The Eastern Caribbean Health Outcomes Research Network (ECHORN) Cohort Study: Design, Methods, and Baseline Characteristics
Abstract
:1. Introduction
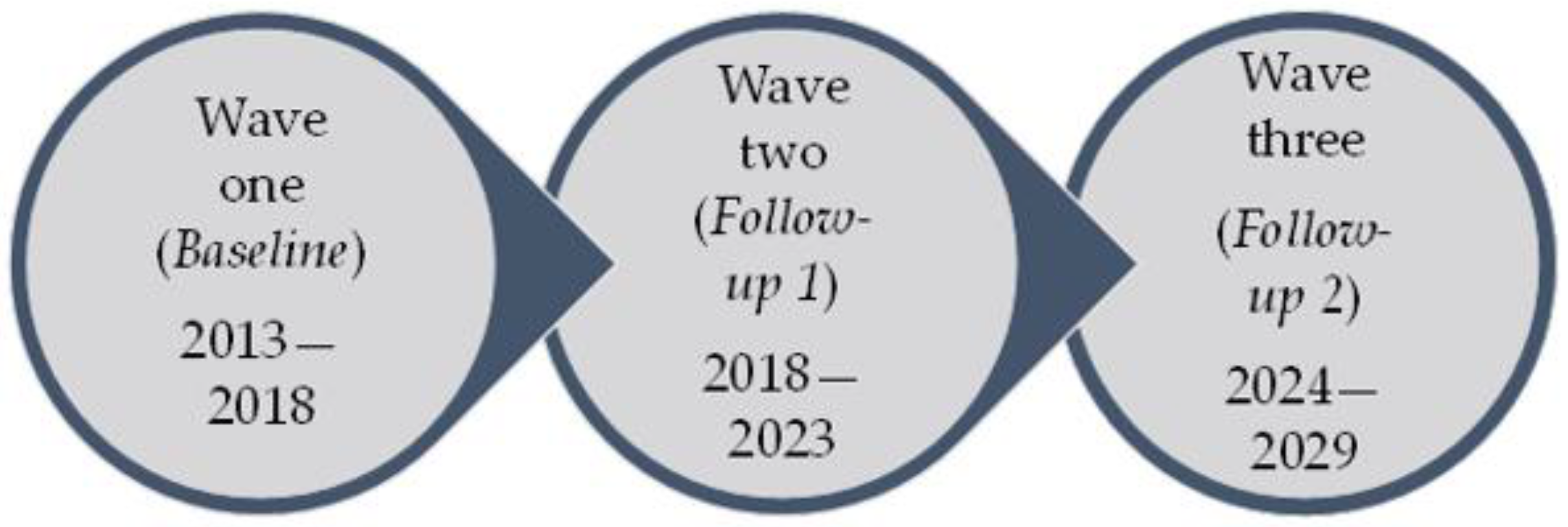
2. The ECHORN Cohort Study (ECS)
3. A Collaborative Approach
4. Methods
4.1. Sampling Strategy
4.2. Eligibility Criteria
Data Elements
4.3. Clinical Exam and Laboratory Tests
4.4. Self-Reported Health Survey
5. Data Quality Assurance Procedures
6. Results
6.1. Description of the ECS Wave One Sample
6.2. The Biobank
7. Discussion
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. Detailed Descriptions of Clinical Observations Included in Wave One of the ECHORN Cohort Study (ECS)
| Clinical Observation | Equipment Used | Measure | Data Available | Definition |
|---|---|---|---|---|
| Medical history | Paper forms (later uploaded to a database) | - | 2961 | A record of the diseases, health conditions, and deaths in the participant’s nuclear and extended family |
| Medication use and adherence | Paper forms (later uploaded to a database) | - | 2961 | Medication use: A record of the medications the participant is currently taking (prescribed and non-prescribed), reason for use, dosage, timing, and frequency Medication adherence: the extent to which the participant conforms to the provider recommendation for a prescription (timing, dosage, and frequency) |
| Weight | Tanita BF-689 digital scale | Kilometers | 2923 | A measure of relative mass |
| Height | Seca 213 mobile Stadiometer | Centimeters | 2923 | A measure of how tall the participant is, taken from a standing position |
| Waist, hip, and neck circumference | Seca 201 tape measure | Centimeters | 2936, 2935, and 1723, respectively | A measure of visceral fat |
| Grip strength | Jama hand dynamometer | Kilograms | 2930 | A measure of the maximum isometric strength of the hand and forearm muscles |
| Resting blood pressure | Watch BP office ABI device Blood pressure cuffs (various sizes) | Millimeters of mercury | 2932 | A measure of the force of blood on the artery walls |
| Ankle brachial index | Watch BP office ABI device Blood pressure cuffs for upper arm and ankle (various sizes) | Ratio | 2898 | A measure of the ankle (posterior tibial artery) systolic pressure divided by the brachial artery systolic pressure |
| Lipid panel | Point of care machine or reference laboratory | - | 2345 | Measures of cholesterol levels and a measure of triglycerides Participants were not required to fast prior to participating |
| Complete blood count | Point of care machine or reference laboratory | - | 1434 | Measures of red and white cells, hemoglobin, hematocrit, and platelets in the blood |
| Hemoglobin A1C | Point of care machine or reference laboratory | - | 2350 | Measure of blood sugar levels Participants were not required to fast prior to participating |
| Metabolic panel | Point of care machine or reference laboratory | - | 1890 | A measure of several substances in the blood, including glucose, calcium, blood urea nitrogen, creatinine, sodium, potassium, and albumin |
Appendix B. Detailed Descriptions of the Sampling Methodology for the Island Sites Included in Wave One of the ECHORN Cohort Study (ECS)
Appendix B.1. Barbados
Appendix B.1.1. Sampling Frame and Methodology: Barbados
Appendix B.1.2. Sample Size/Sampling Fraction
Appendix B.1.3. Enumeration Districts (Primary Sampling Units)
Appendix B.1.4. Sample Stratification
| Strata | Stratum One | Stratum Two | Stratum Three | Stratum Four | Total |
| Parishes in Stratum | St. Michael | Christ Church St. Philip | St. James St. George St. Thomas | St. Lucy St. Peter St. Andrew St. Joseph St. John | |
| Total possible EDs | 181 (31%) | 176 (30%) | 130 (22%) | 96 (17%) | 583 * |
| Number of Sampled EDs (45 EDs) | 14 | 14 | 10 | 8 | 45 |
| Number of Sampled EDs (35 EDs) | 11 | 11 | 8 | 6 | 35 |
| Number of Sampled EDs (25 EDs) | 8 | 8 | 6 | 4 | 25 |
| * The total will be 538 EDs (583–45 being used by the Barbados Statistical service and the Health of the Nation Study). | |||||
Appendix B.1.5. Non-Response Rate
Appendix B.1.6. Sampling Design
Appendix B.2. US Virgin Islands
Appendix B.2.1. Sampling Frame and Methodology: US Virgin Islands
Appendix B.2.2. Sample Size
Appendix B.2.3. Sampling Design
Appendix B.3. Puerto Rico
Appendix B.3.1. Sampling Frame and Methodology: Puerto Rico
Appendix B.3.2. Sample Size
Appendix B.3.3. Enumeration Districts (Primary Sampling Units)
Appendix B.3.4. Non-Response Rate
Appendix B.3.5. Sampling Design
Appendix B.4. Trinidad
Appendix B.4.1. Sampling Frame and Methodology: Trinidad
Appendix B.4.2. Sample Size/Sampling Fraction
Appendix B.4.3. Enumeration Districts (Primary Sampling Units)
- The ethnic diversity of Trinidad with a mix of urban and semi-rural; upper-, mid-, and low-income households; and East-Indian, African, and Mixed-race heritage
- A densely populous area with over 31,000 households
- Approximately, 155 enumeration districts (EDs), and each ED has between 150 and 300 plus households
- A well-developed infrastructure of roads, public transportation, water supply, and telecommunication
- Mature communities with relatively little new construction.
Appendix B.4.4. Non-Response Rate
Appendix B.4.5. Sampling Design
References
- World Health Organization. Noncommunicable Diseases. Available online: https://www.who.int/news-room/fact-sheets/detail/noncommunicable-diseases (accessed on 7 February 2022).
- Luciani, S.; Agurto, I.; Caixeta, R.; Hennis, A. Prioritizing noncommunicable diseases in the Americas region in the era of COVID-19. Rev. Panam. Salud Publica 2022, 46, e83. [Google Scholar] [CrossRef] [PubMed]
- Razzaghi, H.; Martin, D.N.; Quesnel-Crooks, S.; Hong, Y.; Gregg, E.; Andall-Brereton, G.; Gawryszweski, V.; Saraiya, M. 10-year trends in noncommunicable disease mortality in the Caribbean region. Rev. Panam. Salud Publica 2019, 43, e37. [Google Scholar] [CrossRef] [PubMed]
- Yisahak, S.F.; Beagley, J.; Hambleton, I.R.; Narayan, K.V.; on behalf of the IDF Diabetes Atlas. Diabetes in North America and The Caribbean: An update. Diabetes Res. Clin. Pract. 2014, 103, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Pérez, C.; Ailshire, J.A. Aging in Puerto Rico: A Comparison of Health Status Among Island Puerto Rican and Mainland U.S. Older Adults. J. Aging Health 2017, 29, 1056–1078. [Google Scholar] [CrossRef] [PubMed]
- Pabon-Nau, L.P.; Cohen, A.; Meigs, J.B.; Grant, R.W. Hypertension and Diabetes Prevalence Among U.S. Hispanics by Country of Origin: The National Health Interview Survey 2000–2005. J. Gen. Intern. Med. 2010, 25, 847–852. [Google Scholar] [CrossRef] [PubMed]
- Layne, T.M.; Aminawung, J.A.; Soulos, P.R.; Nunez-Smith, M.; Nunez, M.A.; Jones, B.A.; Wang, K.H.; Gross, C.P. Quality of Breast Cancer Care In The US Territories: Insights From Medicare. Health Aff. 2018, 37, 421–428. [Google Scholar] [CrossRef] [PubMed]
- U.S. Census Bureau. National Caribbean-American Heritage Month: June 2023. Census.gov. Available online: https://www.census.gov/newsroom/stories/caribbean-american-heritage-month.html (accessed on 6 June 2023).
- NCD Countdown 2030 Collaborators. NCD Countdown 2030: Worldwide trends in non-communicable disease mortality and progress towards Sustainable Development Goal target 3.4. Lancet 2018, 392, 1072–1088. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, F.; Cheng, X.; Zhou, W.; He, J.; Xiao, S. Increased Mortality Trends in Patients With Chronic Non-communicable Diseases and Comorbid Hypertension in the United States, 2000–2019. Front. Public Health 2022, 10, 753861. [Google Scholar] [CrossRef] [PubMed]
- Tamir, C. Key Findings about Black Immigrants in the U.S. Pew Research Center. Available online: https://www.pewresearch.org/short-reads/2022/01/27/key-findings-about-black-immigrants-in-the-u-s/ (accessed on 6 June 2023).
- Di Cesare, M.; Khang, Y.-H.; Asaria, P.; Blakely, T.; Cowan, M.J.; Farzadfar, F.; Guerrero, R.; Ikeda, N.; Kyobutungi, C.; Msyamboza, K.P.; et al. Inequalities in non-communicable diseases and effective responses. Lancet 2013, 381, 585–597. [Google Scholar] [CrossRef] [PubMed]
- Hospedales, C.J.; Samuels, T.A.; Cummings, R.; Gollop, G.; Greene, E. Raising the priority of chronic noncommunicable diseases in the Caribbean. Rev. Panam. Salud Publica 2011, 30, 393–400. [Google Scholar] [PubMed]
- 2011 High Level Meeting on the Prevention and Control of Non-Communicable Diseases. United Nations. Available online: https://www.un.org/en/ga/ncdmeeting2011/ (accessed on 9 November 2022).
- Global Noncommunicable Disease Programs|Division of Global Health Protection Global Health|CDC. 2022. Available online: https://www.cdc.gov/globalhealth/healthprotection/ncd/about.html (accessed on 8 June 2023).
- Global Noncommunicable Diseases|Division of Global Health Protection|Global Health|CDC. 2022. Available online: https://www.cdc.gov/globalhealth/healthprotection/ncd/index.html (accessed on 8 June 2023).
- Chronic, Noncommunicable Diseases and Disorders Research Training (D43 D71)—Fogarty International Center @ NIH. Fogarty International Center. Available online: https://www.fic.nih.gov:443/Programs/Pages/chronic-lifespan.aspx (accessed on 8 June 2023).
- fundsforNGOs. NIH Offers Grants to Implement Research on Noncommunicable Disease. fundsforNGOs. 2022. Available online: https://www2.fundsforngos.org/latest-funds-for-ngos/nih-offers-grants-to-implement-research-on-noncommunicable-disease/ (accessed on 8 June 2023).
- Targeting Non-Communicable Diseases and Injuries in Mongolia. Millennium Challenge Corporation. Available online: https://www.mcc.gov/resources/doc/evalbrief-0701114-mng-health (accessed on 8 June 2023).
- World Health Organization. Global Action Plan for the Prevention and Control of Noncommunicable Diseases 2013–2020; World Health Organization: Geneva, Switzerland, 2013; Available online: https://apps.who.int/iris/handle/10665/94384 (accessed on 6 June 2023).
- Sempos, C.T.; Bild, D.E.; Manolio, T.A. Overview of the Jackson Heart Study: A Study of Cardiovascular Diseases in African American Men and Women. Am. J. Med. Sci. 1999, 317, 142–146. [Google Scholar] [CrossRef] [PubMed]
- Miller, G.J.; A Beckles, G.L.; Maude, G.H.; Carson, D.C.; Alexis, S.D.; Price, S.G.L.; A Byam, N.T. Ethnicity and Other Characteristics Predictive of Coronary Heart Disease in a Developing Community: Principal Results of the St James Survey, Trinidad. Leuk. Res. 1989, 18, 808–817. [Google Scholar] [CrossRef] [PubMed]
- Daviglus, M.L.; Pirzada, A.; Talavera, G.A. Cardiovascular Disease Risk Factors in the Hispanic/Latino Population: Lessons From the Hispanic Community Health Study/Study of Latinos (HCHS/SOL). Prog. Cardiovasc. Dis. 2014, 57, 230–236. [Google Scholar] [CrossRef] [PubMed]
- Smeeton, N.C.; Corbin, D.O.C.; Hennis, A.J.M.; Hambleton, I.R.; Rose, A.M.C.; Fraser, H.S.; Heuschmann, P.U.; Wolfe, C.D.A. A Comparison of Outcome for Stroke Patients in Barbados and South London. Int. J. Stroke 2010, 6, 112–117. [Google Scholar] [CrossRef] [PubMed]
- Tucker, K.L.; Mattei, J.; Noel, S.E.; Collado, B.M.; Mendez, J.; Nelson, J.; Griffith, J.; Ordovas, J.M.; Falcon, L.M. The Boston Puerto Rican Health Study, a longitudinal cohort study on health disparities in Puerto Rican adults: Challenges and opportunities. BMC Public Health 2010, 10, 107. [Google Scholar] [CrossRef] [PubMed]
- Lorenzi, J.; Batalova, J. Caribbean Immigrants in the United States. migrationpolicy.org. 2022. Available online: https://www.migrationpolicy.org/article/caribbean-immigrants-united-states (accessed on 9 November 2022).
- Tripathy, J.P. Research priorities in non-communicable diseases in developing countries: Time to go beyond prevalence studies. Public Health Action 2018, 8, 98–99. [Google Scholar] [CrossRef] [PubMed]
- United States Virgin Islands Population 2023 (Live). Available online: https://worldpopulationreview.com/countries/united-states-virgin-islands-population (accessed on 13 January 2022).
- Barbados Population 2023 (Live). Available online: https://worldpopulationreview.com/countries/barbados-population (accessed on 13 January 2022).
- Trinidad and Tobago Population 2023 (Live). Available online: https://worldpopulationreview.com/countries/trinidad-and-tobago-population (accessed on 13 January 2022).
- Puerto Rico Population 2023 (Live). Available online: https://worldpopulationreview.com/countries/puerto-rico-population (accessed on 13 January 2022).
- Dietary Screener Questionnaire in the NHANES 2009–10: Background|EGRP/DCCPS/NCI/NIH. Available online: https://epi.grants.cancer.gov/nhanes/dietscreen/ (accessed on 8 June 2022).
- Barbados Statistical Service. Estimated Resident Population* by Age Group and Sex. Stats.gov.bb. 2010. Available online: https://stats.gov.bb/census/2010-census-tables/2010-census-tables-table-b/. (accessed on 6 December 2023).
- Pelaez, M.; Palloni, A.; Albala, C.; Alfonso, J.C.; Ham-Chande, R.; Hennis, A.; Lebrao, M.L.; Lesn-Diaz, E.; Pantelides, E.; Prats, O. SABE—Survey on health, well-being, and aging in Latin America and the Caribbean. 2006. [Google Scholar] [CrossRef]
- Hennis, A.; Wu, S.-Y.; Nemesure, B.; Honkanen, R.; Leske, M.C.; Barbados Eye Studies Group. Awareness of Incident Open-angle Glaucoma in a Population Study: The Barbados Eye Studies. Ophthalmology 2007, 114, 1816–1821. [Google Scholar] [CrossRef] [PubMed]
- U.S. Census Bureau. 2010 Island Areas—U.S. Virgin Islands Dataset. Census.gov. Available online: https://www.census.gov/data/datasets/2010/dec/virgin-islands.html (accessed on 6 December 2023).
- Holtzman, D. The behavioral risk factor surveillance system. In Community-Based Health Research: Issues and Methods; Springer Publishing Co, Inc.: New York, NY, USA, 2004; pp. 115–131. [Google Scholar]
- U.S. Census Bureau. Puerto Rico Municipios Population Totals: 2010–2019. Census.gov. Available online: https://www.census.gov/data/tables/time-series/demo/popest/2010s-total-puerto-rico-municipios.html (accessed on 6 December 2023).
- Central Statistical Office. Population Statistics. 2010 Cso.gov.tt. Available online: https://cso.gov.tt/subjects/population-and-vital-statistics/population/ (accessed on 6 December 2023).
- Miljkovic, I.; Bodnar, L.M.; A Cauley, J.; Bunker, C.H.; Patrick, A.L.; Wheeler, V.W.; Kuller, L.H.; Zmuda, J.M. Low prevalence of vitamin D deficiency in elderly Afro-Caribbean men. Ethn. Dis. 2011, 21, 79–84. [Google Scholar] [PubMed]
- Continuous Sample Survey of Population. Available online: http://laborsta.ilo.org/applv8/data/SSM3/E/TT.html (accessed on 6 December 2013).
- PanAm STEPS Survey Final Report: Trinidad and Tobago. Available online: http://www.health.gov.tt/news/newsitem.aspx?id=394 (accessed on 6 December 2013).
| Components | Clinical Interview | Clinical Exam | Questionnaire |
|---|---|---|---|
| Clinical Observations | |||
| Medical History | X | ||
| Social Network | X | ||
| Medication Use & Adherence | X | ||
| Height and weight | X | ||
| Waist, hip, and neck circumference | X | ||
| Grip strength | X | ||
| Resting blood pressure | X | ||
| Ankle brachial index | X | ||
| Laboratory tests | |||
| Lipid panel | X | ||
| Complete blood count | X | ||
| Hemoglobin A1C | X | ||
| Metabolic panel | X | ||
| Survey Elements | |||
| Demographics (e.g., age, sex, gender identity, marital status, parity, etc.) | X | ||
| Global Health (general health assessment) | X | ||
| Physical and Mental Health | |||
| History of chronic diseases | X | ||
| Health care access and utilization | X | ||
| Reproductive health | X | ||
| Depression | X | ||
| Health behaviors | |||
| Substance use | X | ||
| Tobacco use | X | ||
| Physical activity | X | ||
| Nutritional assessment | X | ||
| Sleep | X | ||
| Social Health | |||
| Incarceration | X | ||
| Social support | X | ||
| Neighborhood factors | X | ||
| Recruitment Method | Site | Encountered | Eligible | Enrolled | Participation Rate |
|---|---|---|---|---|---|
| Household | BB | 2317 | 1415 | 1008 | 71% |
| Household | PR | 1192 | 988 | 771 | 78% |
| Household | TT | 1326 | 1235 | 829 | 67% |
| Random Digit Dialing | USVI | 1022 | 618 | 353 | 57% |
| Total | 5857 | 4256 | 2961 | 70% |
| Characteristic | N (Available Data) | Mean (SD) |
|---|---|---|
| Age (years) | 2961 | 57.3 (10.3) |
| BMI (Kg/m2) | 2923 | 29.2 (6.1) |
| Total cholesterol (mmol/L) | 2372 | 193 (39.3) |
| HDL (mmol/L) | 2345 | 52.6 (14.6) |
| N (%) | ||
| Sex, Female | 2961 | 1935 (65.4) |
| Age (years) | 2961 | |
| 40–49 | 742 (25.1) | |
| 50–59 | 1046 (35.3) | |
| 60–69 | 775 (26.2) | |
| 70+ | 398 (13.4) | |
| Marital status | 2901 | |
| Single | 1181 (40.7) | |
| Married | 1196 (41.2) | |
| Divorced or separated | 304 (10.5) | |
| Widowed | 220 (7.6) | |
| Sexual orientation | 2792 | |
| Heterosexual (‘straight’) | 2488 (89.1) | |
| Gay or lesbian | 25 (0.9) | |
| Bi-sexual | 17 (0.6) | |
| Not sure or questioning | 262 (9.4) | |
| Regular source of care (Is there one place you usually go when you need routine or non-emergency care?) | 2934 | |
| Yes | 2093 (71.3) | |
| I do not seek routine care anywhere | 405 (13.8) | |
| I seek routine care at more than one place | 436 (14.9) | |
| General Health (In general, would you say your health is:) | 2956 | |
| Poor | 84 (2.8) | |
| Fair | 800 (27.1) | |
| Good | 1267 (42.9) | |
| Very Good | 545 (18.4) | |
| Excellent | 260 (8.8) | |
| Comorbidities | ||
| Obese (BMI 30+) | 2923 | 1136 (38.9) |
| Hypertension a | 2953 | 1459 (49.4) |
| Cardiovascular Disease b | 2956 | 410 (13.9) |
| Diabetes c | 2503 | 562 (22.5) |
| Cancer | 2953 | 121 (4.1) |
| Current Smoker | 2843 | 230 (8.1) |
| At least one of the comorbidities above | 2829 | 2213 (78.2) |
| PAD (ABI ≤ 0.9) | 2772 | 121 (4.4) |
| All Sites | BB | PR | USVI | |||||
|---|---|---|---|---|---|---|---|---|
| Biobank participants | 635 | 101 | 439 | 95 | ||||
| Sample type | n | % | n | % | n | % | n | % |
| Blood clot | 633 | 5.93 | 104 | 5.99 | 435 | 5.87 | 94 | 6.13 |
| Serum | 3098 | 29.03 | 472 | 27.20 | 2201 | 29.72 | 425 | 27.72 |
| Whole blood | 6942 | 65.04 | 1159 | 66.80 | 4769 | 64.40 | 1014 | 66.14 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thompson, T.-A.M.; Desai, M.M.; Martinez-Brockman, J.L.; Tessier-Sherman, B.; Nunez, M.; Adams, O.P.; Nazario, C.M.; Maharaj, R.G.; Nunez-Smith, M. The Eastern Caribbean Health Outcomes Research Network (ECHORN) Cohort Study: Design, Methods, and Baseline Characteristics. Int. J. Environ. Res. Public Health 2024, 21, 17. https://doi.org/10.3390/ijerph21010017
Thompson T-AM, Desai MM, Martinez-Brockman JL, Tessier-Sherman B, Nunez M, Adams OP, Nazario CM, Maharaj RG, Nunez-Smith M. The Eastern Caribbean Health Outcomes Research Network (ECHORN) Cohort Study: Design, Methods, and Baseline Characteristics. International Journal of Environmental Research and Public Health. 2024; 21(1):17. https://doi.org/10.3390/ijerph21010017
Chicago/Turabian StyleThompson, Terri-Ann M., Mayur M. Desai, Josefa L. Martinez-Brockman, Baylah Tessier-Sherman, Maxine Nunez, O. Peter Adams, Cruz María Nazario, Rohan G. Maharaj, and Marcella Nunez-Smith. 2024. "The Eastern Caribbean Health Outcomes Research Network (ECHORN) Cohort Study: Design, Methods, and Baseline Characteristics" International Journal of Environmental Research and Public Health 21, no. 1: 17. https://doi.org/10.3390/ijerph21010017
APA StyleThompson, T.-A. M., Desai, M. M., Martinez-Brockman, J. L., Tessier-Sherman, B., Nunez, M., Adams, O. P., Nazario, C. M., Maharaj, R. G., & Nunez-Smith, M. (2024). The Eastern Caribbean Health Outcomes Research Network (ECHORN) Cohort Study: Design, Methods, and Baseline Characteristics. International Journal of Environmental Research and Public Health, 21(1), 17. https://doi.org/10.3390/ijerph21010017






