Abstract
Adverse childhood experiences (ACEs) encompass negative, stressful, and potentially traumatic events during childhood, impacting physical and mental health outcomes in adulthood. Limited studies suggest ACEs can have short-term effects on children’s gut microbiomes and adult cognitive performance under stress. Nevertheless, the long-term effects of ACEs experienced during adulthood remain unexplored. Thus, this study aimed to assess the long-term effects of ACEs on the gut microbiota of adult nursing students. We employed a multidimensional approach, combining 16S rRNA sequencing, bioinformatics tools, and machine learning to predict functional capabilities. High-ACE individuals had an increased abundance of Butyricimonas spp. and Prevotella spp. and decreased levels of Clostridiales, and Lachnospira spp. Prevotella abundance correlated negatively with L-glutamate and L-glutamine biosynthesis, potentially impacting intestinal tissue integrity. While nursing students with high ACE reported increased depression, evidence for a direct gut microbiota–depression relationship was inconclusive. High-ACE individuals also experienced a higher prevalence of diarrhea. These findings highlight the long-lasting impact of ACEs on the gut microbiota and its functions in adulthood, particularly among nursing students. Further research is warranted to develop targeted interventions and strategies for healthcare professionals, optimizing overall health outcomes.
1. Introduction
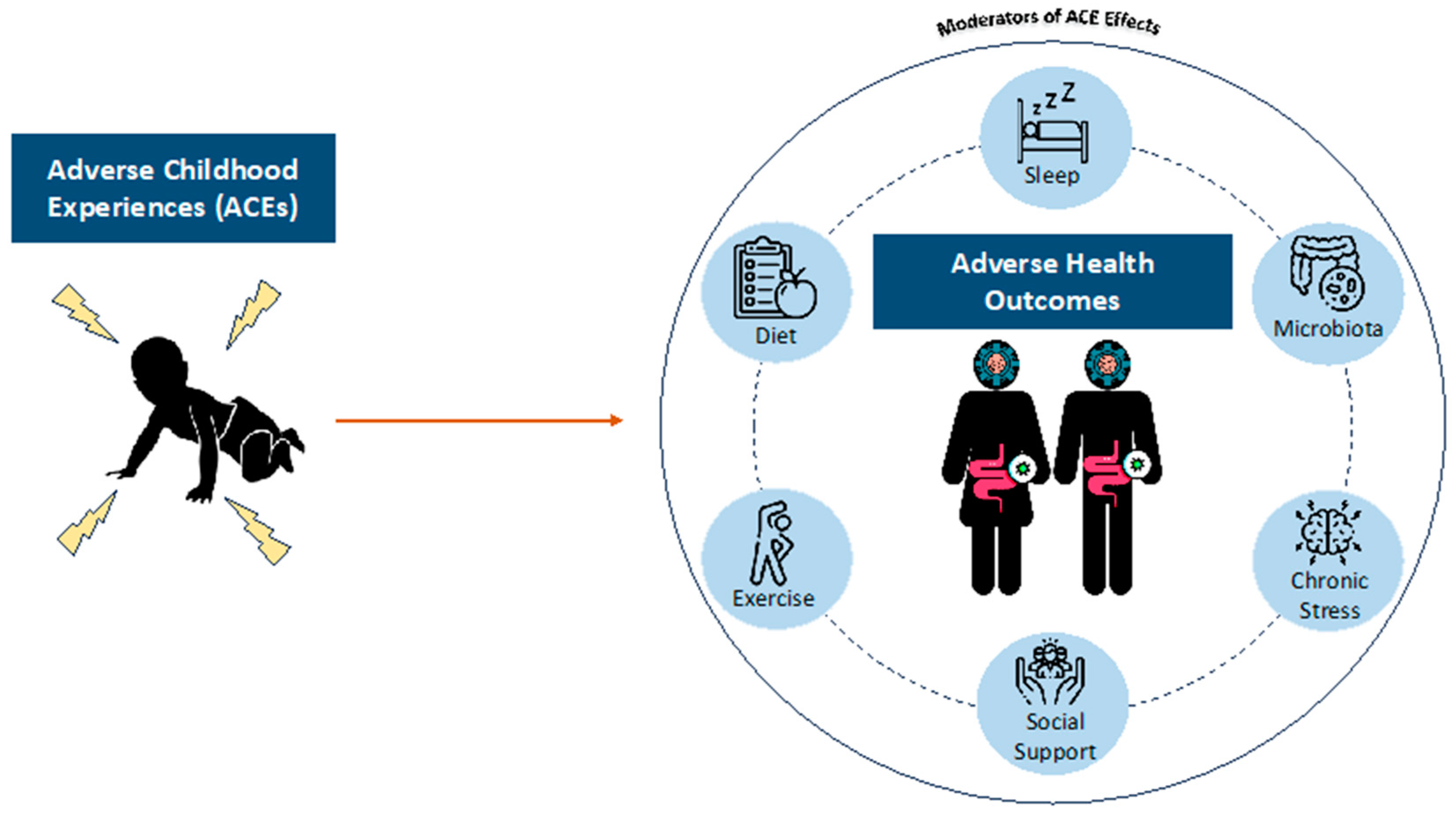
Adverse childhood experiences (ACEs) encompass abuse (emotional, physical, and sexual), neglect, and household dysfunction [1,2,3,4]. Most adults have encountered at least one ACE [5], with prevalence at 65–87% [6,7]. ACE exposure has been associated with various health issues in adulthood, including cardiovascular disease [1,8], diabetes [1], cancer [9,10], depression [11,12], obesity, irritable bowel disease (IBD) [13], sexually transmitted infections [11,14], and premature mortality in adulthood [15]. An ACE history is also associated with risky behaviors such as smoking, inactivity, unsafe sexual practices [14,15], and substance use disorders [16]. Social, behavioral, and environmental factors may also moderate the effects of ACEs on the mental and physical health of individuals (Figure 1). Social support, for example, can buffer ACEs’ effects on health [17], but low socioeconomic status exacerbates stress, impacting sleep, activity, diet, and nutrition [18,19].
Figure 1.
Factors moderating the effects of adverse childhood experiences (ACEs) on the mental and physical health of individuals.
The gut microbiome, influenced by endogenous and exogenous factors and life events such as diet, age, stress, genetics, disease, and antibiotics, varies significantly among individuals [20,21]. The early years of childhood play a crucial role in shaping the gut microbiome, impacting biological and behavioral outcomes throughout life [22]. ACEs may also permanently affect the gut microbiome’s diversity and composition in adulthood [7,8,9]. For example, past studies of rodents have shown that early life stress can alter the gut microbiome [23,24]. Flannery et al. (2020) also demonstrated that caregiving behaviors can modify children’s gut microbiome, influencing both taxonomic and functional compositions [25]. In addition, a history of ACE may lead to impaired immune function [26,27,28] and dysregulated hypothalamic pituitary adrenal (HPA) axis function in adulthood [29,30]. Excitingly, emerging research has revealed the interconnectedness of the gut microbiota, the brain, and various aspects of the gut–brain axis [31,32]. In summary, the gut microbiome’s role in regulating immune homeostasis and its symbiotic relationship with the immune system suggests its potential role as a mediator in the ACE–neuroendocrine–immune function link [33,34,35].
The lifetime impacts of ACE can be even more critical for individuals with increased occupational stress [36]. Nursing is an occupation with an inherently increased exposure to stressors that start at nursing school. Nursing students, for example, often face a heavy academic workload, information overload, rigorous exams, and learning of complex skills in a fast-paced environment, along with long and irregular work hours while caring for patients in clinical settings [37,38]. Rella et al. (2009) conducted a study investigating fatigue and burnout among nursing students and found that 20% of graduates reported serious fatigue/stress and depression [39]. The presence of ACEs in their lives can exacerbate this risk even further, as studies have shown the association of high ACE with increased burnout severity and depressive symptoms among nursing students [40]. Additionally, research suggests that the gut microbiota may play a significant role in influencing the development and severity of depression [41,42,43,44]. For this reason, we hypothesized that the gut microbiota could exacerbate the impact of ACEs on nursing students’ mental health. Although research on stress among nursing students and nurses is abundant, no studies have examined the long-lasting impact of ACEs and considered the relationship between ACEs, the gut microbiota and its functions, and the overall health of nursing students. This insight could support gut–microbiome pathways to prevent depression-related challenges among nursing students, as these conditions may persist or worsen post-graduation and can contribute to increased healthcare costs and lower quality of healthcare [45,46,47].
To address these gaps, this study aimed to examine the impact of ACE exposure on the mental and physical health of nursing students. Specifically, we sought to determine whether ACE exposure has long-term effects on the gut microbiota and its function during adulthood. Lastly, we aimed to identify potential factors moderating the effects of ACEs on the gut microbiome, which could in turn serve as interventional targets to mitigate the lifelong consequences of ACE.
2. Materials and Methods
2.1. Study Design and Participant Recruitment
This study was part of a larger prospective cohort study. Male and female participants (n = 150) were selected from an existing cohort of nursing students enrolled in the Bachelor of Science in Nursing (BSN) program at the University of Texas at El Paso (UTEP) who participated in the Nurse Engagement and Wellness Study (NEWS), a prospective cohort study examining how social, behavioral, and environmental factors may moderate the effects of stress experienced by nursing students and new nurses [48]. Media outlets, posters, flyers, emails, and information sessions held within classes were used to recruit eligible participants, and all participants provided written informed consent at the Biobehavioral Research Laboratory at UTEP. In addition, any participant who transferred to a different university, dropped out of the BSN program, did not graduate from the BSN program, was pregnant or lactating, or had been treated with antibiotics within the month prior to providing a stool sample was excluded from this study. This study was approved by review boards from the Harvard T.H. Chan School of Public Health (16-0080), UTEP (857149-1), and the University of British Columbia (UBC) (H21-03481). All research methods were performed in accordance with the relevant guidelines and regulations.
2.2. Stool Collection and Analyses
Sample collection took place from February to December 2018. Participants donated stool samples using a self-collected stool kit. This stool kit was modelled after the stool kit used by the Human Microbiome Project II and included two pre-labelled Para-Pak sampling vials. One Para-Pak vial was pre-filled with 5 mL of RNAlater preservative. The second Para-Pak vial was pre-filled with 10 mL of 20% glycerol and 2 mL of acid-washed glass beads for bacterial strain isolation and analyses. Participants placed a teaspoon-sized sample of stool (approximately 1 g) into each sampling tube, stored the samples in a designated package within their personal freezer, and then delivered the package to the Biobehavioral Research Laboratory at UTEP within 24 h. Upon arrival at the lab, the stool samples were stored at −80 °C. Once all stool samples had been processed, the samples were stored on dry ice and shipped to the Massachusetts Host-Microbiome Center at Brigham and Women’s Hospital (MGH CCIB DNA core) for bacterial DNA extraction and sequencing. Following the extraction and purification of DNA from each stool sample, the V4 region of the 16S rRNA bacterial gene was amplified by polymerase chain reaction (PCR) using the universal bacterial primers 515F (forward primer) and 806R (reverse primer) as described previously [49,50]. Equal amounts of DNA from each purified PCR product were pooled together to generate an aggregated library for downstream processing. Illumina MiSeq sequencing was performed following standard Illumina MiSeq operation procedures. Demultiplexed, paired-end reads were generated for each sample using the default parameters of the Illumina MiSeq control software and imported into Quantitative Insights into Microbial Ecology (QIIME) version 2 (2021.8) [51]. A total of 10,202,879 raw reads were obtained, with an average of 68,019 reads per sample, and each forward and reverse read was 250 nucleotides in length. A minimum quality control score of 20 was selected: 13 bases were trimmed from the 5′ end of each read, and each read was truncated from the 3′ end at position n = 210 using the DADA2 denoise-paired method in QIIME 2 [52]. The resulting total frequency of amplicon sequence variants (ASVs) was 7,965,440, with an average frequency of 52,103 ASVs per sample. Taxonomy was assigned to each ASV using a pre-trained naïve Bayes classifier and the q2-feature-classifier plugin. This classifier was trained on the Greengenes 13_8 99% OTUs from the 515F/806R region of sequences [53]. Similar sequences were collapsed into single-replicate sequences, or operational taxonomic units (OTUs), to the genus level (Supplementary Table S1). Subsequently, taxa abundances were normalized by the total number of reads sequenced in each sample (Supplementary Table S1). All singletons and doubletons were removed from the normalized OTU table, which was then used to predict the microbiome’s biochemical pathways abundance (MetaCyc) using phylogenetic investigation of communities by reconstruction of unobserved states (PICRUSt2) (version 2.4.1) [54].
2.3. Blood Collection and Analysis
Blood samples were also collected from participants. Serum and plasma were separated from the blood samples by centrifugation within an hour of collection and stored at −80 °C to preserve the integrity of the components for future analysis. Serum samples were then analyzed for levels of triglycerides, high-density lipoprotein cholesterol (HDL), low-density lipoprotein cholesterol (LDL), and glucose. Inflammatory markers were assessed via serum levels of C-reactive protein (CRP) and the inflammatory cytokines interleukin (IL)-1β, IL-6, IL-8, and tumor necrosis factor alpha (TNFα). Serum samples were analyzed in duplicate wells using the Milliplex™ MultiAnalyte Profiling (MAP) Human CVD Panel 3 pre-mixed kit (EMD Millipore Corp, Billerica, MA, USA) for CRP and the Human High-Sensitivity T Cell pre-mixed kit (EMD Millipore Corp, Billerica, MA, USA) for IL-1β, IL-6, IL-8, and TNFα. The plates were read on a Luminex 200 analyzer (Luminex Corporation, Austin, TX, USA) running Milliplex Analyst Version 5.1 software (Vigene Tech Inc. Carlisle, MA, USA) [48]. Concentrations for each biomarker were calculated with reference to a five-point best-fitting standard curve.
2.4. Demographic and Collected Health Endpoints
Specific information pertaining to the different study assessments performed (health endpoints, biomarkers, life stress exposure, behaviors and personal traits, social factors, indicators of engagement and performance, and environmental exposure via clinical measures, biological sample collection, and self-reports) in the NEWS has been described in detail elsewhere [48]. In short, participants were required to complete a series of self-report questionnaires including a demographic questionnaire, the Adverse Childhood Experiences Questionnaire (ACE-Q), and the PHQ9 questionnaire. All questionnaires were completed anonymously in an online format during the first 4 weeks of the participants’ BSN program. Data from incomplete questionnaires were excluded from the analysis. Each participant also provided baseline demographic information including gender, race, ethnicity, and age (see Supplementary Table S1).
2.5. Childhood Adversity
Upon arrival at the Biobehavioral Research Laboratory at UTEP, each participant completed the ACE-Q (approximately 20 min to complete on average) to assess exposure to adverse events that occurred before the age of 18, such as childhood maltreatment and neglect, household dysfunction, and abusive parenting [1]. The Centers for Disease Control and Prevention (CDC) have expanded the original seven-item ACE-Q to address the current ten central ACE categories recognized by the CDC. These ten ACE categories include three types of abuse, two types of neglect, and five types of family dysfunction [55]. ACE scores are calculated by adding the number of different ACE categories reported by an individual. Thus, ACE scores may range from 0 to 10 points and reflect the occurrence of an adverse event, not the severity or frequency of the event. ACE scores greater than or equal to 3 were considered “High ACE” and ACE scores less than 3 were considered “Low ACE” in this study. These parameters were based on the sample distribution.
2.6. Depression Questionnaires
Depression was measured using the patient health (PHQ9) questionnaire. The PHQ9 questionnaire (approximately < 10 min to complete) consists of the nine criteria on which the diagnosis of DSM-IV depressive disorder is based. Results of the PHQ-9 were scored as follows: a score of 5–9 was considered minimal depression; 10–15 was considered mild major depression; 15–19 was considered moderate major depression; and greater than or equal to 20 was considered severe major depression.
2.7. Statistical Analysis
All collected and measured data were utilized to test whether any features were able to differentiate between ACE groups. Specifically, a dimensionality reduction model using principal component analysis (PCA) was used to compute the features which best described the principal components. The top features from this analysis were selected and, after checking normality (using the Shapiro–Wilk test), the Mann–Whitney U test was used to compare the features between ACE groups. All p-values less than 0.05 after false discovery rate (FDR) correction were considered significant.
Using 16S rRNA sequences, the Shannon diversity index for each sample was determined using the normalized OTU table with the singletons and doubletons removed and the Vegan: Community Ecology Package [56] within R (version 2021.09.1). The R packages ggplot2 [57], preseqR [58], and reshape2 [59] were used to construct a boxplot, and the t-test was performed to determine if any significant differences in alpha diversity existed within the data. Differences among bacterial community structures between samples (beta diversity) were determined using Bray–Curtis Dissimilarity from the Vegan: Community Ecology Package within R (version 4.0.3) [56]. Significant differences in beta diversity were evaluated using the ANOSIM test, and any p-value less than 0.05 was considered significant.
To test whether certain gut microbiota and functional biochemical pathways were predictive of ACE, a random forest (RF) model [60] was trained on high- and low-ACE samples at the genus level, and again separately for the biochemical pathways using the Python library Scikit-learn [61]. A dimensionality reduction model was utilized to ensure that the trained RF model avoided overfitting and generalized better on the data [62]. PCA was used to compute the features which best described the principal components. The top twenty features from this analysis were selected and used in the training process for the RF model. The leave-one-out (LOO) cross-validation method was then implemented to assess how effectively the trained classifier generalized in the event of unseen data. All measured test errors were averaged to determine the cross-validation error value. The receiver operating characteristic (ROC) curve was plotted for each LOO dataset. Then, Fisher’s exact statistical significance test was utilized to compare the RF classifiers with the greatest validation scores and determine the differences between the predictive performances of the top RF candidates [63]. The RF model was run 100 times, and the average mean decrease in impurity (MDI) of the most notable features was determined [64]. The linear discriminant analysis (LDA) effect size (LEfSe) method was also performed to identify key taxa and biochemical pathways that may contribute to the observed differences in gut microbial communities using relative abundances [65]. The LEfSe algorithm coupled the non-parametric Kruskal–Wallis test and the unpaired Wilcoxon test with LDA scores to estimate the LDA effect sizes of differentially abundant bacterial OTUs and functional biochemical pathways with statistical significance [65]. The overlapping microbial taxa and biochemical pathways from RF and LEfSe analysis were then used for further analysis and comparisons between groups. Using multiple machine-learning algorithms and finding consensus between their results increases the reliability, generalization, and robustness of the analysis, reducing bias and overfitting. It allows for model selection, performance comparison, and more accurate insights into the underlying patterns and relationships within the data. Gut microbiomes and biochemical pathways were considered to be significantly different if the p-value was less than 0.05 and if the LDA score (log10) threshold was greater than 3.0.
Lastly, network analysis was utilized to assess the relationships between various features differentiating low- and high-ACE groups with potential factors that could moderate the effects of ACEs. The k-nearest neighbor (KNN) algorithm based on Euclidean distance was used to estimate and substitute missing data (k = 5) [66]. The network was constructed based on Spearman’s rank correlation coefficients whose rho values were more than 0.3 and p-adjusted < 0.05 (using FDR).
3. Results
3.1. Demographic Characteristics and Features Differentiating ACE Groups
This study included a total of 150 adult male and female nursing students who were enrolled in the BSN program at UTEP and members of the original NEWS cohort [48]. The average participant’s age was 26 years (SD = 6.5), and the average BMI score was 26.3 (SD = 5.6) (Table 1). The majority of the study participants self-identified as female (83%), Hispanic (87%), and White (88%) (Table 1). Furthermore, 53% of the participants reported “High ACE” scores (≥3), and 47% of the participants reported “Low ACE” scores (<3) (Table 1).

Table 1.
Summary of the sample population.
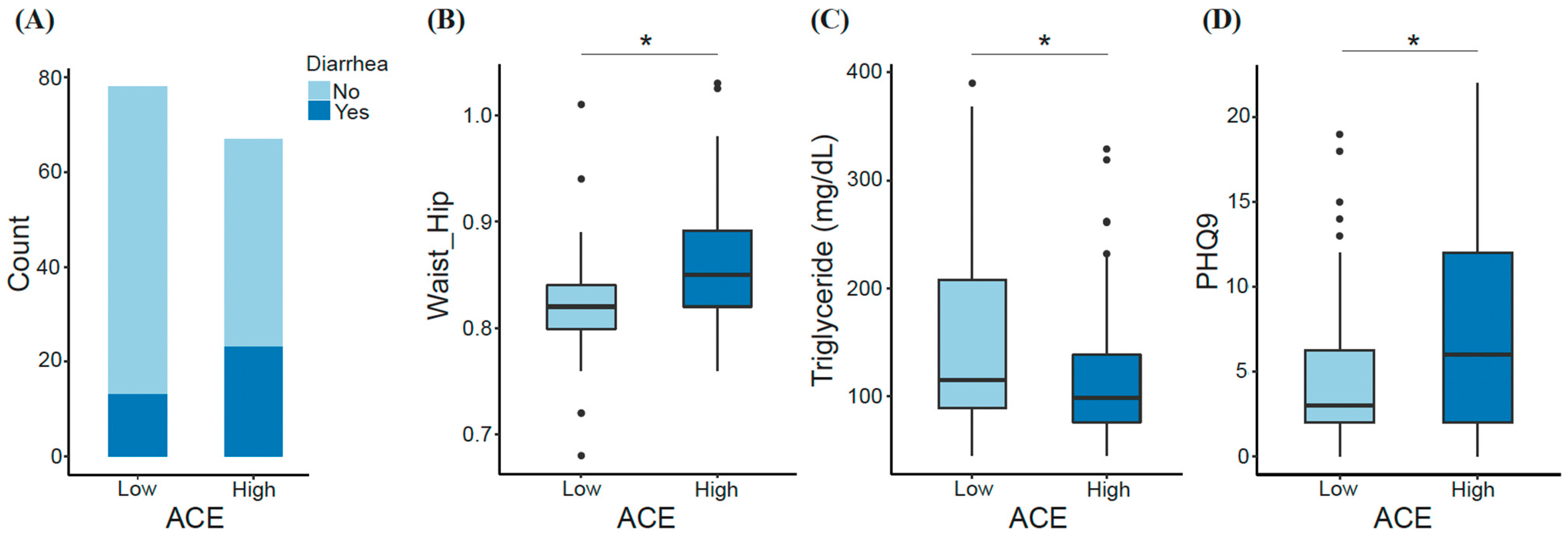
We observed some key differences between low- and high-ACE groups. For instance, having experienced diarrhea within the last two weeks prior to sample collection was among one of the factors differentiating ACE groups. Diarrhea was experienced by 16.7% of the low-ACE group compared with 34.4% of the high-ACE group (Figure 2A). Also, triglycerides were significantly decreased in individuals with high ACE (Mann–Whitney U; p-adjusted = 0.03), while waist-to-hip ratio (Mann–Whitney U; p-adjusted = 0.024) and depression (Mann–Whitney U; p-adjusted = 0.025) were significantly increased in individuals with high ACE (Figure 2B–D). Inflammatory markers were not significantly different between ACE groups.
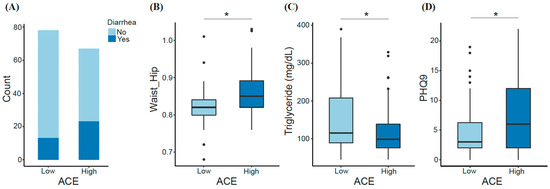
Figure 2.
Significant features differentiating the low- and high-ACE groups. Factors including (A) diarrhea episodes within two weeks prior to sample collection, (B) waist-to-hip ratio, (C) triglyceride (mg/dL), and (D) depression index (PHQ9) were compared between ACE groups. Significant differences were determined using the Mann–Whitney U test and FDR correction. * p-adjusted < 0.05. ACE, adverse childhood experience.
3.2. Gut Microbiome Diversity Analysis
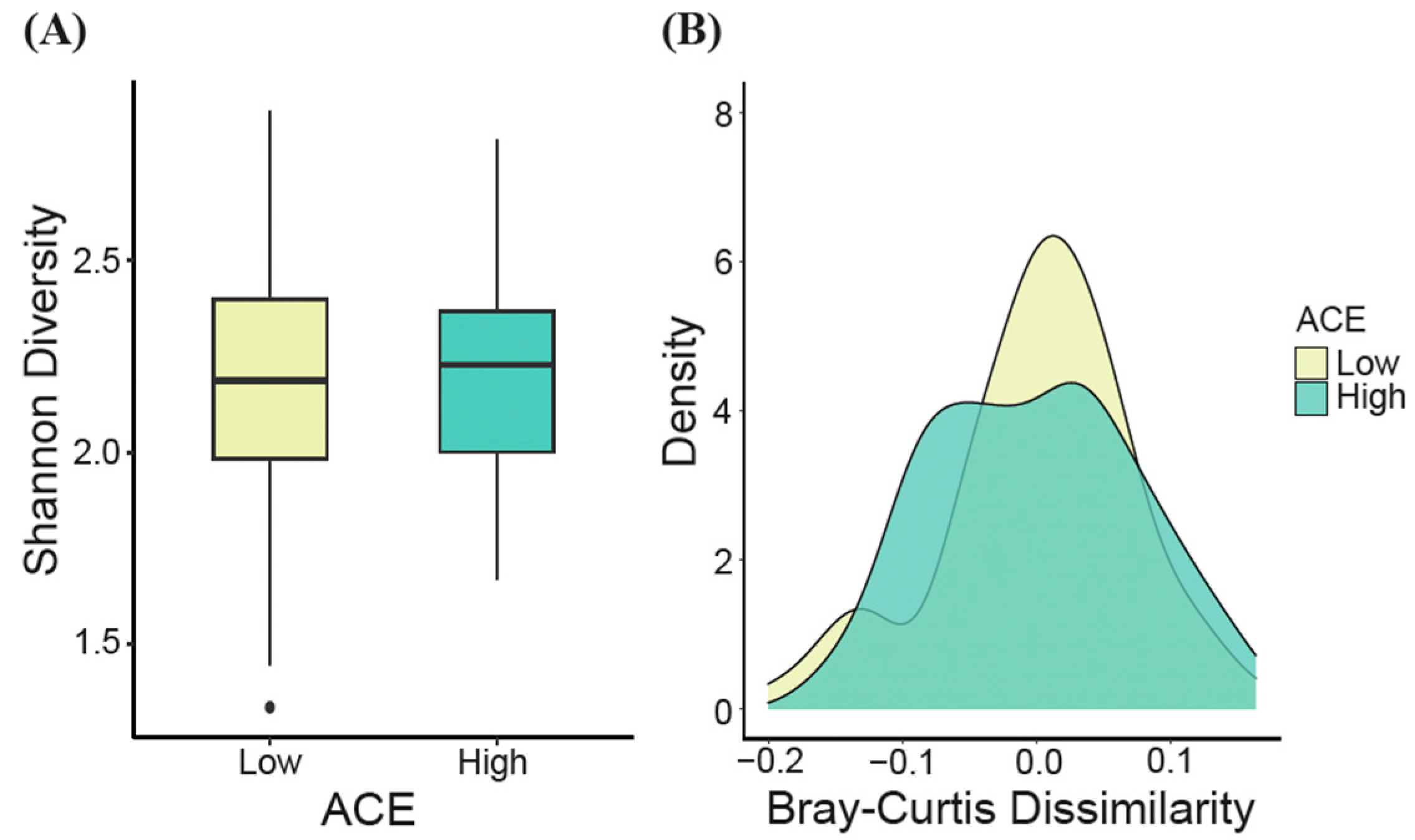
At the genus level, no significant differences in alpha diversity, as measured by the Shannon diversity index, were observed between the ACE groups (t-test, p > 0.05, Figure 3A). However, when generating density plots based on the estimated Bray–Curtis dissimilarity values, significant differences in beta diversity were detected for the ACE groups (ANOSIM, p = 0.04, Figure 3B).
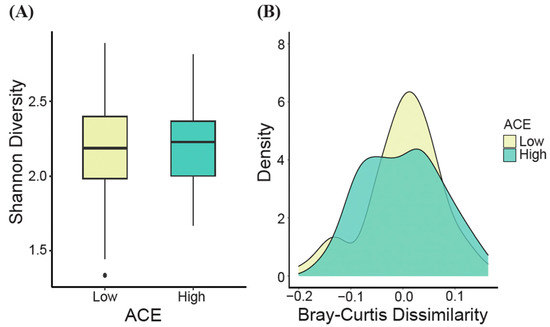
Figure 3.
Gut microbial diversity (genus level). (A) The alpha diversity (Shannon’s diversity index) and (B) the beta diversity density plot (Bray–Curtis dissimilarity) for ACE groups were determined. Significant differences were determined using the t-test and ANOSIM for alpha and beta diversity, respectively. ACE, adverse childhood experience.
3.3. Bacteria Differentiating ACE Groups
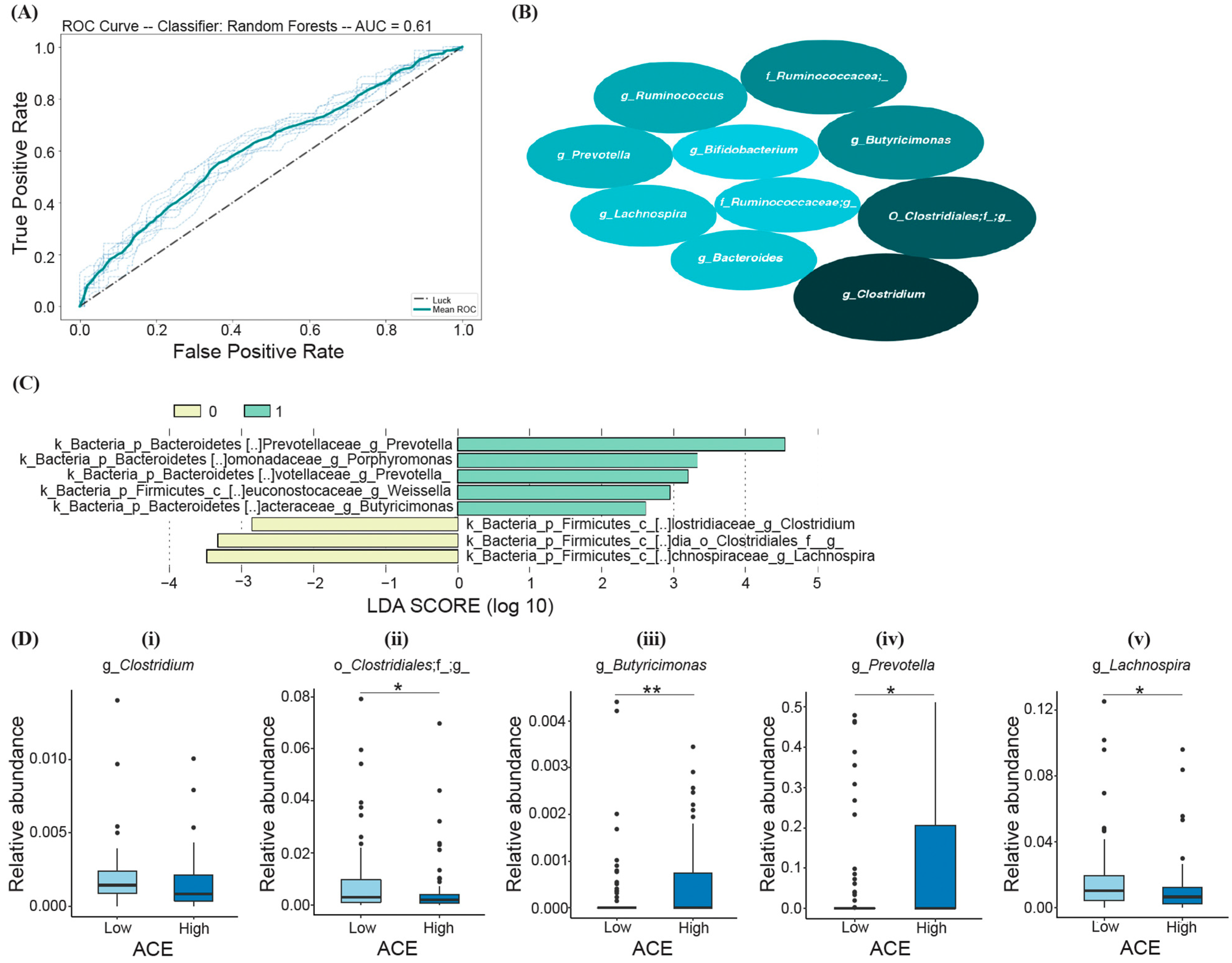
Based on the RF model, the following top ten most important bacteria were identified to differentiate ACE groups (listed in the order of decreasing average mean decrease in impurity (MDI)): Clostridium spp., members of Clostridiales, Butyricimonas spp., Ruminococcacea., Ruminococcus spp. Prevotella spp., Lachnospira spp., Bacteroides spp., members of Ruminococcaceae, and Bifidobacterium spp. (Figure 4B); however, the prediction model was determined to be not significant (Fisher’s exact test; p > 0.05) (Figure 4A).
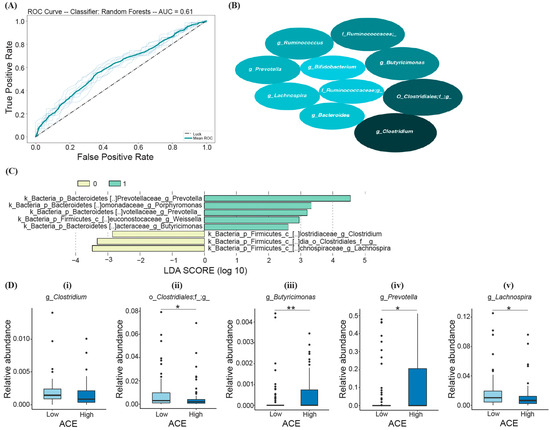
Figure 4.
RF and LEfSe analysis characterizing the significant bacterial taxa in the high- and low-ACE groups. The implemented RF model led to (A) the ROC curve displaying an AUC of 61% (p > 0.05). (B) The top ten most important bacteria differentiating ACE groups, where the size of the ellipses represents the average mean decrease in impurity (MDI). (C) Histogram of the LDA scores (log10) calculated for taxa with differential abundances in high- and low-ACE individuals. The high-ACE group is represented in green (1.0) and the low-ACE group is represented in yellow (0.0). (D) Overlapping bacterial taxa from both random forest and LEfSe compared between ACE groups using the Mann–Whitney U test and FDR correction. * p-adjusted < 0.05; ** p-adjusted < 0.01. ACE, adverse childhood experience.
LEfSe analysis was also performed in comparison with RF to test which groups of bacteria differentiate low- and high-ACE individuals and are predictive of ACE. From the calculated LDA scores, certain taxa were identified for the “High ACE” group (Prevotella, Porphyromonas, Weissella, and Butyricimonas), and three taxa were identified for the “Low ACE” group (Lachnospira, Clostridiales, and Clostridium) (Figure 4C). The taxa with the greatest relative abundance (characterized by the LDA score with the highest magnitude) were the genera Prevotella and Lachnospira for the high- and low-ACE groups, respectively (Figure 4C).
Random forest and LEfSe analysis revealed five overlapping taxa of bacteria that can differentiate between ACE groups, including Clostridium, Clostridiales, Butyricimonas, Prevotella, and Lachnospira (Figure 4D). High-ACE individuals exhibited decreased levels of Clostridiales (Mann–Whitney U, p-adjusted = 0.042, Figure 4D-ii) and Lachnospira (Mann–Whitney U, p-adjusted = 0.041, Figure 4D-v), as well as increased levels of Prevotella (Mann–Whitney U, p-adjusted = 0.043, Figure 4D-iv) and Butyricimonas (Mann–Whitney U, p-adjusted = 0.008, Figure 4D-iii). However, there was no significant difference in Clostridium levels between the two groups after correction (Mann–Whitney U, p-adjusted > 0.05, Figure 4D-i).
3.4. Functional Differences between ACE Groups
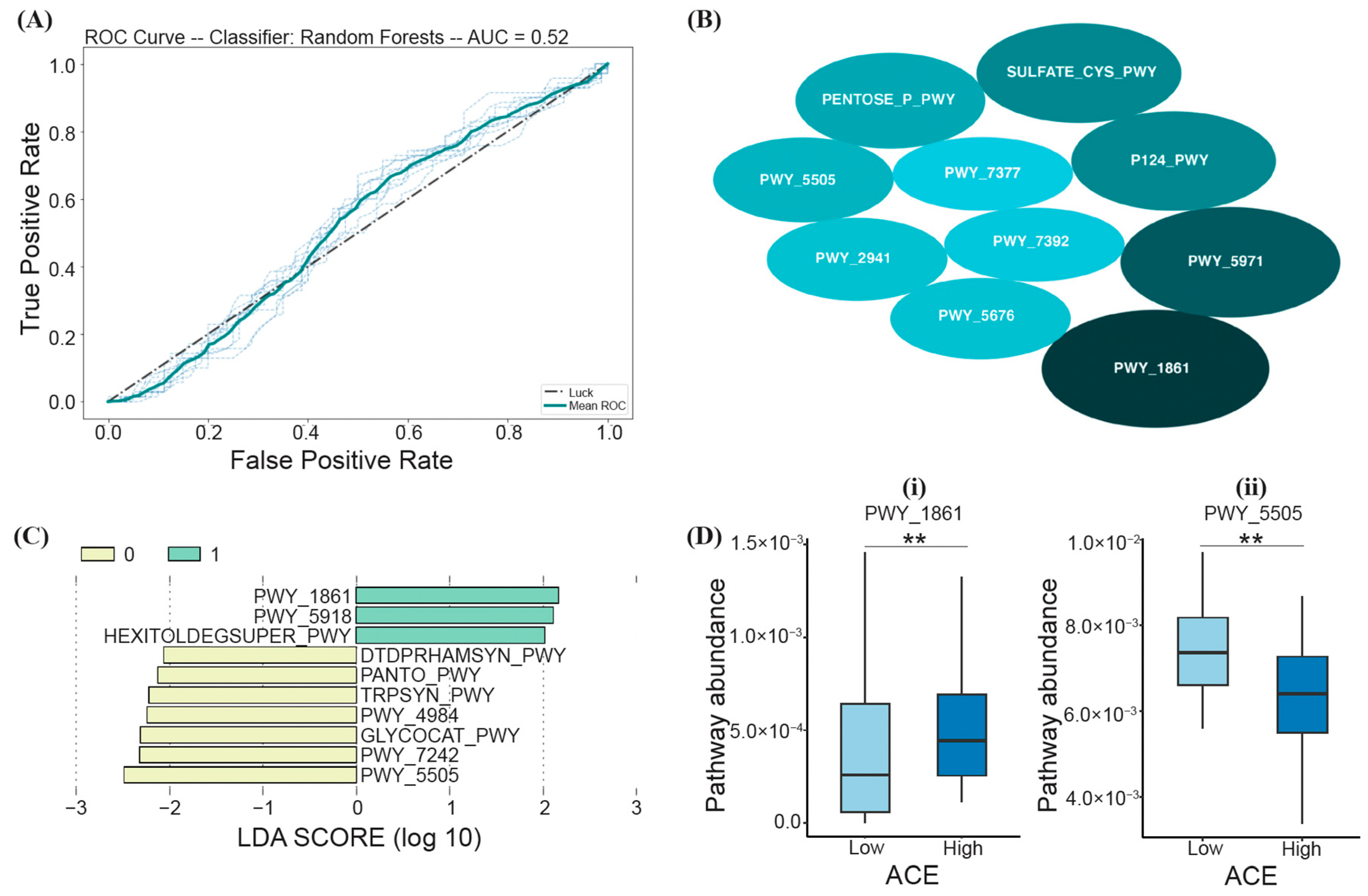
Based on the RF model trained on samples from both high- and low-ACE individuals, a PCA identified the top ten most important biochemical pathways that may predict ACE (listed in the order of decreasing average MDI), including PWY_1861 (formaldehyde assimilation II-assimilatory RuMP Cycle), PWY_5971 (palmitate biosynthesis II), P124_PWY (Bifidobacterium shunt), SULFATE-CYS-PWY (sulfate assimilation and cysteine biosynthesis), PENTOSE_P_PWY (the pentose phosphate pathway), PWY_5505 (L-glutamate and L-glutamine biosynthesis), PWY_2941 (L-lysine biosynthesis II), PWY_5676 (acetyl-CoA fermentation to butanoate), PWY_7392 (taxadiene biosynthesis), and PWY_7377 (cob(II)yrinate a,c-diamide biosynthesis I) (Figure 5B); however, based on LOO cross-validation, the prediction model was not significant (Fisher’s exact test; p > 0.05) (Figure 5A).
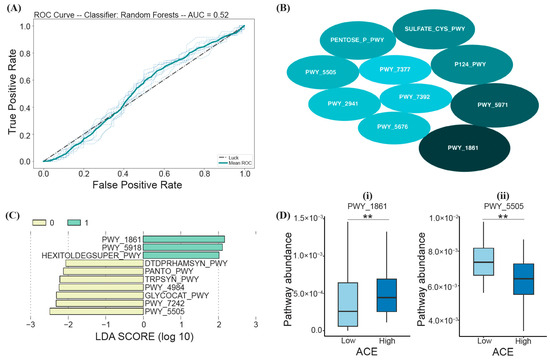
Figure 5.
RF and LEfSe analysis characterizing the significant biochemical pathways (via PICRUSt2) in the high- and low-ACE groups. The implemented RF model led to (A) the ROC curve displaying an AUC of 52% (p > 0.05), (B) the top ten most important pathways differentiating ACE groups, where the size of the ellipses represents the average mean decrease in impurity (MDI), (C) a histogram of the LDA scores (log10) calculated for pathways with differential abundances in high- and low-ACE individuals (the high-ACE group is represented in green (1.0), and the low-ACE group is represented in yellow (0.0)), and (D) overlapping pathways from both RF and LEfSe compared between ACE groups using the Mann–Whitney U test and FDR correction. ** p-adjusted < 0.01. ACE, adverse childhood experience.
LEfSe analysis was also performed to assess the functional differences between low- and high-ACE individuals. From the calculated LDA scores, three functional pathways were identified for the “High ACE” group (PWY_1861 (the formaldehyde assimilation II-assimilatory RuMP cycle), PWY_5918 (the superpathway of heme b biosynthesis from glutamate), and HEXITOLDEGSUPER_PWY (the superpathway of hexitol degradation (bacteria))) (Figure 5C). Seven functional pathways were identified for the “Low ACE” group (DTDPRHAMSYN_PWY (dTDP-L-rhamnose biosynthesis), PANTO_PWY (phosphopantothenate biosynthesis I), TRPSYN_PWY (L-tryptophan biosynthesis), PWY_4984 (the urea cycle), GLYCOCAT_PWY (glycogen degradation I), PWY_7242 (D-fructuronate degradation), and PWY_5505 (L-glutamate and L-glutamine biosynthesis)) (Figure 5C). The functional pathways with the greatest relative abundance (characterized by the LDA scores with the highest magnitude) were PWY_1861 and PWY_5505 for the high- and low-ACE groups, respectively (Figure 5C).
Random forest and LEfSe analysis led to two overlapping pathways that can differentiate between ACE groups, PWY_1861 and PWY_5505 (Figure 5D). High-ACE individuals had decreased PWY_5505 (L-glutamate and L-glutamine biosynthesis; Mann–Whitney U; p-adjusted = 0.01) and increased PWY_1861 (formaldehyde assimilation II-assimilatory RuMP Cycle; Mann–Whitney U; p-adjusted = 0.01) (Figure 5D).
Lastly, using network analysis (rho > 0.3 or rho < −0.3), we assessed the relationships between various features differentiating the low- and high-ACE groups (i.e., bacterial taxa, biochemical pathways, diarrhea, depression, waist-to-hip ratio, and triglycerides), with potential factors that could moderate the effects of ACEs (i.e., sleep, diet, and physical activity). We found that the bacterial genus Prevotella was negatively correlated with L-glutamate and L-glutamine biosynthesis (PWY-5505) (Spearman correlation; rho = 0.33; p-adjusted = 0.001), with no significant correlations with potential moderators. On the other hand, decreased sleep quality was found to be significantly related to depression (Spearman correlation; rho = 0.43; p-adjusted = 0.001), but not linked to any affected bacterial taxa or functions.
4. Discussion
In our quest to investigate the long-lasting impacts of ACEs, we examined the gut microbiota and its functions in adulthood. We observed distinct differences between individuals with low and high ACE exposure in gut microbiota composition and the relative abundance of specific bacterial taxa. Notably, high-ACE individuals displayed an increased relative abundance of Butyricimonas spp. and Prevotella spp. while exhibiting decreased levels of Clostridiales and Lachnospira spp. These observations align with a previous study where pregnant women with a history of high ACE showed a higher abundance of Prevotella [35]. It is worth noting that an overabundance of certain Prevotella spp. has been associated with autoimmune diseases, chronic inflammatory conditions, hypertension, and heart failure [67,68,69,70], suggesting potential health implications of ACE-related microbial changes. Furthermore, we observed a negative correlation between Prevotella abundance and L-glutamate and L-glutamine biosynthesis, which were found to be decreased in the high-ACE group. Glutamine plays a vital role in maintaining intestinal tissue integrity by promoting enterocyte proliferation and inducing the expression of tight-junction proteins [71]. Depletion of glutamine stores may occur during trauma, sepsis, and inflammatory bowel disease (IBD) [71], providing a potential link between stressful events in childhood and decreased gut health later in life.
The higher prevalence of diarrhea among individuals in the high-ACE group suggests that changes in gut microbiota, particularly the increased abundance of Prevotella, may play a key role in gut health across the lifespan. Prevotella contributes to inflammation by producing lipopolysaccharide (LPS), which can trigger Toll-like receptor 4 (TLR-4), leading to low-grade inflammation and diarrhea-predominant irritable bowel syndrome (IBS) [72,73]. Increased diarrhea can also be attributed to the effects of stress on the gastrointestinal system. For instance, when the stress hormone corticotropin-releasing hormone (CRH) was injected into the hypothalamus of rats, it resulted in a significant 84% reduction in colonic transit time [74]. Consequently, this accelerated passage of stool through the colon can lead to greater excretion of water and nutrients from the body, potentially contributing to an increase in diarrhea episodes [75]. Hence, the role of diarrhea in the connections between ACEs, the gut microbiota, and its functions warrants further investigation.
We also observed increased depression among nursing students with high ACE, further highlighting the potential to explore the gut–brain axis in individuals with ACEs. Previous research has also shown a relationship between ACE and depression among nursing students [40]. A study performed by Chapman et al. (2004) observed a strong positive relationship between ACE score and the likelihood of developing chronic depressive disorder in adulthood [12]. In addition, previous research has suggested a potential relationship between the gut microbiome and depression, indicating that exploring the gut–brain axis may shed light on the mechanisms underlying the association between gut health and depressive disorders. For example, in a study examining the impact of ACE and caregiver stress on the gut microbiota in children (ages 5–7), researchers discovered that the microbial composition of the gut was significantly altered by socioeconomic risk, dysfunction in the relationship between the caregiver and the child, and dysregulation in the child’s behavioral patterns [25]. The increased level of Butyricmonas and reduced levels of Clostridiales have also been associated with stress [76,77] and depression [78]. However, contrary to previous studies, our findings did not reveal a direct relationship between the gut microbiome and depression. This could be because ACEs may also affect the overall adaptiveness of the body’s immune response and lead to increased levels of pro-inflammatory molecules [27,79]. Gut microbiota metabolites, such as neuropeptides and short-chain fatty acids (SCFAs), play a key role in the gut–brain axis by interacting with the central nervous system, activating microglia, which modulate HPA activity and, in turn, cytokine release [80]. Interestingly, multiple rodent studies have revealed that exposure to early life stress (ELS), the functional equivalent of ACE in rodents, has been linked to increased intestinal permeability, bacterial translocation across the gut epithelial barrier, and alterations in the composition of the gut microbiota [81,82,83]. These changes may elicit an immune response and affect the overall body system. Therefore, further exploration of the connections between ACEs and the gut–brain axis may provide valuable insights into the mechanisms through which stress in childhood affects health and well-being across the lifespan.
Lastly, our study explored potential moderators that might mitigate the lasting health effects of ACEs. Improving sleep quality may help combat depression among nursing students with high ACE, given the well-established link between depression and sleep disturbances [84,85]. Sleep disturbances can have wide-ranging effects on gastrointestinal, psychiatric, and neurological health [86,87,88]. Notably, sleep quality has also been found to influence the gut microbiota, with circadian clock misalignment and sleep deprivation affecting the microbial community structure in the gut [89,90]. Maltreated children, who are at an elevated risk of sleep problems, may also experience increased inflammation levels and metabolic disruptions in the gut, significantly impacting overall health [91]. Therefore, enhancing sleep quality could serve as an intervention for high–ACE individuals, potentially reducing depressive symptoms and improving overall health.
This research is the first study investigating the long-term effects of ACES on the gut microbiota and its associated functions in adult nursing students. Our comprehensive methodology allowed us to gain a holistic understanding of the impact of ACEs on the gut microbiota and its potential implications for nursing students’ well-being. The limitations of the study include the low racial and ethnic diversity of the student cohort, as 88% of the participants self-identified as White and 87% self-identified as Hispanic. Thus, additional studies with an increased number of participants and diverse demographic characteristics are needed to provide a more accurate representation of the general student population of the world. Furthermore, instances of ACEs are self-reported on the ACE questionnaire. Since everyone responds differently to chronic stress and trauma, instances of ACEs may be over- or under-reported on the ACE questionnaire. Therefore, ACE scores can only be used to demonstrate correlations or associations between childhood adversity and the gut microbiome and functional changes in adulthood. Future studies are encouraged to incorporate a longitudinal approach with the collection of comprehensive health histories, specifically of gastrointestinal disorders, to better understand the long-term impacts of ACEs. In addition, detailed data collection can serve as an extensive control for potential confounding factors in order to address the complex landscape of microbiome assessments. It is crucial to acknowledge that further research and comprehensive assessments are needed to fully understand the intricate interplay between ACE exposure, the gut microbiome, and various adverse health and mental health outcomes in adulthood.
5. Conclusions
In conclusion, this study substantiates that adult nursing students with high ACE display a notable alteration in gut microbiotal composition and functionality, with implications for intestinal health and potential associations with psychological well-being. Focusing on functional interactions within the gut microbiome and the metabolites produced provides a more comprehensive understanding of the intricate mechanisms underlying the relationship between ACE and adverse health outcomes. Such understanding can help inform potential therapeutic strategies to restore microbial balance and promote intestinal health in individuals with high ACE. Probiotic use may offer a promising approach to alleviate diarrhea symptoms associated with ACEs and improve overall gut health. Through further exploration of the gut–brain axis and potential interventions, we can pave the path for future healthcare approaches catered to individuals with high ACE, ultimately improving their well-being and quality of life. Collaboration and ongoing research are vital in fully unravelling ACE’s impact and devising effective strategies to promote optimal health for all those affected by early-life adversity.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijerph21010068/s1, Table S1: Study metadata and normalized and non-normalized feature tables.
Author Contributions
Writing—original draft preparation, N.K.; writing—review and editing, S.P., H.A.O.A., J.G.C.L., A.N. and N.K.; formal analysis, N.K., T.Z. and N.C.; visualization, N.K. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by a JPB Environmental Health Fellowship award granted to Hector A. Olvera Alvarez and Jose Guillermo Cedeño Laurent by the JPB Foundation and managed by the Harvard T.H. Chan School of Public Health; by Grant 5G12MD007592 from the National Institute on Minority Health and Health Disparities (NIMHD), a component of the National Institutes of Health (NIH), to Hector Olvera Alvarez; and by the Hoffman Program on Chemicals and Health at the Harvard T.H. Chan School of Public Health.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the review boards of the Harvard T.H. Chan School of Public Health (16-0080), UTEP (857149-1), and UBC (H21-03481).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The metadata and normalized and non-normalized feature tables are available in the supplementary data. The raw 16S rRNA sequence data are available at https://www.ncbi.nlm.nih.gov/sra/PRJNA1024544 (accessed on 7 January 2024).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Felitti, V.J.; Anda, R.F.; Nordenberg, D.; Williamson, D.F.; Spitz, A.M.; Edwards, V.; Koss, M.P.; Marks, J.S. Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults. The Adverse Childhood Experiences (ACE) Study. Am. J. Prev. Med. 1998, 14, 245–258. [Google Scholar] [CrossRef] [PubMed]
- Cronholm, P.F.; Forke, C.M.; Wade, R.; Bair-Merritt, M.H.; Davis, M.; Harkins-Schwarz, M.; Pachter, L.M.; Fein, J.A. Adverse childhood experiences: Expanding the concept of adversity. Am. J. Prev. Med. 2015, 49, 354–361. [Google Scholar] [CrossRef]
- Wade, R.; Cronholm, P.F.; Fein, J.A.; Forke, C.M.; Davis, M.B.; Harkins-Schwarz, M.; Pachter, L.M.; Bair-Merritt, M.H. Household and community-level adverse childhood experiences and adult health outcomes in a diverse urban population. Child Abus. Negl. 2016, 52, 135–145. [Google Scholar] [CrossRef] [PubMed]
- Anda, R.F.; Butchart, A.; Felitti, V.J.; Brown, D.W. Building a framework for global surveillance of the public health implications of adverse childhood experiences. Am. J. Prev. Med. 2010, 39, 93–98. [Google Scholar] [CrossRef] [PubMed]
- LaNoue, M.; Graeber, D.A.; Helitzer, D.L.; Fawcett, J. Negative affect predicts adults’ ratings of the current, but not childhood, impact of adverse childhood events. Community Ment. Health J. 2013, 49, 560–566. [Google Scholar] [CrossRef] [PubMed]
- Dong, M.; Anda, R.F.; Dube, S.R.; Giles, W.H.; Felitti, V.J. The relationship of exposure to childhood sexual abuse to other forms of abuse, neglect, and household dysfunction during childhood. Child Abuse Negl. 2003, 27, 625–639. [Google Scholar] [CrossRef] [PubMed]
- LaNoue, M.; Graeber, D.; de Hernandez, B.U.; Warner, T.D.; Helitzer, D.L. Direct and indirect effects of childhood adversity on adult depression. Community Ment. Health J. 2012, 48, 187–192. [Google Scholar] [CrossRef]
- Dong, M.; Giles, W.H.; Felitti, V.J.; Dube, S.R.; Williams, J.E.; Chapman, D.P.; Anda, R.F. Insights into causal pathways for ischemic heart disease: Adverse childhood experiences study. Circulation 2004, 110, 1761–1766. [Google Scholar] [CrossRef]
- Brown, D.W.; Anda, R.F.; Felitti, V.J.; Edwards, V.J.; Malarcher, A.M.; Croft, J.B.; Giles, W.H. Adverse childhood experiences are associated with the risk of lung cancer: A prospective cohort study. BMC Public Health 2010, 10, 311. [Google Scholar] [CrossRef]
- van der Meer, L.; van Duijn, E.; Wolterbeek, R.; Tibben, A. Adverse childhood experiences of persons at risk for huntington’s disease or BRCA1/2 hereditary breast/ovarian cancer. Clin. Genet. 2012, 81, 18–23. [Google Scholar] [CrossRef]
- Jewkes, R.K.; Dunkle, K.; Nduna, M.; Jama, P.; Puren, A. Associations between childhood adversity and depression, substance abuse and HIV and HSV2 incident infections in rural South African youth. Child Abuse Negl. 2010, 34, 833–841. [Google Scholar] [CrossRef] [PubMed]
- Chapman, D.P.; Whitfield, C.L.; Felitti, V.J.; Dube, S.R.; Edwards, V.J.; Anda, R.F. Adverse childhood experiences and the risk of depressive disorders in adulthood. J. Affect. Disord. 2004, 82, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Dube, S.R.; Fairweather, D.; Pearson, W.S.; Felitti, V.J.; Anda, R.F.; Croft, J.B. Cumulative childhood stress and autoimmune diseases in adults. Psychosom. Med. 2009, 71, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Hillis, S.D.; Anda, R.F.; Felitti, V.J.; Nordenberg, D.; Marchbanks, P.A. Adverse childhood experiences and sexually transmitted diseases in men and women: A retrospective study. Virus Res. 2000, 106, e11. [Google Scholar] [CrossRef] [PubMed]
- Brown, D.W.; Anda, R.F. Adverse childhood experiences: Origins of behaviors that sustain the HIV epidemic. AIDS 2009, 23, 2231–2233. [Google Scholar] [CrossRef]
- McLaughlin, K.A.; Greif Green, J.; Gruber, M.J.; Sampson, N.A.; Zaslavsky, A.M.; Kessler, R.C. Childhood adversities and first onset of psychiatric disorders in a national sample of US adolescents. Arch. Gen. Psychiatry 2012, 69, 1151–1160. [Google Scholar] [CrossRef]
- Hatch, V.; Swerbenski, H.; Gray, S.A.O. Family social support buffers the intergenerational association of maternal adverse childhood experiences and preschoolers’ externalizing behavior. Am. J. Orthopsychiatry 2020, 90, 489–501. [Google Scholar] [CrossRef]
- Gellis, L.A.; Lichstein, K.L.; Scarinci, I.C.; Durrence, H.H.; Taylor, D.J.; Bush, A.J.; Riedel, B.W. Socioeconomic status and insomnia. J. Abnorm. Psychol. 2005, 114, 111–118. [Google Scholar] [CrossRef]
- Janssen, I.; Boyce, W.F.; Simpson, K.; Pickett, W. Influence of individual- and area-level measures of socioeconomic status on obesity, unhealthy eating, and physical inactivity in Canadian adolescents. Am. J. Clin. Nutr. 2006, 83, 139–145. [Google Scholar] [CrossRef]
- RodrÍguez, J.M.; Murphy, K.; Stanton, C.; Ross, R.P.; Kober, O.I.; Juge, N.; Avershina, E.; Rudi, K.; Narbad, A.; Jenmalm, M.C.; et al. The composition of the gut microbiota throughout life, with an emphasis on early life. Microb. Ecol. Health Dis. 2015, 26, 26050. [Google Scholar] [CrossRef]
- Cowan, C.S.M.; Dinan, T.G.; Cryan, J.F. Annual Research Review: Critical windows—The microbiota-gut-brain axis in neurocognitive development. J. Child Psychol. Psychiatry 2020, 61, 353–371. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Nakayama, J. Development of the gut microbiota in infancy and its impact on health in later life. Allergol. Int. 2017, 66, 515–522. [Google Scholar] [CrossRef] [PubMed]
- Jašarević, E.; Howard, C.D.; Misic, A.M.; Beiting, D.P.; Bale, T.L. Stress during pregnancy alters temporal and spatial dynamics of the maternal and offspring microbiome in a sex-specific manner. Sci. Rep. 2017, 7, 44182. [Google Scholar] [CrossRef] [PubMed]
- Jašarević, E.; Howerton, C.L.; Howard, C.D.; Bale, T.L. Alterations in the vaginal microbiome by maternal stress are associated with metabolic reprogramming of the offspring gut and brain. Endocrinology 2015, 156, 3265–3276. [Google Scholar] [CrossRef] [PubMed]
- Flannery, J.E.; Stagaman, K.; Burns, A.R.; Hickey, R.J.; Roos, L.E.; Giuliano, R.J.; Fisher, P.A.; Sharpton, T.J. Gut feelings begin in childhood: The gut metagenome correlates with early environment, caregiving, and behavior. mBio 2020, 11, e02780-19. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, L.L.; Gawuga, C.E.; Tyrka, A.R.; Lee, J.K.; Anderson, G.M.; Price, L.H. Association between plasma IL-6 response to acute stress and early-life adversity in healthy adults. Neuropsychopharmacology 2010, 35, 2617–2623. [Google Scholar] [CrossRef]
- Danese, A.; Pariante, C.M.; Caspi, A.; Taylor, A.; Poulton, R. Childhood maltreatment predicts adult inflammation in a life-course study. Proc. Natl. Acad. Sci. USA 2007, 104, 1319–1324. [Google Scholar] [CrossRef]
- Fagundes, C.P.; Glaser, R.; Kiecolt-Glaser, J.K. Stressful early life experiences and immune dysregulation across the lifespan. Brain Behav. Immun. 2013, 27, 8–12. [Google Scholar] [CrossRef]
- Ehrlich, K.B.; Ross, K.M.; Chen, E.; Miller, G.E. Testing the biological embedding hypothesis: Is early life adversity associated with a later proinflammatory phenotype? Dev. Psychopathol. 2016, 28 Pt 2, 1273–1283. [Google Scholar] [CrossRef]
- Lovallo, W.R.; Farag, N.H.; Sorocco, K.H.; Cohoon, A.J.; Vincent, A.S. Lifetime adversity leads to blunted stress axis reactivity: Studies from the oklahoma family health patterns project. Biol. Psychiatry 2012, 71, 344–349. [Google Scholar] [CrossRef]
- Mayer, E.A.; Savidge, T.; Shulman, R.J. Brain-gut microbiome interactions and functional bowel disorders. Gastroenterology 2014, 146, 1500–1512. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.; Wei, Y.; Hashimoto, K. Brain–gut–microbiota axis in depression: A historical overview and future directions. Brain Res. Bull. 2022, 182, 44–56. [Google Scholar] [CrossRef]
- Zheng, D.; Liwinski, T.; Elinav, E. Interaction between microbiota and immunity in health and disease. Cell Res. 2020, 30, 492–506. [Google Scholar] [CrossRef] [PubMed]
- Nusslock, R.; Miller, G.E. Early-life adversity and physical and emotional health across the lifespan: A neuroimmune network hypothesis. Biol. Psychiatry 2016, 80, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Hantsoo, L.; Jašarević, E.; Criniti, S.; Mcgeehan, B.; Tanes, C.; Sammel, M.D.; Elovitz, M.; Compher, C.; Wu, G.; Epperson, C.N. Childhood adversity impact on gut microbiota and inflammatory response to stress during pregnancy. Brain Behav. Immun. 2019, 75, 240–250. [Google Scholar] [CrossRef] [PubMed]
- Huerta-Franco, M.R.; Vargas-Luna, M.; Tienda, P.; Delgadillo-Holtfort, I.; Balleza-Ordaz, M.; Flores-Hernandez, C. Effects of occupational stress on the gastrointestinal tract. World J. Gastrointest. Pathophysiol. 2013, 4, 108–118. [Google Scholar] [CrossRef] [PubMed]
- Lavoie-Tremblay, M.; Sanzone, L.; Aubé, T.; Paquet, M. Sources of stress and coping strategies among undergraduate nursing students across all years. Can. J. Nurs. Res. 2021, 54, 261–271. [Google Scholar] [CrossRef]
- Jimenez, C.; Navia-Osorio, M.P.; Diaz, C.V. Stress and health in novice and experienced nursing students. J. Adv. Nurs. 2010, 66, 442–455. [Google Scholar] [CrossRef]
- Rella, S.; Winwood, P.C.; Lushingtoon, K. When does nursing burnout begin? An investigation of the fatigue experience of australian nursing students. J. Nurs. Manag. 2009, 17, 886–897. [Google Scholar] [CrossRef]
- McKee-Lopez, G.; Robbins, L.; Provencio-Vasquez, E.; Olvera, H. The relationship of childhood adversity on burnout and depression among BSN students. J. Prof. Nurs. 2019, 35, 112–119. [Google Scholar] [CrossRef]
- Limbana, T.; Khan, F.; Eskander, N. Gut microbiome and depression: How microbes affect the way we think. Curēus 2020, 12, e9966. [Google Scholar] [CrossRef] [PubMed]
- Chevalier, G.; Siopi, E.; Guenin-Macé, L.; Pascal, M.; Laval, T.; Rifflet, A.; Boneca, I.G.; Demangel, C.; Colsch, B.; Pruvost, A.; et al. Effect of gut microbiota on depressive-like behaviors in mice is mediated by the endocannabinoid system. Nat. Commun. 2020, 11, 6363. [Google Scholar] [CrossRef] [PubMed]
- Cheung, S.G.; Goldenthal, A.R.; Uhlemann, A.; Mann, J.J.; Miller, J.M.; Sublette, M.E. Systematic review of gut microbiota and major depression. Front. Psychiatry 2019, 10, 34. [Google Scholar] [CrossRef] [PubMed]
- Petrak, F.; Herpertz, S.; Hirsch, J.; Röhrig, B.; Donati-Hirsch, I.; Juckel, G.; Meier, J.J.; Gatermann, S. Gut microbiota differs in composition between adults with type 1 diabetes with or without depression and healthy control participants: A case-control study. BMC Microbiol. 2022, 22, 169. [Google Scholar] [CrossRef]
- Gibbons, C. Stress, coping and burn-out in nursing students. Int. J. Nurs. Stud. 2010, 47, 1299–1309. [Google Scholar] [CrossRef] [PubMed]
- Hwang, E.; Kim, J. Factors affecting academic burnout of nursing students according to clinical practice experience. BMC Med. Educ. 2022, 22, 346. [Google Scholar] [CrossRef] [PubMed]
- Njim, T.; Mbanga, C.; Mouemba, D.; Makebe, H.; Toukam, L.; Kika, B.; Mulango, I. Determinants of depression among nursing students in cameroon: A cross-sectional analysis. BMC Nurs. 2020, 19, 26. [Google Scholar] [CrossRef] [PubMed]
- Olvera, H.A.; Provencio-Vasquez, E.; Slavich, G.M.; Laurent, J.G.C.; Browning, M.; McKee-Lopez, G.; Robbins, L.; Spengler, J.D. Stress and health in nursing students: The nurse engagement and wellness study. Nurs. Res. 2019, 68, 453–463. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Lauber, C.L.; Walters, W.A.; Berg-Lyons, D.; Lozupone, C.A.; Turnbaugh, P.J.; Fierer, N.; Knight, R. Global patterns of 16S rRNA diversity at a depth of millions of sequences per sample. Proc. Natl. Acad. Sci. USA 2011, 108, 4516–4522. [Google Scholar] [CrossRef]
- Kozich, J.J.; Westcott, S.L.; Baxter, N.T.; Highlander, S.K.; Schloss, P.D. Development of a dual-index sequencing strategy and curation pipeline for analyzing amplicon sequence data on the MiSeq illumina sequencing platform. Appl. Environ. Microbiol. 2013, 79, 5112–5120. [Google Scholar] [CrossRef]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef] [PubMed]
- Callahan, B.J.; Mcmurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef] [PubMed]
- Bokulich, N.A.; Kaehler, B.D.; Rideout, J.R.; Dillon, M.; Bolyen, E.; Knight, R.; Huttley, G.A.; Gregory Caporaso, J. Optimizing taxonomic classification of marker-gene amplicon sequences with QIIME 2′s q2-feature-classifier plugin. Microbiome 2018, 6, 90. [Google Scholar] [CrossRef] [PubMed]
- Douglas, G.M.; Maffei, V.J.; Zaneveld, J.R.; Yurgel, S.N.; Brown, J.R.; Taylor, C.M.; Huttenhower, C.; Langille, M.G.I. PICRUSt2 for prediction of metagenome functions. Nat. Biotechnol. 2020, 38, 685–688. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. Adverse Childhood Experiences (ACEs). 2021. Available online: https://www.cdc.gov/violenceprevention/aces/index.html (accessed on 1 September 2023).
- Oksanen, J.; Simpson, G.L.; Blanchet, F.G.; Kindt, R.; Legendre, P.; Minchin, P.R.; O’Hara, R.B.; Solymos, P.; Stevens, M.H.H.; Szoecs, E.; et al. Vegan: Community Ecology Package. 2020. Available online: https://cran.r-project.org/web/packages/vegan/index.html (accessed on 1 September 2023).
- Wickham, H.; Chang, W.; Henry, L.; Pedersen, T.; Takahashi, K.; Wilke, C.; Woo, K.; Yutani, H.; Dunnington, D. Ggplot2: Create Elegant Data Visualizations Using the Grammar of Graphics. 2020. Available online: https://cran.r-project.org/web/packages/ggplot2/index.html (accessed on 1 September 2023).
- Deng, C.; Daley, T.; Smith, A. preseqR: Predicting Species Accumulation Curves. 2018. Available online: https://CRAN.R-project.org/package=preseqR (accessed on 1 September 2023).
- Wickham, H. Reshape2: Flexibly Reshape Data: A Reboot of the Reshape Package. 2020. Available online: https://cran.r-project.org/web/packages/reshape2/index.html (accessed on 1 September 2023).
- Cutler, D.R.; Edwards, T.C.J.; Beard, K.H.; Cutler, A.; Hess, K.T.; Gibson, J.; Lawler, J.J. Random forests for classification in ecology. Ecology 2007, 88, 2783–2792. [Google Scholar] [CrossRef] [PubMed]
- Pedregosa, F.; Varoquaux, G.; Gramfort, A.; Michel, V.; Thirion, B.; Grisel, O.; Blondel, M.; Prettenhofer, P.; Weiss, R.; Dubourg, V.; et al. Scikit-learn: Machine learning in Python. J. Mach. Learn. Res. 2011, 12, 2825–2830. [Google Scholar]
- Cao, L.J.; Chua, K.S.; Chong, W.K.; Lee, H.P.; Gu, Q.M. A comparison of PCA, KPCA and ICA for dimensionality reduction in support vector machine. Neurocomputing 2003, 55, 321–336. [Google Scholar] [CrossRef]
- Dietterich, T.G. Approximate statistical tests for comparing supervised classification learning algorithms. Neural Comput. 1998, 10, 1895–1923. [Google Scholar] [CrossRef]
- Louppe, G.; Wehenkel, L.; Sutera, A.; Geurts, P. Understanding variable importances in forests of randomized trees. Neural Inf. Process. Syst. 2013, 2013, 431–439. [Google Scholar]
- Segata, N.; Izard, J.; Waldron, L.; Gevers, D.; Miropolsky, L.; Garrett, W.S.; Huttenhower, C. Metagenomic biomarker discovery and explanation. Genome Biol. 2011, 12, R60. [Google Scholar] [CrossRef]
- Batista, G.E.; Monard, M.C. A study of k-nearest neighbour as an imputation method. Soft Comput. Syst. Des. Manag. Appl. 2002, 48, 251–260. [Google Scholar]
- Iljazovic, A.; Roy, U.; Gálvez, E.J.C.; Lesker, T.R.; Zhao, B.; Gronow, A.; Amend, L.; Will, S.E.; Hofmann, J.D.; Pils, M.C.; et al. Perturbation of the gut microbiome by Prevotella spp. enhances host susceptibility to mucosal inflammation. Mucosal Immunol. 2021, 14, 113–124. [Google Scholar] [CrossRef] [PubMed]
- Larsen, J.M. The immune response to Prevotella bacteria in chronic inflammatory disease. Immunology 2017, 151, 363–374. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhao, F.; Wang, Y.; Chen, J.; Tao, J.; Tian, G.; Wu, S.; Liu, W.; Cui, Q.; Geng, B.; et al. Gut microbiota dysbiosis contributes to the development of hypertension. Microbiome 2017, 5, 14. [Google Scholar] [CrossRef] [PubMed]
- Kummen, M.; Mayerhofer, C.C.K.; Vestad, B.; Broch, K.; Awoyemi, A.; Storm-Larsen, C.; Ueland, T.; Yndestad, A.; Hov, J.R.; Trøseid, M. Gut microbiota signature in heart failure defined from profiling of 2 independent cohorts. J. Am. Coll. Cardiol. 2018, 71, 1184–1186. [Google Scholar] [CrossRef]
- Kim, M.; Kim, H. The roles of glutamine in the intestine and its implication in intestinal diseases. Int. J. Mol. Sci. 2017, 18, 1051. [Google Scholar] [CrossRef]
- Graham, C.; Mullen, A.; Whelan, K. Obesity and the gastrointestinal microbiota: A review of associations and mechanisms. Nutr. Rev. 2015, 73, 376–385. [Google Scholar] [CrossRef]
- Wu, K.; Sun, W.; Li, N.; Chen, Y.; Wei, Y.; Chen, D. Small intestinal bacterial overgrowth is associated with diarrhea-predominant irritable bowel syndrome by increasing mainly Prevotella abundance. Scand. J. Gastroenterol. 2019, 54, 1419–1425. [Google Scholar] [CrossRef]
- Mönnikes, H.; Schmidt, B.G.; Taché, Y. Psychological stress-induced accelerated colonic transit in rats involves hypothalamic corticotropin-releasing factor. Gastroenterology 1993, 104, 716. [Google Scholar] [CrossRef]
- Osadchiy, V.; Martin, C.R.; Mayer, E.A. The Gut–Brain axis and the microbiome: Mechanisms and clinical implications. Clin. Gastroenterol. Hepatol. 2019, 17, 322–332. [Google Scholar] [CrossRef]
- Toh, T.S.; Wei Jie Lee, J.; Toh, K.Y.; Ho, J.P.; Fung Yen Lim, J.; Tan, A.H.; Chong, C.W. IDDF2021-ABS-0165 Psychological well-being and sleep quality among healthy stool donors in Singapore: A cross-sectional study. Gut 2021, 70, A50–A51. [Google Scholar]
- Qu, Y.; Yang, C.; Ren, Q.; Ma, M.; Dong, C.; Hashimoto, K. Comparison of (R)-ketamine and lanicemine on depression-like phenotype and abnormal composition of gut microbiota in a social defeat stress model. Sci. Rep. 2017, 7, 15725. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Qu, Y.; Fujita, Y.; Ren, Q.; Ma, M.; Dong, C.; Hashimoto, K. Possible role of the gut microbiota-brain axis in the antidepressant effects of (R)-ketamine in a social defeat stress model. Transl. Psychiatry 2017, 7, 1294. [Google Scholar] [CrossRef] [PubMed]
- Hartwell, K.J.; Moran-Santa Maria, M.M.; Twal, W.O.; Shaftman, S.; Desantis, S.M.; Mcrae-Clark, A.L.; Brady, K.T. Association of elevated cytokines with childhood adversity in a sample of healthy adults. J. Psychiatr. Res. 2013, 47, 604–610. [Google Scholar] [CrossRef] [PubMed]
- Rea, K.; Dinan, T.G.; Cryan, J.F. The microbiome: A key regulator of stress and neuroinflammation. Neurobiol. Stress 2016, 4, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Moussaoui, N.; Larauche, M.; Biraud, M.; Molet, J.; Million, M.; Mayer, E.; Taché, Y. Limited nesting stress alters maternal behavior and in vivo intestinal permeability in male wistar pup rats. PLoS ONE 2016, 11, e0155037. [Google Scholar] [CrossRef]
- De Palma, G.; Blennerhassett, P.; Lu, J.; Deng, Y.; Park, A.J.; Green, W.; Denou, E.; Silva, M.A.; Santacruz, A.; Sanz, Y.; et al. Microbiota and host determinants of behavioural phenotype in maternally separated mice. Nat. Commun. 2015, 6, 7735. [Google Scholar] [CrossRef]
- O’Mahony, S.M.; Marchesi, J.R.; Scully, P.; Codling, C.; Ceolho, A.-M.; Quigley, E.M.M.; Cryan, J.F.; Dinan, T.G. Early life stress alters behavior, immunity, and microbiota in rats: Implications for irritable bowel syndrome and psychiatric illnesses. Biol. Psychiatry 2009, 65, 263–267. [Google Scholar] [CrossRef]
- Agargun, M.Y.; Kara, H.; Solmaz, M. Sleep disturbances and suicidal behavior in patients with major depression. J. Clin. Psychiatry 1997, 58, 249–251. [Google Scholar]
- Paterson, L.M.; Nutt, D.J.; Wilson, S.J. NAPSAQ-1: National patient sleep assessment questionnaire in depression. Int. J. Psychiatry Clin. Pract. 2009, 13, 48–58. [Google Scholar] [CrossRef]
- Khanijow, V.; Prakash, P.; Emsellem, H.A.; Borum, M.L.; Doman, D.B. Sleep dysfunction and gastrointestinal diseases. Gastroenterol. Hepatol. 2015, 11, 817–825. [Google Scholar]
- Graham, C.D.; Kyle, S.D. A preliminary investigation of sleep quality in functional neurological disorders: Poor sleep appears common, and is associated with functional impairment. J. Neurol. Sci. 2017, 378, 63–166. [Google Scholar] [CrossRef] [PubMed]
- Hyun, M.K.; Baek, Y.; Lee, S. Association between digestive symptoms and sleep disturbance: A cross-sectional community-based study. BMC Gastroenterol. 2019, 19, 34. [Google Scholar] [CrossRef] [PubMed]
- Thaiss, C.A.; Levy, M.; Korem, T.; Dohnalová, L.; Shapiro, H.; Jaitin, D.A.; David, E.; Winter, D.R.; Gury-BenAri, M.; Tatirovsky, E.; et al. Microbiota diurnal rhythmicity programs host transcriptome oscillations. Cell 2016, 167, 1495–1510.e12. [Google Scholar] [CrossRef]
- Davies, S.; Ang, J.; Revell, V.; Holmes, B.; Mann, A.; Robertson, F.; Cui, N.; Middleton, B.; Ackermann, K.; Kayser, M.; et al. Effect of sleep deprivation on the human metabolome. Proc. Natl. Acad. Sci. USA 2014, 111, 10761–10766. [Google Scholar] [CrossRef]
- Baldwin, J.R.; Danese, A. Pathways from childhood maltreatment to cardiometabolic disease: A research review. Adopt. Foster. 2019, 43, 329–339. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).