Gastrointestinal Conditions Affect Chronic Pain and Quality of Life in Women
Abstract
1. Introduction
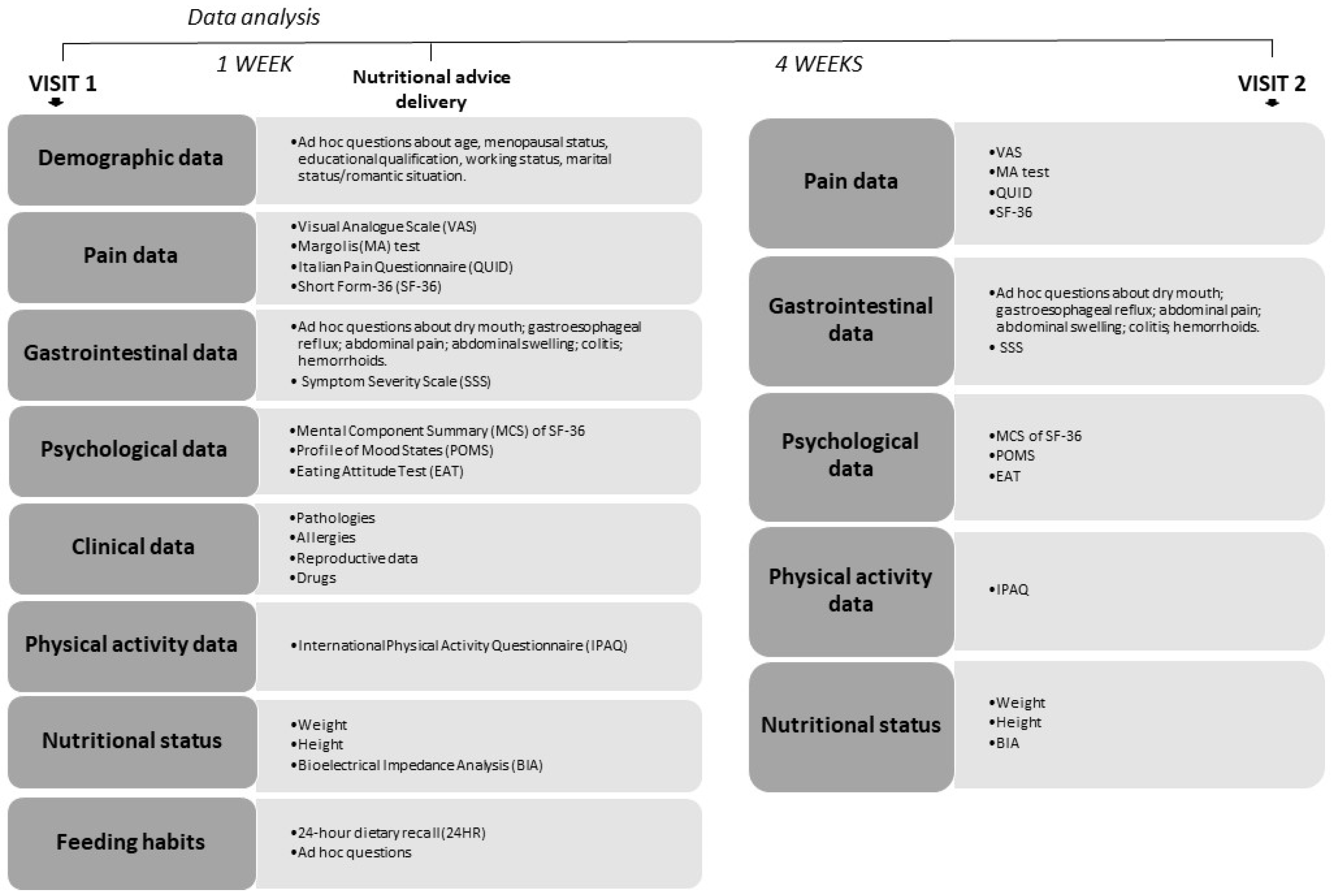
2. Subjects and Methods
3. Experimental Procedure
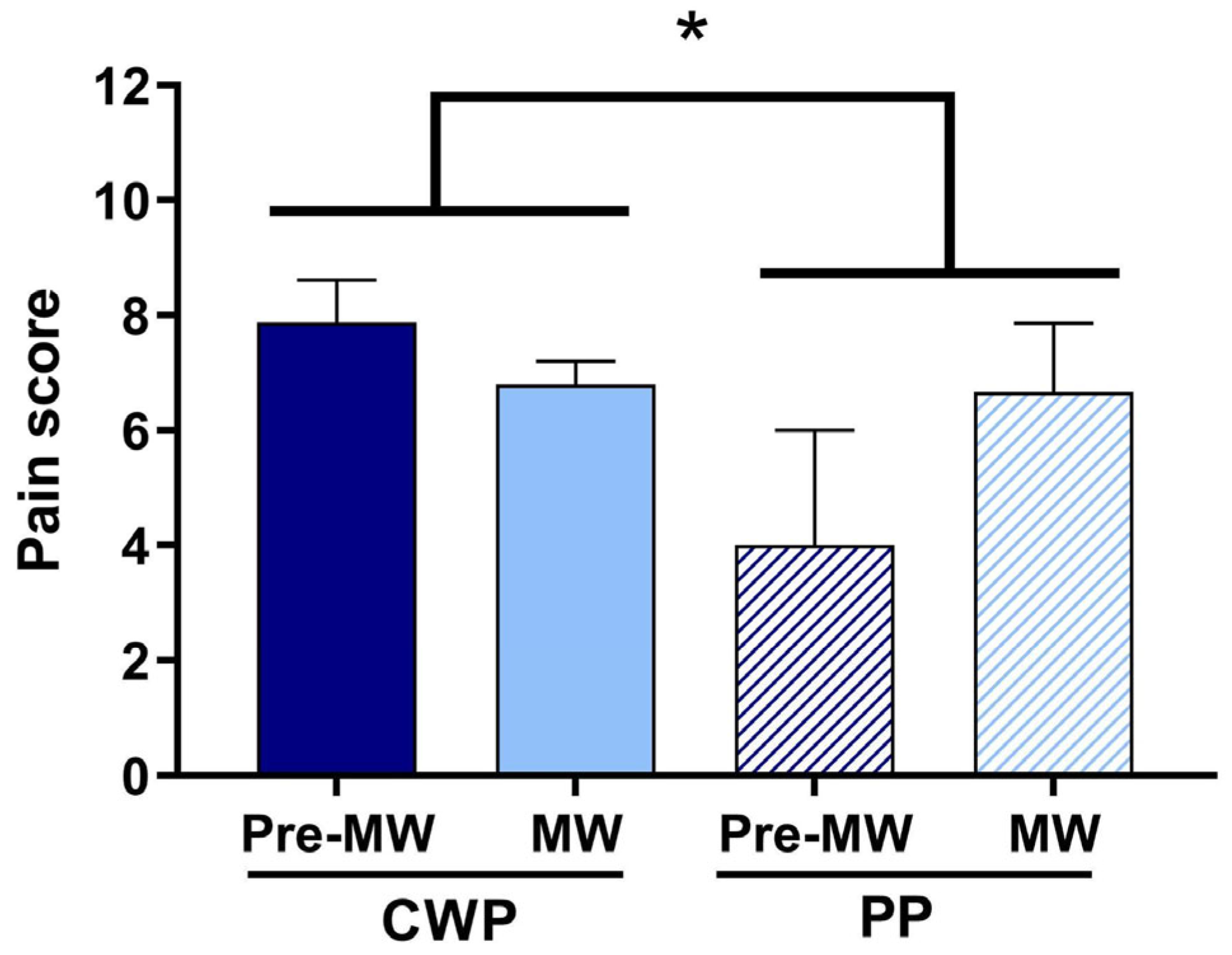
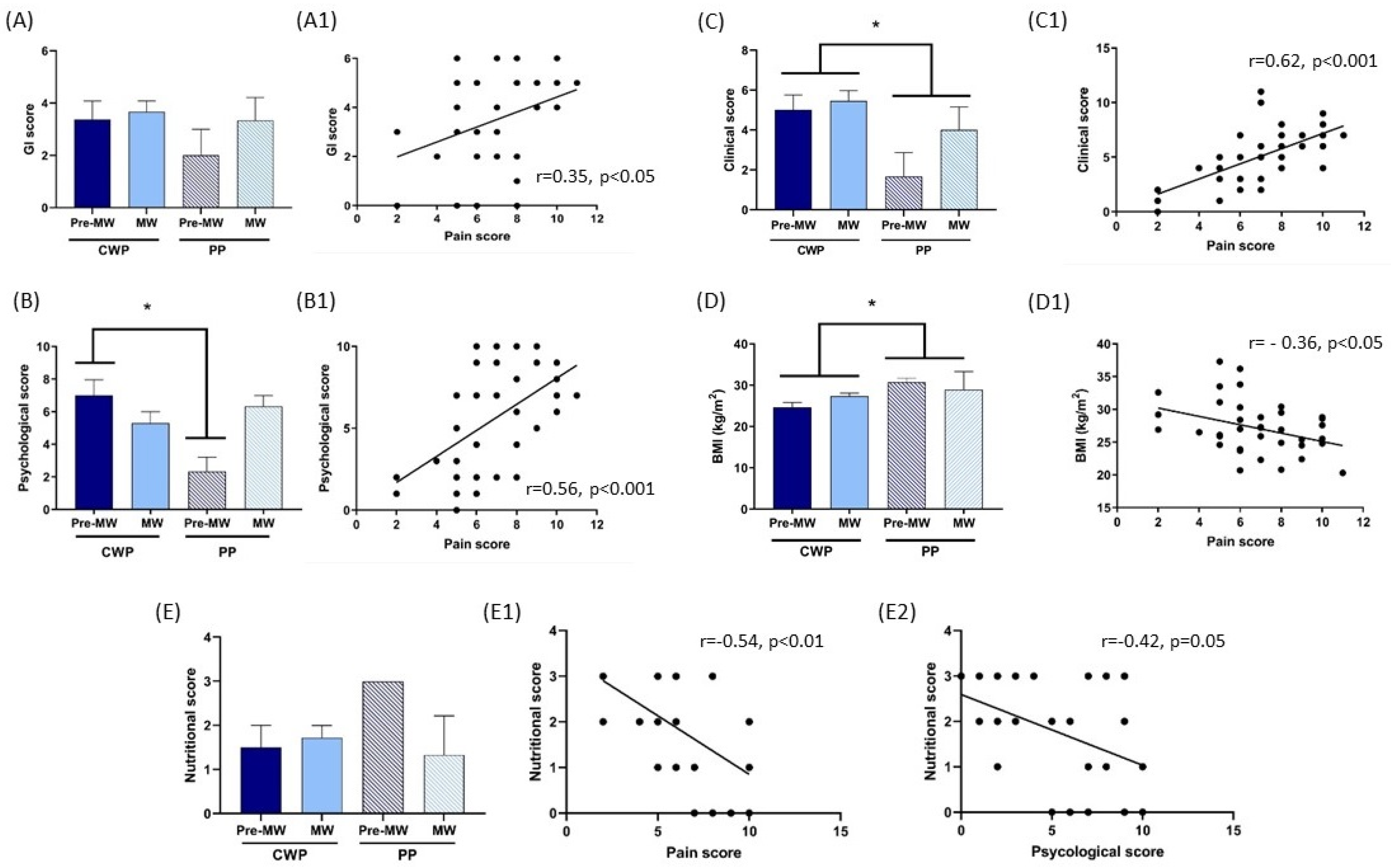
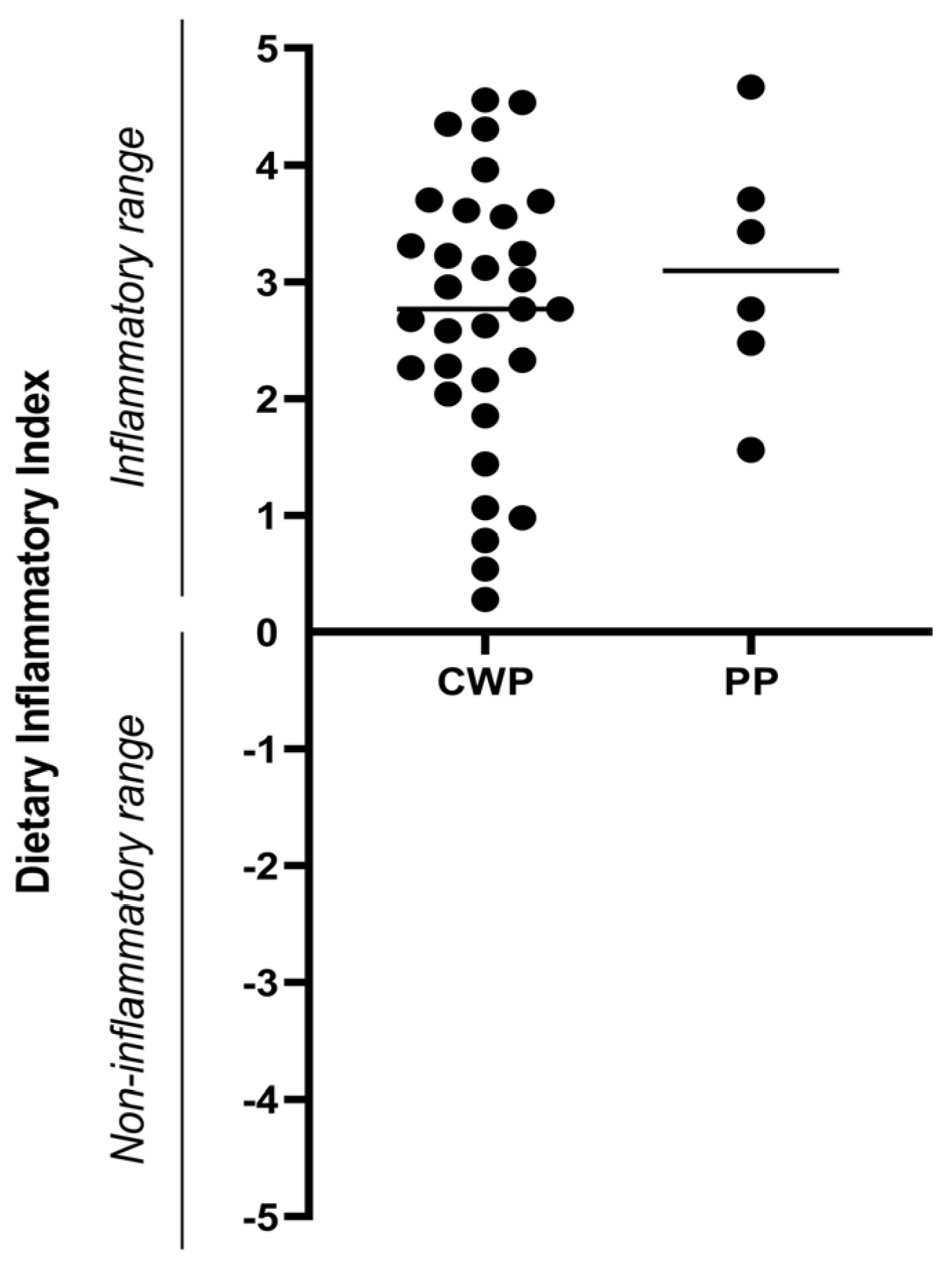
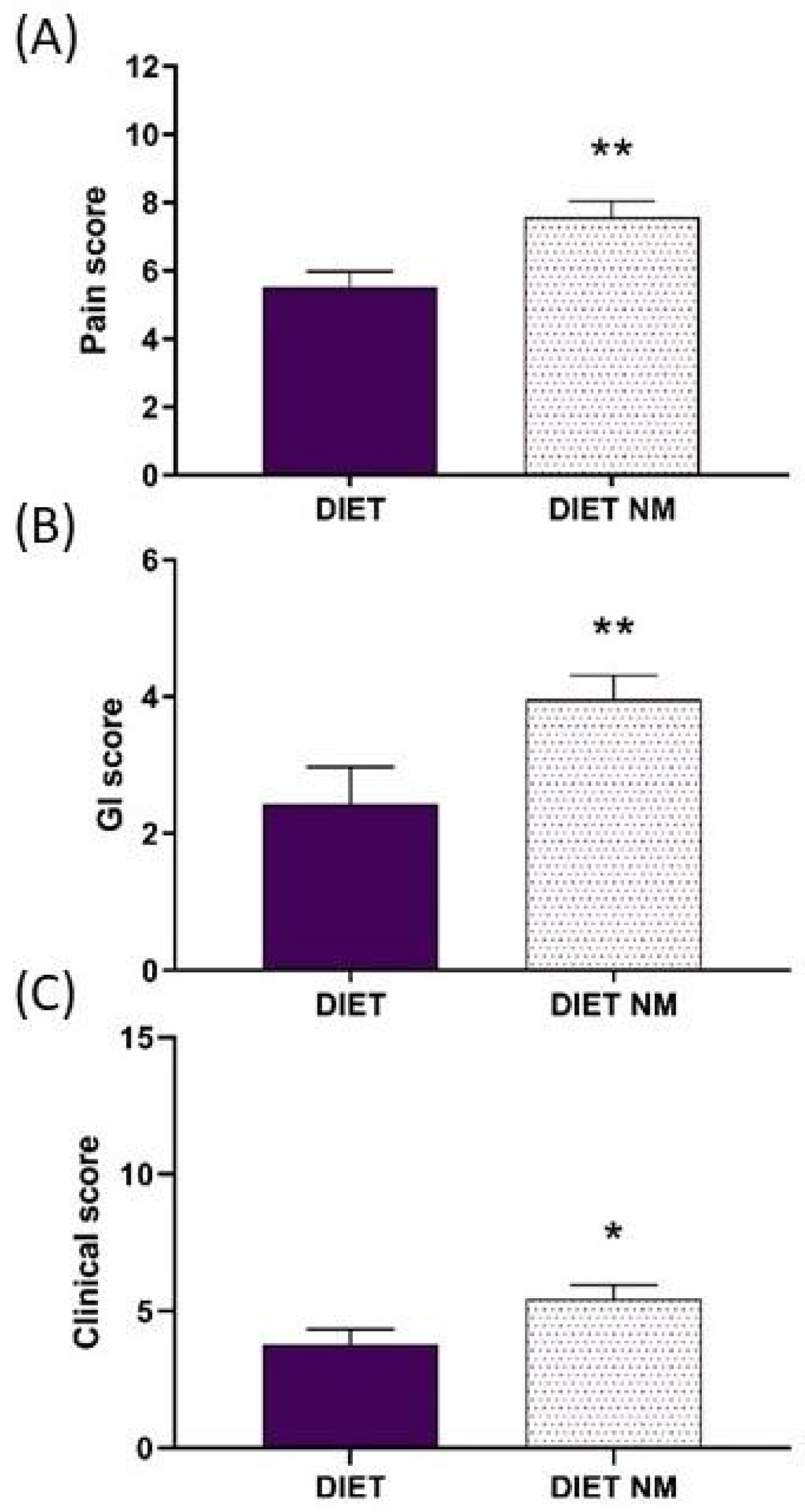
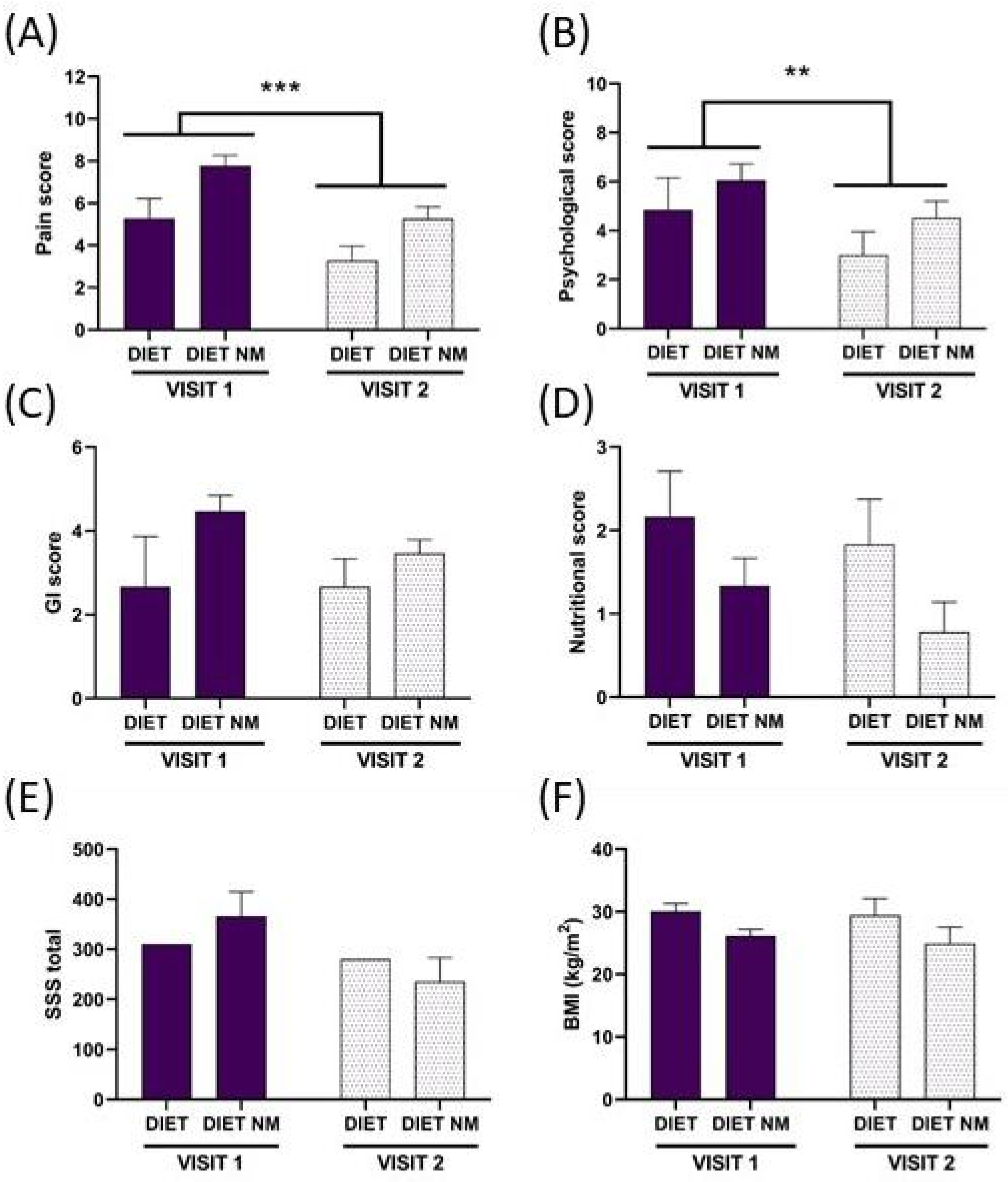
4. Results
5. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 24HR | 24-h dietary recall |
| AGEs | advanced glycation end products |
| AH | abdominal heaviness |
| ALEs | advanced lipoxidation end products |
| ANOVA | analysis of variance |
| APF | abdominal pain frequency |
| API | abdominal pain intensity |
| AS | abdominal swelling |
| BCMI | body cell mass index |
| BIA | bioelectrical impedance analysis |
| BMI | body mass index |
| BP | bodily pain |
| CWP | chronic widespread pain |
| DIET NM | diet without milk |
| DIET | diet with milk |
| DII | Dietary Inflammatory Index |
| EAT | Eating Attitude Test |
| ES | evacuation satisfaction |
| FFM | fat free mass |
| FM | fat mass |
| FODMAP | fermentable oligosaccharides, disaccharides, monosaccharides and polyols |
| GI | Gastrointestinal |
| IBS | irritable bowel syndrome |
| IgG4 | immunoglobulin G4 |
| IPAQ | international physical activity questionnaire |
| IQL | interference with quality of life |
| LSD | least significant difference |
| MA | Margolis |
| MCS | Mental Component Summary: vitality (V), social functioning (SF), emotional role (ER), mental health (MH) |
| MW | menopausal women |
| NFkB | nuclear factor kappa-light-chain-enhancer of activated B cells |
| NSAIDs | nonsteroidal anti-inflammatory drugs |
| POMS | Profile of Mood States: Tension-Anxiety (T), Depression-Dejection (D), Anger-Hostility (A), Vigor-Activity (V), Fatigue-Inertia (F), Confusion-Bewilderment (C) |
| PP | pelvic pain |
| Pre-MW | premenopausal women |
| PRIr-T | Pain Rating Index rank-Total |
| PUFA | polyunsaturated fatty acid |
| QUID | Italian Pain Questionnaire: sensory (S), affective (A), emotional (E), miscellaneous (M) |
| SEM | standard error of the mean |
| SF-36 | Short Form-36 |
| SSS | symptom severity scale |
| TBW | total body water |
| VAS | visual analogue scale |
References
- Casini, I.; Fatighenti, E.; Giannantoni, A.; Massai, L.; Pieretti, S.; Ceccarelli, I.; Aloisi, A.M. Food-specific IgG4 antibody-guided exclusion diet improves conditions of patients with chronic pain. Pain Ther. 2022, 11, 873–906. [Google Scholar] [CrossRef] [PubMed]
- Casini, I.; Ladisa, V.; Clemente, L.; Delussi, M.; Rostanzo, E.; Peparini, S.; Aloisi, A.M.; De Tommaso, M. A personalized Mediterranean diet improves pain and quality of life in patients with fibromyalgia. Pain Ther. 2024, 13, 609–620. [Google Scholar] [CrossRef] [PubMed]
- Philpott, H.L.; Nandurkar, S.; Lubel, J.; Gibson, P.R. Drug-induced gastrointestinal disorders. Frontline Gastroenterol. 2014, 5, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Sivri, A.; Cindaş, A.; Dinçer, F.; Sivri, B. Bowel dysfunction and irritable bowel syndrome in fibromyalgia patients. Clin. Rheumatol. 1996, 15, 283–286. [Google Scholar] [CrossRef] [PubMed]
- Chang, L. The association of functional gastrointestinal disorders and fibromyalgia. Eur. J. Surg. Suppl. 1998, 583, 32–36. [Google Scholar] [CrossRef]
- König, J.; Wells, J.; Cani, P.D.; García-Ródenas, C.L.; MacDonald, T.; Mercenier, A.; Whyte, J.; Troost, F.; Brummer, R.J. Human intestinal barrier function in health and disease. Clin. Transl. Gastroenterol. 2016, 7, e196. [Google Scholar] [CrossRef]
- Mahapatro, M.; Erkert, L.; Becker, C. Cytokine-mediated crosstalk between immune cells and epithelial cells in the gut. Cells 2021, 10, 111. [Google Scholar] [CrossRef]
- Pagliai, G.; Giangrandi, I.; Dinu, M.; Sofi, F.; Colombini, B. Nutritional interventions in the management of fibromyalgia syndrome. Nutrients 2020, 12, 2525. [Google Scholar] [CrossRef]
- Dragan, S.; Șerban, M.C.; Damian, G.; Buleu, F.; Valcovici, M.; Christodorescu, R. Dietary patterns and interventions to alleviate chronic pain. Nutrients 2020, 12, 2510. [Google Scholar] [CrossRef]
- Huskisson, E.C.; Jones, J.; Scott, P.J. Application of visual-analogue scales to the measurement of functional capacity. Rheumatol. Rehabil. 1976, 15, 185–187. [Google Scholar] [CrossRef]
- Thong, I.S.K.; Jensen, M.P.; Miró, J.; Tan, G. The validity of pain intensity measures: What do the NRS, VAS, VRS, and FPS-R measure? Scand. J. Pain. 2018, 18, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Margolis, R.B.; Tait, R.C.; Krause, S.J. A rating system for use with patient pain drawings. Pain 1986, 24, 57–65. [Google Scholar] [CrossRef] [PubMed]
- De Benedittis, G.; Massel, R.; Nobili, R.; Pieri, A. The Italian Pain Questionnaire. Pain 1988, 33, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Ware, J.E.; Sherbourne, C.D., Jr. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med. Care 1992, 30, 473–483. [Google Scholar] [CrossRef]
- Francis, C.Y.; Morris, J.; Whorwell, P.J. The irritable bowel severity scoring system: A simple method of monitoring irritable bowel syndrome and its progress. Aliment. Pharmacol. Ther. 1997, 11, 395–402. [Google Scholar] [CrossRef]
- McNair, D.; Lorr, M.; Doppleman, L. Manual for the Profile of Mood States; Educational and Industrial Testing Service: San Diego, CA, USA, 1971. [Google Scholar]
- Dotti, A.; Lazzari, R. Validation and reliability of the Italian EAT-26. Eat. Weight Disord. Stud. Anorex. Bulim. Obes. 1998, 3, 188–194. [Google Scholar] [CrossRef]
- Craig, C.L.; Marshall, A.L.; Sjöström, M.; Bauman, A.E.; Booth, M.L.; Ainsworth, B.E.; Pratt, M.; Ekelund, U.; Yngve, A.; Sallis, J.F.; et al. International physical activity questionnaire: 12-country reliability and validity. Med. Sci. Sports Exerc. 2003, 35, 1381–1395. [Google Scholar] [CrossRef]
- Eknoyan, G. Adolphe Quetelet (1796–1874)-the average man and indices of obesity. Nephrol. Dial. Transplant. 2008, 23, 47–51. [Google Scholar] [CrossRef]
- Borga, M.; West, J.; Bell, J.D.; Harvey, N.C.; Romu, T.; Heymsfield, S.B.; Leinhard, O.D. Advanced body composition assessment: From body mass index to body composition profiling. J. Investig. Med. 2018, 66, 1–9. [Google Scholar] [CrossRef]
- Shivappa, N.; Steck, S.E.; Hurley, T.G.; Hussey, J.R.; Hébert, J.R. Designing and developing a literature-derived, population-based dietary inflammatory index. Public Health Nutr. 2014, 17, 1689–1696. [Google Scholar] [CrossRef]
- SINU—Italian Society of Human Nutrition. Dietary Reference Values of Nutrients and Energy for the Italian Population—IV Revision; SINU—Italian Society of Human Nutrition: Milano, Italy, 2014. [Google Scholar]
- Fitó, M.; Cladellas, M.; de la Torre, R.; Martí, J.; Muñoz, D.; Schröder, H.; Alcántara, M.; Pujadas-Bastardes, M.; Marrugat, J.; López-Sabater, M.C.; et al. Anti-inflammatory effect of virgin olive oil in stable coronary disease patients: A randomized, crossover, controlled trial. Eur. J. Clin. Nutr. 2008, 62, 570–574. [Google Scholar] [CrossRef] [PubMed]
- Maiorino, M.I.; Bellastella, G.; Petrizzo, M.; Scappaticcio, L.; Giugliano, D.; Esposito, K. Anti-inflammatory effect of Mediterranean diet in type 2 diabetes is durable: 8-year follow-up of a controlled trial. Diabetes Care 2016, 39, e44–e45. [Google Scholar] [CrossRef] [PubMed]
- Schönenberger, K.A.; Schüpfer, A.C.; Gloy, V.L.; Hasler, P.; Stanga, Z.; Kaegi-Braun, N.; Reber, E. Effect of anti-inflammatory diets on pain in rheumatoid arthritis: A systematic review and meta-analysis. Nutrients 2021, 13, 4221. [Google Scholar] [CrossRef] [PubMed]
- Pieretti, S.; Di Giannuario, A.; Di Giovannandrea, R.; Marzoli, F.; Piccaro, G.; Minosi, P.; Aloisi, A.M. Gender differences in pain and its relief. Ann. Ist. Super. Sanita 2016, 52, 184–189. [Google Scholar] [PubMed]
- Rustøen, T.; Wahl, A.K.; Hanestad, B.R.; Lerdal, A.; Paul, S.; Miaskowski, C. Age and the experience of chronic pain: Differences in health and quality of life among younger, middle-aged, and older adults. Clin. J. Pain 2005, 21, 513–523. [Google Scholar] [CrossRef]
- Elma, Ö.; Brain, K.; Dong, H.J. The importance of nutrition as a lifestyle factor in chronic pain management: A narrative review. J. Clin. Med. 2022, 11, 5950. [Google Scholar] [CrossRef]
- Erdrich, S.; Hawrelak, J.A.; Myers, S.P.; Harnett, J.E. A systematic review of the association between fibromyalgia and functional gastrointestinal disorders. Ther. Adv. Gastroenterol. 2020, 13, 1756284820977402. [Google Scholar] [CrossRef]
- Guo, R.; Chen, L.H.; Xing, C.; Liu, T. Pain regulation by gut microbiota: Molecular mechanisms and therapeutic potential. Br. J. Anaesth. 2019, 123, 637–654. [Google Scholar] [CrossRef]
- Clos-Garcia, M.; Andrés-Marin, N.; Fernández-Eulate, G.; Abecia, L.; Lavín, J.L.; van Liempd, S.; Cabrera, D.; Royo, F.; Valero, A.; Errazquin, N.; et al. Gut microbiome and serum metabolome analyses identify molecular biomarkers and altered glutamate metabolism in fibromyalgia. EBioMedicine 2019, 46, 499–511. [Google Scholar] [CrossRef]
- Chen, P.; Wang, C.; Ren, Y.N.; Ye, Z.J.; Jiang, C.; Wu, Z.B. Alterations in the gut microbiota and metabolite profiles in the context of neuropathic pain. Mol. Brain 2021, 14, 50. [Google Scholar] [CrossRef]
- Ricker, M.A.; Haas, W.C. Anti-inflammatory diet in clinical practice: A review. Nutr. Clinical Pract. 2017, 32, 318–325. [Google Scholar] [CrossRef] [PubMed]
- Khansari, M.; Sohrabi, M.; Zamani, F. The useage of opioids and their adverse effects in gastrointestinal practice: A review. Middle East J. Dig. Dis. 2013, 5, 5–16. [Google Scholar] [PubMed]
- Utzeri, E.; Usai, P. Role of non-steroidal anti-inflammatory drugs on intestinal permeability and nonalcoholic fatty liver disease. World J. Gastroenterol. 2017, 23, 3954–3963. [Google Scholar] [CrossRef] [PubMed]
- Targownik, L.E.; Bolton, J.M.; Metge, C.J.; Leung, S.; Sareen, J. Selective serotonin reuptake inhibitors are associated with a modest increase in the risk of upper gastrointestinal bleeding. Am. J. Gastroenterol. 2009, 104, 1475–1482. [Google Scholar] [CrossRef]
- Kapka-Skrzypczak, L.; Niedźwiecka, J.; Skrzypczak, M.; Kruszewski, M. Udzial skladnikow diety w modulacji procesow zapalnych [Nutrients as inflammatory state modulators]. Pediatr. Endocrinol. Diabetes. Metab. 2013, 19, 39–43. [Google Scholar]
- Rönnbäck, C.; Hansson, E. The importance and control of low-grade inflammation due to damage of cellular barrier systems that may lead to systemic inflammation. Front. Neurol. 2019, 10, 533. [Google Scholar] [CrossRef]
- Di Tommaso, N.; Gasbarrini, A.; Ponziani, F.R. Intestinal barrier in human health and disease. Int. J. Environ. Res. Public Health 2021, 18, 12836. [Google Scholar] [CrossRef]
- Weiss, G.A.; Hennet, T. Mechanisms and consequences of intestinal dysbiosis. Cell. Mol. Life Sci. 2017, 74, 2959–2977. [Google Scholar] [CrossRef]
- Vistoli, G.; De Maddis, D.; Cipak, A.; Zarkovic, N.; Carini, M.; Aldini, G. Advanced glycoxidation and lipoxidation end products (AGEs and ALEs): An overview of their mechanisms of formation. Free Radic. Res. 2013, 47 (Suppl. S1), 3–27. [Google Scholar] [CrossRef]
- Tóbon-Velasco, J.C.; Cuevas, E.; Torres-Ramos, M.A. Receptor for AGEs (RAGE) as mediator of NF-kB pathway activation in neuroinflammation and oxidative stress. CNS Neurol. Disord. Drug Targets 2014, 13, 1615–1626. [Google Scholar] [CrossRef]
- Bengmark, S. Advanced glycation and lipoxidation end products-amplifiers of inflammation: The role of food. J. Parenter. Enter. Nutr. 2007, 31, 430–440. [Google Scholar] [CrossRef] [PubMed]

| Groups | Number | Age | Pain |
|---|---|---|---|
| Pre-MW | 11 | 34.18 | CWP = 8 |
| (±2.74) | PP = 3 | ||
| MW | 27 | 60.04 (±1.23) | CWP = 24 PP = 3 |
| Cod Sub | P/O | Age | Pre-MW MW | BMI V1–V2 | Pain Scores V1–V2 | GI Scores V1–V2 | Psycho Scores V1–V2 | Clinical Scores | Reprod Scores | Allergy Scores | Drugs Scores | Activity Scores V1–V2 | Nutri Scores V1–V2 | Diet Sugg Yes/No |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | P | 26 | PRE-MW | 29–na | 2–na | 3–na | 2–na | 0 | 5 | 2 | 1 | 1–na | 3–na | No |
| 2 | P | 50 | PRE-MW | 30–na | 8–na | 0–na | 4–na | 4 | 3 | 0 | 4 | 2–na | 3–na | No |
| 3 | P | 60 | MW | 22–23 | 9–5 | 5–na | 5–2 | 6 | 3 | 0 | 1 | 1–1 | 0–0 | Yes |
| 4 | P | 31 | PRE-MW | 32–32 | 2–1 | 3–na | 1–1 | 1 | 4 | 1 | 0 | 1–2 | 3–3 | Yes |
| 5 | P | 59 | MW | 37–36 | 5–3 | 3–na | 7–3 | 4 | 3 | 0 | 0 | 2–3 | 3–3 | Yes |
| 6 | P | 67 | MW | 27–na | 6–na | 2–na | 7–na | 2 | 2 | 0 | 2 | 2–na | 1–na | No |
| 7 | P | 56 | MW | 36–34 | 6–3 | 0–na | 9–4 | 5 | 2 | 0 | 0 | 2–2 | 3–3 | Yes |
| 8 | P | 60 | MW | 26–na | 5–na | 0–na | 2–na | 1 | 1 | 0 | 1 | 1–na | 2–na | No |
| 9 | P | 55 | MW | 31–na | 5–2 | 4–na | 0–0 | 3 | 3 | 1 | 1 | 2–na | 3–na | Yes |
| 10 | P | 37 | PRE-MW | 28–na | 6–na | 2–na | 9–na | 2 | 3 | 0 | 1 | 2–na | 2–na | No |
| 11 | P | 49 | MW | 26–na | 2–1 | 0–na | 1–0 | 2 | 2 | 0 | 0 | 2–na | 2–na | Yes |
| 12 | P | 57 | MW | 22–na | 7–4 | 3–na | 7–2 | 2 | 5 | 1 | 1 | 1–2 | 0–na | Yes |
| 13 | P | 72 | MW | 24–23 | 5–3 | 5–na | 5–4 | 5 | 3 | 0 | 2 | 1–na | 2–1 | Yes |
| 14 | P | 19 | PRE-MW | 20–21 | 6–9 | 0–na | 10–8 | 3 | 4 | 0 | 0 | 0–na | 1–2 | Yes |
| 15 | P | 56 | MW | 25–na | 5–na | 6–na | 3–na | 5 | 7 | 1 | 1 | 1–na | 3–na | No |
| 16 | P | 54 | MW | 33–32 | 6–3 | 5–na | 4–1 | 5 | 5 | 1 | 1 | 1–na | 3–3 | Yes |
| 17 | P | 61 | MW | 29–28 | 8–5 | 1–2 | 8–7 | 6 | 3 | 1 | 2 | 2–na | 3–1 | Yes |
| 18 | P | 69 | MW | 27–23 | 10–5 | 5–4 | 8–4 | 4 | 5 | 0 | 2 | 1–na | 1–0 | Yes |
| 19 | P | 57 | MW | 20–20 | 8–5 | 2–2 | 6–5 | 5 | 5 | 2 | 2 | 2–1 | 0–0 | Yes |
| 20 | O | 26 | PRE-MW | 27–na | 7–na | 2–na | 9–na | 6 | 2 | 3 | 2 | 1–na | na–na | No |
| 21 | P | 62 | MW | 28–na | 7–5 | 6–5 | 9–2 | 11 | 3 | 2 | 3 | 3–na | na–na | Yes |
| 22 | O | 64 | MW | 25–na | 9–na | 4–na | 10–na | 7 | 4 | 1 | 2 | 2–na | 0–na | No |
| 23 | P | 39 | PRE-MW | 23–na | 6–2 | 3–3 | 4–0 | 5 | 4 | 0 | 3 | 3–3 | na–na | Yes |
| 24 | P | 68 | MW | 23–23 | 6–5 | 5–4 | 2–1 | 7 | 7 | 0 | 4 | 1–1 | 1–1 | Yes |
| 25 | P | 73 | MW | 24–23 | 10–8 | 4–3 | 9–6 | 9 | 4 | 1 | 2 | 1–na | 0–0 | Yes |
| 26 | P | 67 | MW | 28–28 | 10–7 | 4–2 | 6–6 | 7 | 4 | 0 | 2 | 1–na | 2–1 | Yes |
| 27 | O | 47 | PRE-MW | 20–na | 11–5 | 5–4 | 7–6 | 7 | 6 | 0 | 2 | 2–2 | na–na | Yes |
| 28 | O | 62 | MW | 24–na | 9–7 | 5–3 | 9–9 | 6 | 5 | 0 | 4 | 1–na | na–na | Yes |
| 29 | O | 35 | PRE-MW | 28–na | 10–10 | 6–5 | 7–7 | 8 | 5 | 2 | 2 | 1–na | na–na | Yes |
| 30 | O | 34 | PRE-MW | 22–na | 7–na | 4–na | 2–na | 3 | 4 | 3 | 3 | 2–na | na–na | No |
| 31 | O | 32 | PRE-MW | 25–na | 10–8 | 5–4 | 8–8 | 6 | 5 | 4 | 4 | 1–na | na–na | Yes |
| 32 | P | 57 | MW | 26–25 | 4–4 | 2–1 | 3–1 | 4 | 5 | 3 | 2 | 2–2 | 2–0 | Yes |
| 33 | O | 57 | MW | 26–na | 8–4 | 5–3 | 2–6 | 7 | 3 | 2 | 2 | 2–2 | na–na | Yes |
| 34 | O | 57 | MW | 24–na | 8–7 | 6–5 | 10–8 | 8 | 3 | 1 | 2 | 2–na | na–na | Yes |
| 35 | O | 46 | MW | 27–na | 7–na | 6–na | 10–na | 10 | 5 | 0 | 3 | 3–na | na–na | No |
| 36 | P | 55 | MW | 25–22 | 7–2 | 2–3 | 2–6 | 5 | 5 | 2 | 2 | 2–2 | 1–0 | Yes |
| 37 | P | 61 | MW | 33–na | 5–na | 6–na | 1–na | 5 | 4 | 1 | na | na–na | na–na | No |
| 38 | P | 60 | MW | 30–na | 6–na | 2–na | 1–na | 2 | 5 | 0 | na | na–na | 3–na | No |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Casini, I.; Massai, L.; Solomita, E.; Ortenzi, K.; Pieretti, S.; Aloisi, A.M. Gastrointestinal Conditions Affect Chronic Pain and Quality of Life in Women. Int. J. Environ. Res. Public Health 2024, 21, 1435. https://doi.org/10.3390/ijerph21111435
Casini I, Massai L, Solomita E, Ortenzi K, Pieretti S, Aloisi AM. Gastrointestinal Conditions Affect Chronic Pain and Quality of Life in Women. International Journal of Environmental Research and Public Health. 2024; 21(11):1435. https://doi.org/10.3390/ijerph21111435
Chicago/Turabian StyleCasini, Ilenia, Lauretta Massai, Erminia Solomita, Kathleen Ortenzi, Stefano Pieretti, and Anna Maria Aloisi. 2024. "Gastrointestinal Conditions Affect Chronic Pain and Quality of Life in Women" International Journal of Environmental Research and Public Health 21, no. 11: 1435. https://doi.org/10.3390/ijerph21111435
APA StyleCasini, I., Massai, L., Solomita, E., Ortenzi, K., Pieretti, S., & Aloisi, A. M. (2024). Gastrointestinal Conditions Affect Chronic Pain and Quality of Life in Women. International Journal of Environmental Research and Public Health, 21(11), 1435. https://doi.org/10.3390/ijerph21111435








