Nematollahi, A.; Evans, D.; Anderson, K.A.; et al. Differential exposure to organophosphate flame retardants in mother-child pairs. Chemosphere 2019, 219, 567–573. [Google Scholar] [CrossRef] [PubMed]
- Xie, Q.; Guan, Q.; Li, L.; Pan, X.; Ho, C.-L.; Liu, X.; Hou, S.; Chen, D. Exposure of children and mothers to organophosphate esters: Prediction by house dust and silicone wristbands. Environ. Pollut. 2021, 282, 117011. [Google Scholar] [CrossRef] [PubMed]
- Vidi, P.-A.; Anderson, K.A.; Chen, H.; Anderson, R.; Salvador-Moreno, N.; Mora, D.C.; Poutasse, C.; Laurienti, P.J.; Daniel, S.S.; Arcury, T.A. Personal samplers of bioavailable pesticides integrated with a hair follicle assay of DNA damage to assess environmental exposures and their associated risks in children. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2017, 822, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Veludo, A.F.; Röösli, M.; Dalvie, M.A.; Fišerová, P.S.; Prokeš, R.; Přibylová, P.; Šenk, P.; Kohoutek, J.; Mugari, M.; Klánová, J.; et al. Child exposure to organophosphate and pyrethroid insecticides measured in urine, wristbands, and household dust and its implications for child health in South Africa: A panel study. Environ. Epidemiol. 2024, 8, e282. [Google Scholar] [CrossRef]
- Levasseur, J.L.; Hammel, S.C.; Hoffman, K.; Phillips, A.L.; Zhang, S.; Ye, X.; Calafat, A.M.; Webster, T.F.; Stapleton, H.M. Young children’s exposure to phenols in the home: Associations between house dust, hand wipes, silicone wristbands, and urinary biomarkers. Environ. Int. 2021, 147, 106317. [Google Scholar] [CrossRef]
- Quintana, P.J.E.; Lopez-Galvez, N.; Dodder, N.G.; Hoh, E.; Matt, G.E.; Zakarian, J.M.; Vyas, M.; Chu, L.; Akins, B.; Padilla, S.; et al. Nicotine, Cotinine, and Tobacco-Specific Nitrosamines Measured in Children’s Silicone Wristbands in Relation to Secondhand Smoke and E-cigarette Vapor Exposure. Nicotine Tob. Res. 2021, 23, 592–599. [Google Scholar] [CrossRef]
- Hammel, S.C.; Phillips, A.L.; Hoffman, K.; Stapleton, H.M. Evaluating the Use of Silicone Wristbands To Measure Personal Exposure to Brominated Flame Retardants. Environ. Sci. Technol. 2018, 52, 11875–11885. [Google Scholar] [CrossRef]

| Characteristic | Full Cohort | Lower Exposed Participants | Higher Exposed Participants |
|---|---|---|---|
| N = 40 1 | N = 10 1 | N = 12 1 | |
| Gender | |||
| Female | 20 (50%) | 5 (50%) | 9 (75%) |
| Male | 20 (50%) | 5 (50%) | 3 (25%) |
| Child age (years) | |||
| 5 | 11 (28%) | 3 (30%) | 2 (17%) |
| 6 | 29 (73%) | 7 (70%) | 10 (83%) |
| Body Mass Index | 14.34 (13.54, 15.19) | 13.38 (12.96, 13.88) | 15.11 (13.90, 16.05) |
| Birth Order | |||
| 1 | 10 (25%) | 3 (30%) | 1 (8.3%) |
| 2 | 14 (35%) | 2 (20%) | 7 (58%) |
| 3 | 8 (20%) | 2 (20%) | 2 (17%) |
| 4 | 3 (7.5%) | 1 (10%) | 1 (8.3%) |
| 5 | 5 (13%) | 2 (20%) | 1 (8.3%) |
| Health | |||
| Almost Always Unwell | 6 (15%) | 3 (30%) | 1 (8.3%) |
| Sometimes Quite Ill | 3 (7.5%) | 1 (10%) | 0 (0%) |
| Healthy | 31 (78%) | 6 (60%) | 11 (92%) |
| Very Healthy | 0 (0%) | 0 (0%) | 0 (0%) |
| Mother Smokers | |||
| Yes | 0 (0%) | 0 (0%) | 0 (0%) |
| No | 40 (100%) | 10 (100%) | 12 (100%) |
| Father Smokes | |||
| Yes | 19 (48%) | 4 (40%) | 6 (50%) |
| No | 21 (53%) | 6 (60%) | 6 (50%) |
| Other Smokers in the House | |||
| Yes | 11 (28%) | 4 (40%) | 3 (25%) |
| No | 29 (73%) | 6 (60%) | 9 (75%) |
| Own Farm Land | |||
| Yes | 23 (58%) | 4 (40%) | 9 (75%) |
| No | 17 (43%) | 6 (60%) | 3 (25%) |
| Father Employed in Garment Industry | |||
| Yes | 13 (33%) | 1 (10%) | 6 (50%) |
| No | 27 (68%) | 9 (90%) | 6 (50%) |
| Television Ownership | |||
| Yes | 32 (80%) | 8 (80%) | 6 (50%) |
| No | 8 (20%) | 2 (20%) | 6 (50%) |
| Toilet Type | |||
| Pit Latrine | 21 (53%) | 7 (70%) | 5 (42%) |
| Hanging Toilet | 9 (23%) | 1 (10%) | 3 (25%) |
| Flush | 10 (25%) | 2 (20%) | 4 (33%) |
| Maternal Educational Attainment | |||
| No formal education | 2 (5%) | 1 (10%) | 0 (0%) |
| Up to primary | 11 (28%) | 3 (30%) | 2 (17%) |
| Secondary school certificate | 25 (63%) | 5 (50%) | 9 (75%) |
| Higher secondary certificate or higher | 0 (0%) | 0 (0%) | 0 (0%) |
| Unknown | 2 (5%) | 1 (10%) | 1 (8%) |
| Chemical | Median (25th Percentile, 75th Percentile) | Range |
|---|---|---|
| Total | 27.5 (23, 29.25) | (14, 37) |
| Chemicals in Commerce | 9 (8, 11) | (5, 14) |
| Consumer Products | 1 (0, 1) | (0, 4) |
| Dioxins and Furans | 0 (0, 0) | (0, 1) |
| Flame Retardant | 0 (0, 1) | (0, 2) |
| Personal Care | 6 (4, 7) | (1, 11) |
| Pesticides | 6 (5, 7) | (3, 9) |
| Pharmacological | 0 (0, 0) | (0, 1) |
| Polycyclic Aromatic Hydrocarbons (PAHs) | 13 (12, 14) | (5, 20) |
| Volatile Organic Compounds (VOC) | 1 (1, 2) | (0, 2) |
| Chemical | CASN | CID | % Detected | Median (25th and 75th Percentile) | MyExposome Classification |
|---|---|---|---|---|---|
| Bis(2-ethylhexyl)phthalate | 117-81-7 | 8343 | 100 | 1540 (874, 2360) | Chemicals in Commerce and Pesticides |
| Benz[a]anthracene | 56-55-3 | 5954 | 97.5 | 28.2 (20.8, 34.9) | Polycyclic Aromatic Hydrocarbons |
| Pyrene | 129-00-0 | 31,423 | 97.5 | 176 (133, 207) | Chemicals in Commerce and Polycyclic Aromatic Hydrocarbons |
| Acenaphthylene | 208-96-8 | 9161 | 95 | 17.8 (12.5, 24.8) | Polycyclic Aromatic Hydrocarbons |
| Benzyl salicylate | 118-58-1 | 8363 | 95 | 374 (170, 822) | Personal Care |
| Diisobutyl phthalate | 84-69-5 | 6782 | 95 | 1260 (601, 2960) | Chemicals in Commerce |
| Diethyl phthalate | 84-66-2 | 6781 | 92.5 | 436 (238, 1090) | Chemicals in Commerce and Pesticides |
| Naphthalene | 91-20-3 | 931 | 92.5 | 11.6 (9.04, 16) | Polycyclic Aromatic Hydrocarbons and Volatile Organic Compounds |
| Di-n-nonyl phthalate | 84-76-4 | 6787 | 90 | 328 (177, 572) | Chemicals in Commerce |
| Permethrin | 52645-53-1 | 40,326 | 87.5 | 207 (92.4, 584) | Pesticides |
| Di-n-butyl phthalate | 84-74-2 | 3026 | 85 | 3080 (618, 9200) | Chemicals in Commerce, Personal Care, and Pesticides |
| Benzo[a]pyrene | 50-32-8 | 2336 | 82.5 | 18.6 (10.5, 22.2) | Polycyclic Aromatic Hydrocarbons |
| Anthracene | 120-12-7 | 8418 | 77.5 | 124 (39.7, 183) | Polycyclic Aromatic Hydrocarbons |
| Cyclopenta[cd]pyrene | 27208-37-3 | 33,743 | 77.5 | 59.1 (30.2, 82.1) | Polycyclic Aromatic Hydrocarbons |
| 2-Methylphenanthrene | 2531-84-2 | 17,321 | 72.5 | 17.4 (0, 23.2) | Polycyclic Aromatic Hydrocarbons |
| Fluoranthene | 206-44-0 | 9154 | 70 | 152 (0, 202) | Polycyclic Aromatic Hydrocarbons |
| Galaxolide | 1222-05-5 | 91,497 | 70 | 91.8 (0, 279) | Chemicals in Commerce and Personal Care |
| Benzophenone | 119-61-9 | 3102 | 65 | 21.7 (0, 30.4) | Chemicals in Commerce and Personal Care |
| Tonalide | 1506-02-1 | 89,440 | 60 | 29.4 (0, 61.6) | Personal Care |
| Triphenylene | 217-59-4 | 9170 | 52.5 | 9.48 (0, 30.4) | Polycyclic Aromatic Hydrocarbons |
| Benzyl benzoate | 120-51-4 | 2345 | 50 | 18.9 (0, 176) | Pesticides |
| Class-Specific Cumulative Exposure Score (Beta Coefficient and 95% Confidence Interval) | Overall Cumulative Exposure Score | |||||
|---|---|---|---|---|---|---|
| Commerce | Personal Care Product | Pesticide | PAHs | VOCs | ||
| Male Sex at Birth | −1.05 (−2.48, 0.37) | −1.07 (−1.79, −0.344) | −0.838 (−1.51, −0.166) | −1.23 (−3.18, 0.73) | 0.0135 (−0.325, 0.351) | −2.42 (−5.24, 0.399) |
| Age | 0.279 (−1.13, 1.69) | 0.933 (0.0836, 1.78) | 0.107 (−0.734, 0.948) | 0.563 (−1.93, 3.05) | 0.0983 (−0.265, 0.461) | 1.39 (−2.08, 4.86) |
| Child Health | −0.528 (−1.07, 0.0113) | |||||
| BMI | 0.831 (0.349, 1.31) | 0.718 (0.381, 1.06) | 0.358 (0.0788, 0.637) | 1.01 (0.152, 1.87) | 0.114 (−0.00212, 0.230) | 1.81 (0.587, 3.03) |
| Birth Order | −0.332 (−0.830, 0.167) | −0.129 (−0.262, 0.00435) | ||||
| Father Smoke | 0.710 (−0.356, 1.78) | 0.540 (−0.160, 1.24) | 1.87 (−0.170, 3.92) | 2.74 (−0.0113, 5.49) | ||
| Other Smokers | −2.09 (−4.39, 0.215) | −2.36 (−5.43, 0.707) | ||||
| Own Farm Land | 0.610 (−0.206, 1.43) | |||||
| Garment | 0.776 (−0.394, 1.95) | 0.500 (−0.247, 1.25) | 0.652 (−0.0657, 1.37) | 2.23 (0.153, 4.31) | 3.14 (0.209, 6.07) | |
| Rice | −0.141 (−0.323, 0.0407) | 0.700 (0.130, 1.27) | ||||
| Beans | 0.0764 (0.013, 0.140) | 0.0402 (−0.00376, 0.0842) | 0.0397 (0.00102, 0.0783) | 0.115 (−0.0365, 0.267) | ||
| Meat | 0.0133 (−0.00182, 0.0284) | −0.166 (−0.367, 0.0355) | ||||
| Vegetables | −0.0657 (−0.146, 0.0148) | −0.0591 (−0.109, −0.00940) | −0.0799 (−0.219, 0.0593) | 0.0859(−0.0434, 0.215) | ||
| Sweets | −0.0502 (−0.109, 0.00886) | −0.0331 (−0.0735, 0.00724) | −0.0320 (−0.0694, 0.00550) | −0.113 (−0.27, 0.0443) | ||
| Milk | −0.0302 (−0.0652, 0.00474) | −0.0344 (−0.0685, −0.000298) | ||||
| Juice | −0.0885 (−0.151, −0.0257) | −0.119 (−0.284, 0.0463) | −0.179 (−0.418, 0.0591) | |||
| Water | −0.499 (−1.06, 0.0569) | |||||
| TV in House | −1.92 (−3.36, −0.474) | −2.95 (−6.52, 0.628) | ||||
| Hanging Toilet | −0.442 (−1.42, 0.535) | −2.13 (−5.04, 0.793) | −3.1 (−7.05, 0.852) | |||
| Flush | −0.815 (−1.71, 0.0752) | −3.62 (−6.25, −0.979) | −3.26 (−6.61, 0.0967) | |||
| Mother Education | −0.147 (−0.358, 0.0645) | 0.0465 (−0.0105, 0.104) | ||||
| R-Squared | 0.471 | 0.529 | 0.366 | 0.217 | 0.246 | 0.411 |
| PC1 | PC2 | PC3 | PC4 | PC5 | PC6 | PC7 | PC8 | PC9 | |
|---|---|---|---|---|---|---|---|---|---|
| Bis(2-ethylhexyl)phthalate | −0.018 | −0.240 | −0.193 | 0.049 | 0.110 | −0.231 | −0.002 | 0.668 | 0.247 |
| Benz[a]anthracene | −0.126 | −0.048 | 0.071 | 0.055 | −0.132 | 0.174 | 0.764 | 0.010 | 0.113 |
| Pyrene | −0.051 | −0.024 | −0.038 | −0.070 | −0.018 | −0.854 | −0.011 | 0.050 | 0.089 |
| Acenaphthylene | 0.036 | −0.139 | 0.006 | −0.023 | 0.739 | 0.002 | 0.053 | 0.056 | 0.068 |
| Benzyl salicylate | −0.111 | 0.052 | 0.021 | −0.894 | 0.020 | −0.036 | −0.038 | −0.181 | 0.038 |
| Diisobutyl phthalate | 0.078 | −0.103 | 0.340 | −0.810 | −0.023 | −0.110 | 0.005 | 0.107 | −0.064 |
| Diethyl phthalate | 0.018 | −0.268 | 0.073 | −0.001 | −0.201 | 0.321 | −0.676 | −0.034 | 0.093 |
| Naphthalene | −0.183 | −0.078 | −0.112 | 0.039 | 0.770 | −0.025 | −0.018 | 0.109 | −0.107 |
| Di-n-nonyl phthalate | 0.209 | 0.154 | 0.009 | −0.023 | −0.156 | 0.001 | 0.021 | −0.289 | −0.780 |
| Permethrin | 0.514 | −0.015 | 0.324 | 0.187 | −0.018 | −0.074 | 0.032 | −0.483 | −0.285 |
| Di-n-butyl phthalate | 0.423 | −0.226 | 0.555 | −0.148 | −0.062 | −0.089 | 0.042 | −0.032 | −0.024 |
| Benzo[a]pyrene | −0.056 | −0.105 | 0.324 | −0.031 | 0.390 | 0.022 | 0.081 | 0.688 | −0.188 |
| Anthracene | 0.800 | 0.196 | −0.152 | 0.105 | −0.065 | 0.002 | −0.098 | 0.074 | −0.067 |
| Cyclopenta[cd]pyrene | 0.060 | −0.489 | 0.162 | −0.055 | 0.177 | 0.033 | 0.652 | 0.151 | 0.057 |
| 2-Methylphenanthrene | −0.172 | −0.865 | 0.050 | −0.094 | 0.199 | −0.010 | 0.031 | 0.086 | −0.096 |
| Fluoranthene | −0.222 | 0.354 | −0.002 | 0.161 | −0.362 | 0.144 | 0.315 | 0.584 | −0.035 |
| Galaxolide | 0.765 | −0.041 | 0.000 | −0.128 | −0.096 | 0.079 | −0.042 | −0.173 | −0.101 |
| Benzophenone | 0.270 | −0.309 | −0.448 | −0.584 | 0.105 | 0.256 | −0.005 | 0.258 | −0.025 |
| Tonalide | 0.056 | −0.300 | −0.018 | −0.007 | 0.185 | 0.119 | −0.125 | 0.170 | −0.815 |
| Triphenylene | −0.185 | 0.039 | 0.864 | −0.138 | −0.009 | 0.152 | 0.071 | 0.011 | 0.025 |
| Benzyl benzoate | −0.286 | 0.446 | 0.203 | −0.147 | 0.520 | 0.357 | 0.146 | −0.068 | 0.104 |
| Eigenvalue | 3.1 | 2.6 | 2 | 1.8 | 1.6 | 1.3 | 1.2 | 1 | 1 |
| Variance Percent Explained | 14.9% | 12.3% | 9.7% | 8.4% | 7.6% | 6.1% | 5.8% | 5.0% | 4.9% |
| Cumulative Variance Explained | 14.9% | 27.2% | 36.9% | 45.3% | 52.9% | 59.0% | 64.8% | 69.8% | 74.6% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quaid, M.; Haque, S.E.; Islam, T.; Shahriar, M.H.; Sarwar, G.; Ahmed, A.; O’Connell, S.; Jasmine, F.; Kibriya, M.G.; Ahsan, H.; et al. An Assessment of Multipollutant Exposures Using Silicone Wristbands Among Bangladeshi Youth. Int. J. Environ. Res. Public Health 2024, 21, 1691. https://doi.org/10.3390/ijerph21121691
Quaid M, Haque SE, Islam T, Shahriar MH, Sarwar G, Ahmed A, O’Connell S, Jasmine F, Kibriya MG, Ahsan H, et al. An Assessment of Multipollutant Exposures Using Silicone Wristbands Among Bangladeshi Youth. International Journal of Environmental Research and Public Health. 2024; 21(12):1691. https://doi.org/10.3390/ijerph21121691
Chicago/Turabian StyleQuaid, Margaret, Syed Emdadul Haque, Tariqul Islam, Mohammad Hasan Shahriar, Golam Sarwar, Alauddin Ahmed, Steven O’Connell, Farzana Jasmine, Muhammad G. Kibriya, Habibul Ahsan, and et al. 2024. "An Assessment of Multipollutant Exposures Using Silicone Wristbands Among Bangladeshi Youth" International Journal of Environmental Research and Public Health 21, no. 12: 1691. https://doi.org/10.3390/ijerph21121691
APA StyleQuaid, M., Haque, S. E., Islam, T., Shahriar, M. H., Sarwar, G., Ahmed, A., O’Connell, S., Jasmine, F., Kibriya, M. G., Ahsan, H., & Argos, M. (2024). An Assessment of Multipollutant Exposures Using Silicone Wristbands Among Bangladeshi Youth. International Journal of Environmental Research and Public Health, 21(12), 1691. https://doi.org/10.3390/ijerph21121691






