Trends in the Epidemiology of Non-Typhoidal Salmonellosis in Israel between 2010 and 2021
Abstract
1. Introduction
2. Materials and Methods
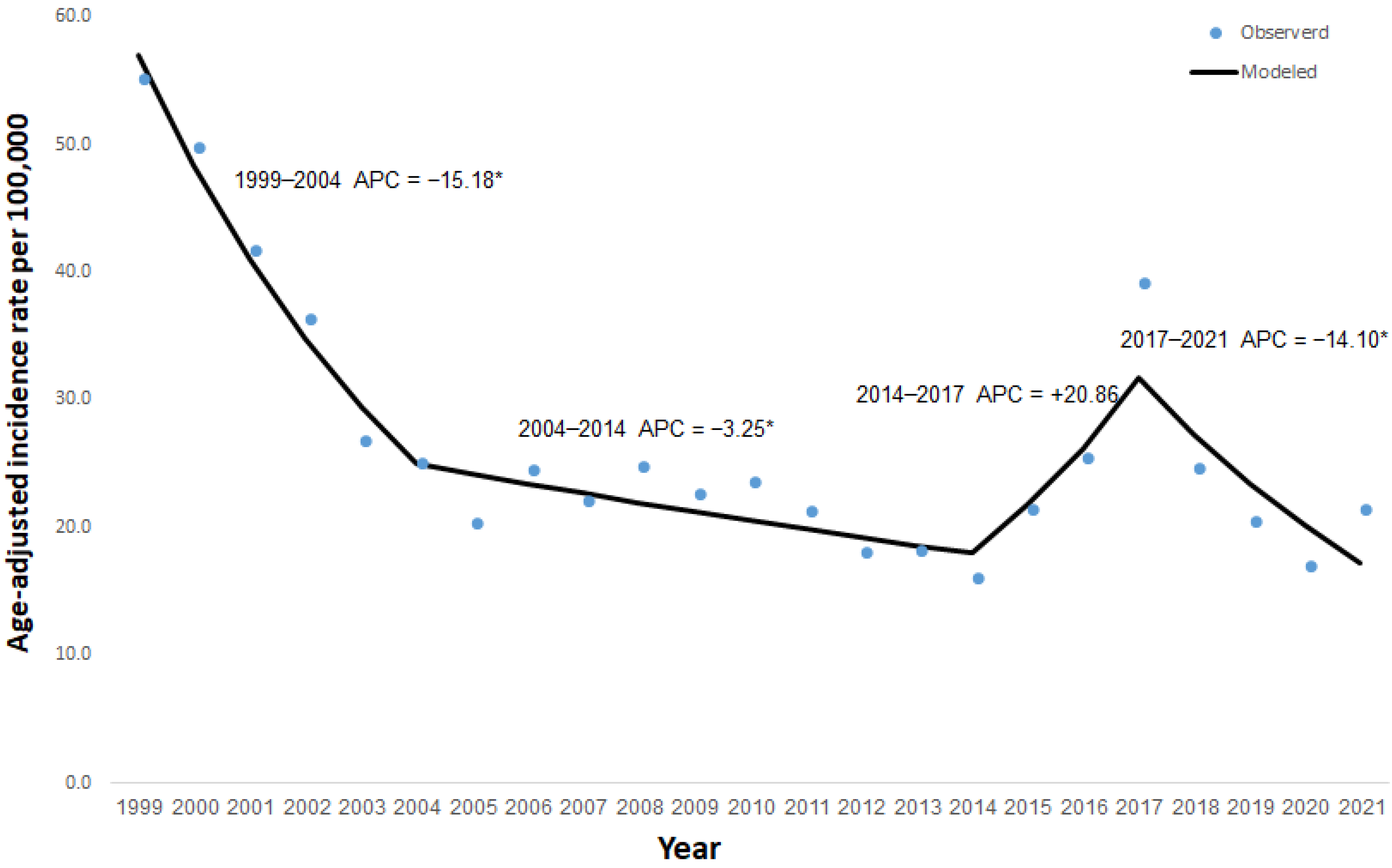
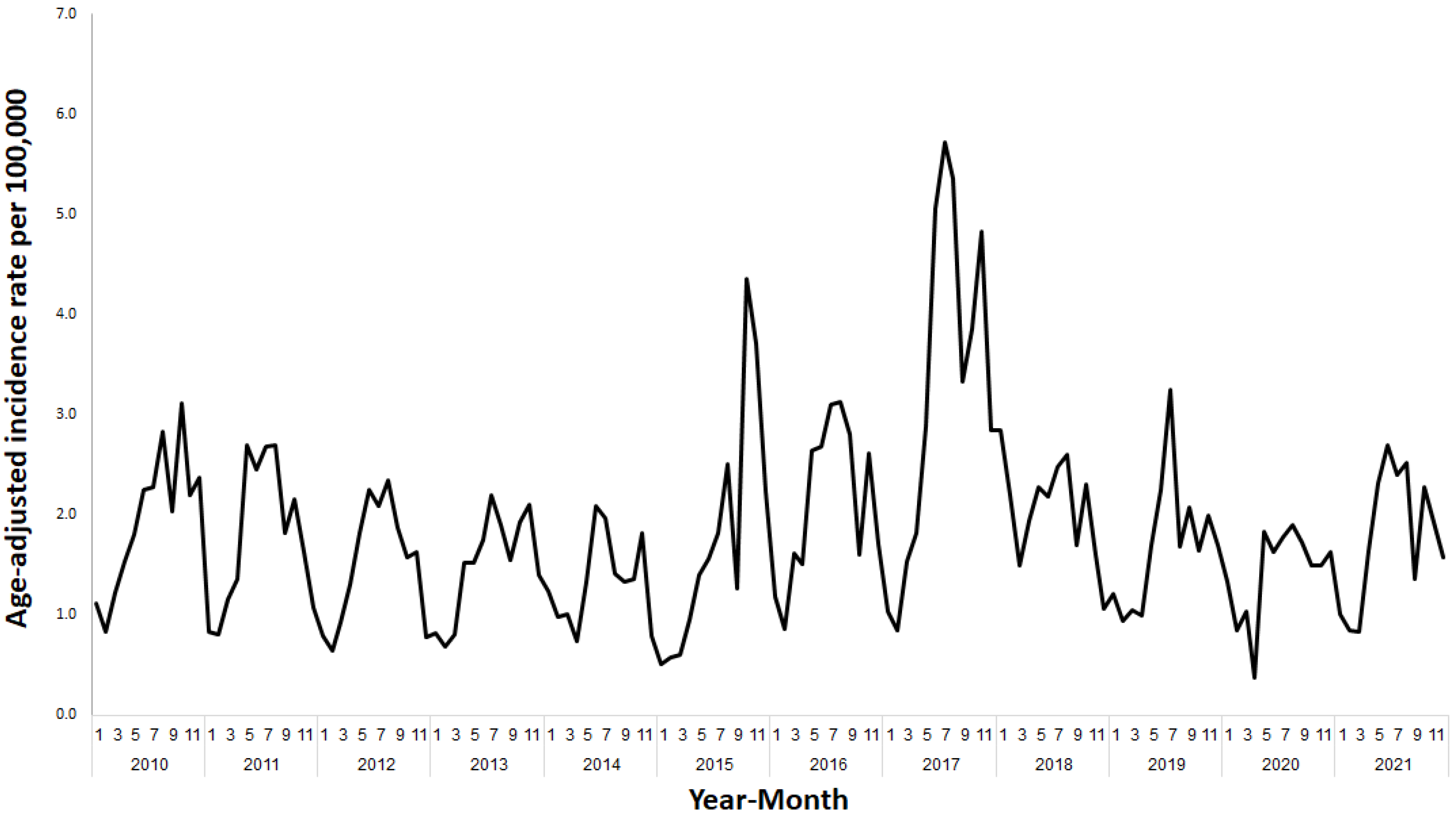
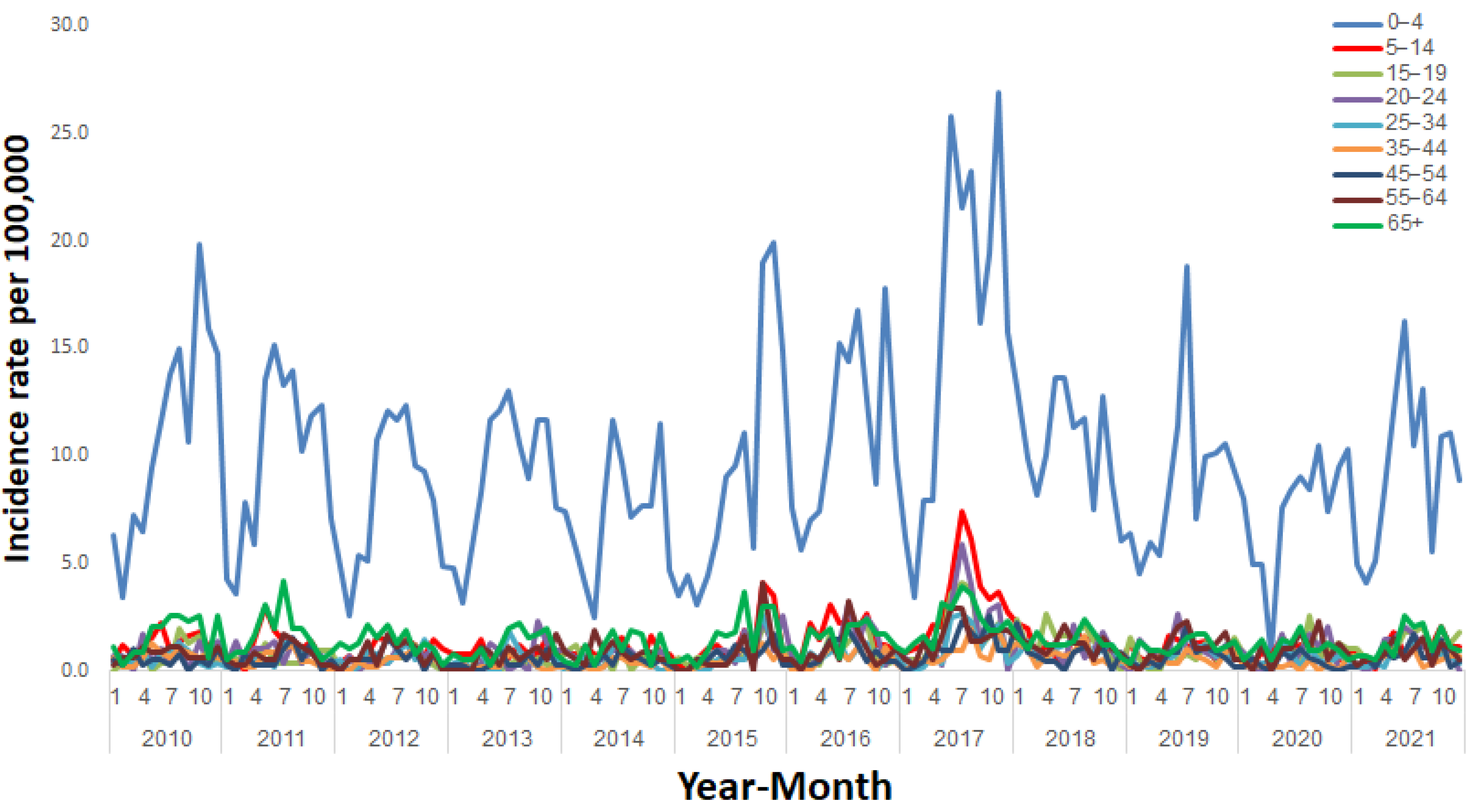
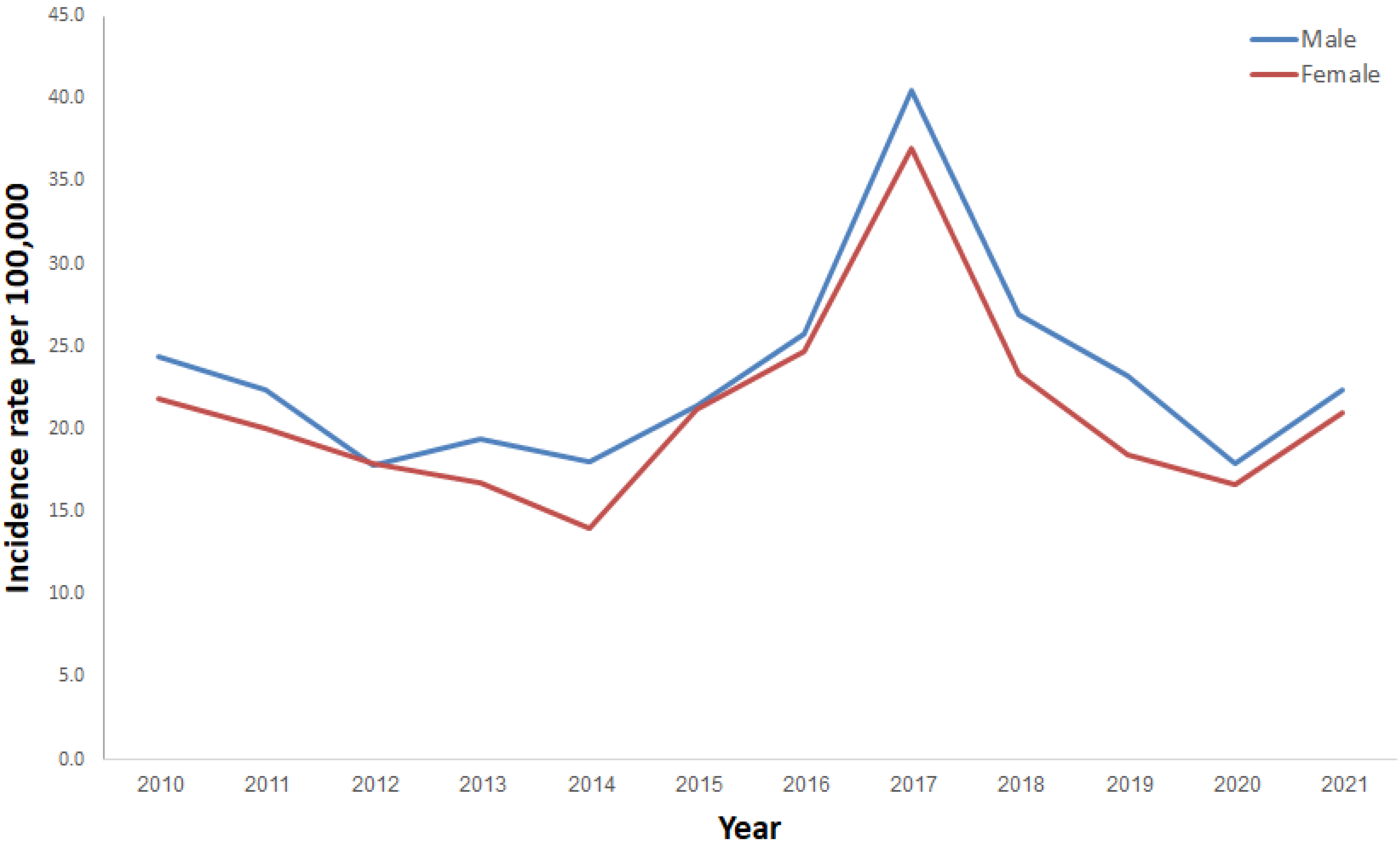
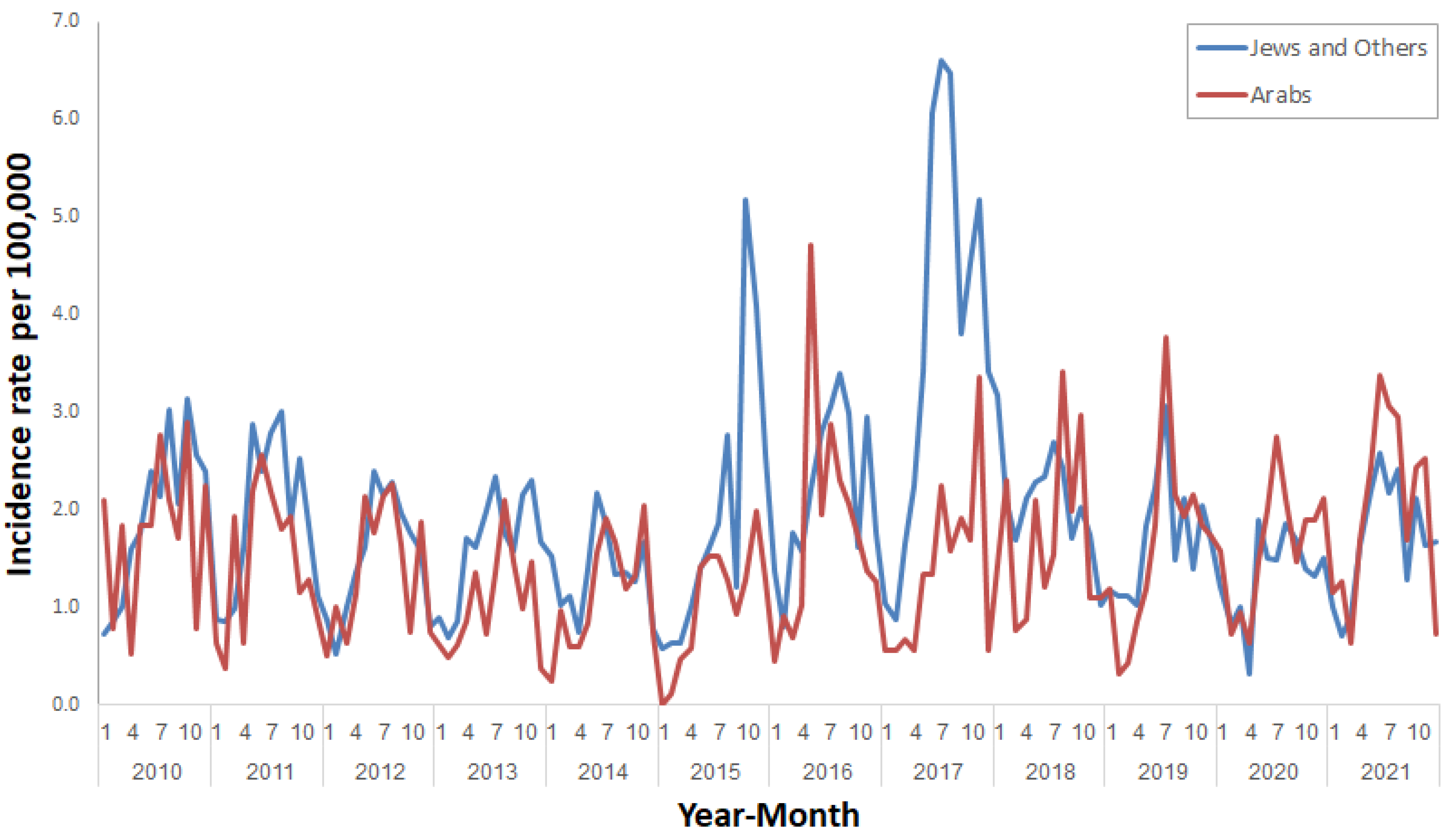
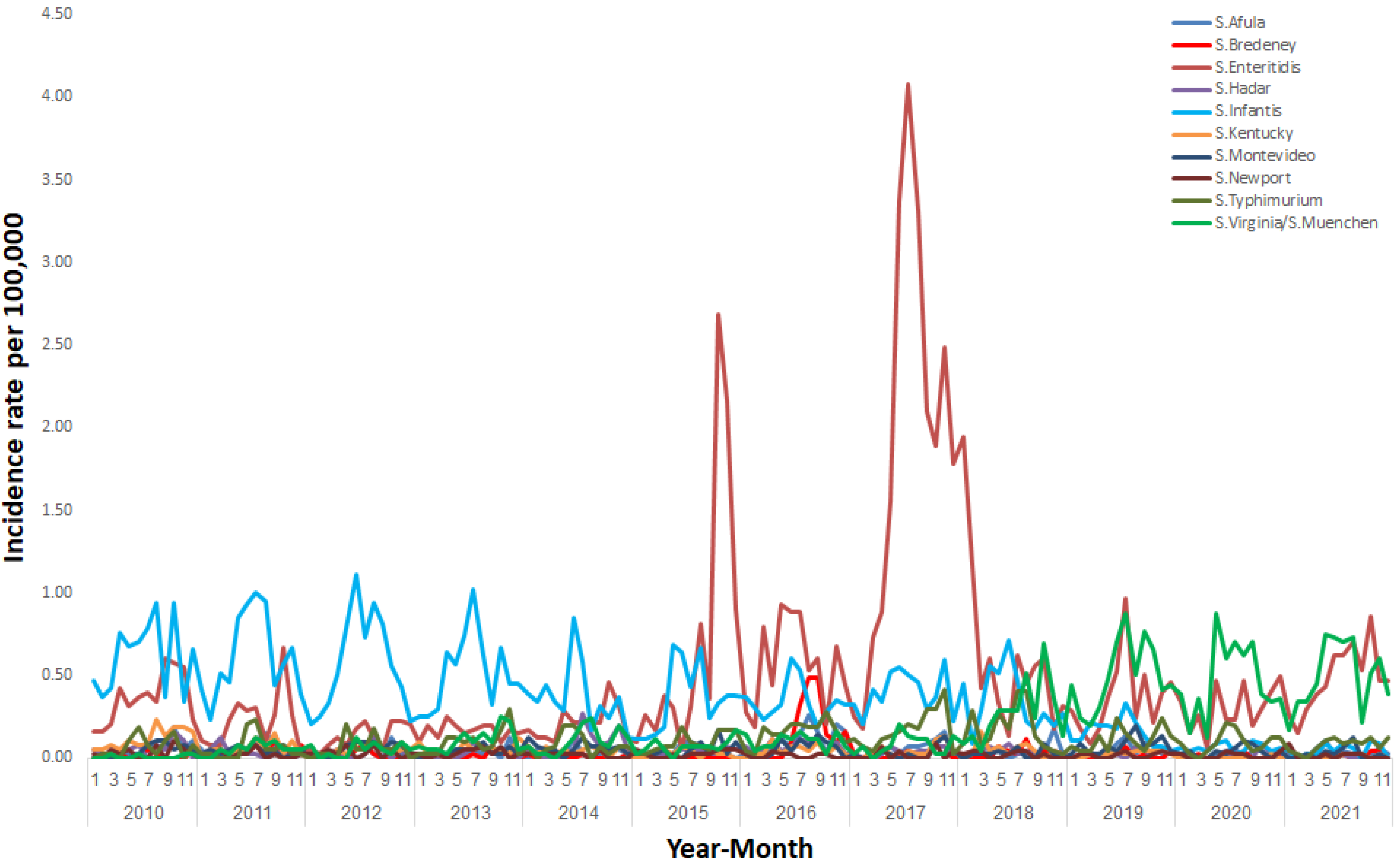
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Besser, J.M. Salmonella epidemiology: A whirlwind of change. Food Microbiol. 2018, 71, 55–59. [Google Scholar] [CrossRef] [PubMed]
- Guibourdenche, M.; Roggentin, P.; Mikoleit, M.; Fields, P.I.; Bockemühl, J.; Grimont, P.A.; Weill, F.-X. Supplement 2003–2007 (No. 47) to the White-Kauffmann-Le Minor scheme. Res. Microbiol. 2010, 161, 26–29. [Google Scholar] [CrossRef]
- WHO—World Health Organization. Salmonella (Non-Typhoidal). Available Online: https://www.who.int/news-room/fact-sheets/detail/salmonella-(non-typhoidal) (accessed on 10 December 2018).
- Center for Disease Control. Salmonella Homepage. 2016. Available Online: https://www.cdc.gov/salmonella/index.html (accessed on 15 January 2021).
- United States Food and Drug Administration. Salmonella (Salmonellosis). Available Online: https://www.fda.gov/food/foodborne-pathogens/salmonella-salmonellosis (accessed on 28 February 2021).
- Salmonella Surveillance Program in Laying Hens in Israel, 2017–2018. The Annual Conference of the Association of Public Health Physicians and Schools of Public Health in Israel, Israel, 3 June 2019. Kfar Maccabiah Convention Center, Ramat Gan, Israel. Available Online: https://program.eventact.com/lecture?id=198837&code=3945265 (accessed on 18 April 2023).
- Bassal, R.; Reisfeld, A.; Andorn, N.; Yishai, R.; Nissan, I.; Agmon, V.; Peled, N.; Block, C.; Keller, N.; Kenes, Y.; et al. Recent trends in the epidemiology of non-typhoidal Salmonella in Israel, 1999–2009. Epidemiol. Infect. 2011, 140, 1446–1453. [Google Scholar] [CrossRef]
- Grimont, P.; Weill, F.-X. Antigenic Formulae of the Salmonella serovars, 9th ed; WHO Collaborating Centre for Reference and Research on Salmonella: Paris, France; Institute Pasteur: Paris, France, 2007; pp. 1–166. [Google Scholar]
- Characterization and classification of local authorities by the socio-economic level of the population in 2015. Local Councils and Municipalities—Rank, Cluster Membership, Population, Variable Values, Standardized Values and Ranking for the Variables Used in the Computation of the Index. Available Online: https://www.cbs.gov.il/en/publications/Pages/2019/Characterization-and-Classification-of-Geographical-Units-by-the-Socio-Economic-Level-of-the-Population-2015.aspx (accessed on 18 April 2023).
- Epidemiologic, Environmental and Laboratory Investigation in Response to an Outbreak of Salmonella Enteritidis Infection in Israel. The 6th International Jerusalem Conference on Health Policy, Health Policy: From Local Experience to Global Patterns and Back Again. Available Online: https://israelhpr.org.il/wp-content/uploads/2019/11/The-6th-International-Jerusalem-Conference-on-Health-Policy-Program-Book-of-Abstract.pdf (accessed on 18 April 2023).
- Jerusalem Central Laboratories, Department of Laboratories., Israel Ministry of Health. Annual Report 2017. Available Online: https://www.gov.il/BlobFolder/reports/lab-jer2017/he/files_publications_units_labs_LAB_JER2017.pdf (accessed on 18 April 2023).
- Ministry of Health. Marketing Cessation of “Yesh Maof” Eggs. Available Online: https://www.health.gov.il/English/News_and_Events/Spokespersons_Messages/Pages/23102017_1.aspx (accessed on 18 April 2023).
- Center for Disease Control and Prevention. Outbreak of Salmonella Infections Linked to Tahini from Achdut Ltd. Available Online: https://www.cdc.gov/salmonella/concord-11-18/index.html (accessed on 18 April 2023).
- Li, X.; Singh, N.; Beshearse, E.; Blanton, J.L.; DeMent, J.; Havelaar, A.H. Spatial Epidemiology of Salmonellosis in Florida, 2009–2018. Front. Public Health 2021, 8. [Google Scholar] [CrossRef] [PubMed]
- Jeffs, E.; Williman, J.; Martin, N.; Brunton, C.; Walls, T. The epidemiology of non-viral gastroenteritis in New Zealand children from 1997 to 2015: An observational study. BMC Public Health 2019, 19, 18. [Google Scholar] [CrossRef] [PubMed]
- EFSA; ECDC. The European Union One Health 2019 Zoonoses Report. EFSA J. 2021, 19. [Google Scholar] [CrossRef]
- Dmochowska, P.; von Brzezinski, M.S.; Żelazowski, J.; Wojtkiewicz, J.; Jung, S.; Harazny, J.M. Epidemiological Survey and Retrospective Analysis of Salmonella Infections between 2000 and 2017 in Warmia and Masuria Voivodship in Poland. Medicina 2019, 55, 74. [Google Scholar] [CrossRef]
- Ford, L.; Glass, K.; Veitch, M.; Wardell, R.; Polkinghorne, B.; Dobbins, T.; Lal, A.; Kirk, M.D. Increasing Incidence of Salmonella in Australia, 2000–2013. PLOS ONE 2016, 11, e0163989. [Google Scholar] [CrossRef]
- Jacob, J.J.; Solaimalai, D.; Sethuvel, D.P.M.; Rachel, T.; Jeslin, P.; Anandan, S.; Veeraraghavan, B. A nineteen-year report of serotype and antimicrobial susceptibility of enteric non-typhoidal Salmonella from humans in Southern India: Changing facades of taxonomy and resistance trend. Gut Pathog. 2020, 12, 49. [Google Scholar] [CrossRef]
- Tack, D.M.; Ray, L.; Griffin, P.M.; Cieslak, P.R.; Dunn, J.; Rissman, T.; Jervis, R.; Lathrop, S.; Muse, A.; Duwell, M.; et al. Preliminary Incidence and Trends of Infections with Pathogens Transmitted Commonly Through Food — Foodborne Diseases Active Surveillance Network, 10 U.S. Sites, 2016–2019. MMWR. Morb. Mortal. Wkly. Rep. 2020, 69, 509–514. [Google Scholar] [CrossRef]
- Bassal, R.; Keinan-Boker, L.; Cohen, D. A Significant Decrease in the Incidence of Shigellosis in Israel during COVID-19 Pandemic. Int. J. Environ. Res. Public Health 2021, 18, 3070. [Google Scholar] [CrossRef] [PubMed]
- Davis, B.P.F.; Amin, J.; Franklin, N.; Beggs, P.J. Salmonellosis in Australia in 2020: Possible impacts of COVID-19 related public health measures. Commun. Dis. Intell. 2022, 46. [Google Scholar] [CrossRef]
- Dallal, M.M.S.; Ehrampoush, M.H.; Aminharati, F.; Tafti, A.A.D.; Yaseri, M.; Memariani, M. Associations between climatic parameters and the human salmonellosis in Yazd province, Iran. Environ. Res. 2020, 187, 109706. [Google Scholar] [CrossRef] [PubMed]
- Simpson, R.B.; Zhou, B.; Naumova, E.N. Seasonal synchronization of foodborne outbreaks in the United States, 1996–2017. Sci. Rep. 2020, 10, 17500. [Google Scholar] [CrossRef]
- Kynčl, J.; Špačková, M.; Fialová, A.; Kyselý, J.; Malý, M. Influence of air temperature and implemented veterinary measures on the incidence of human salmonellosis in the Czech Republic during 1998–2017. BMC Public Health 2021, 21, 55. [Google Scholar] [CrossRef]
- Liu, J.; Bai, L.; Li, W.; Han, H.; Fu, P.; Ma, X.; Bi, Z.; Yang, X.; Zhang, X.; Zhen, S.; et al. Trends of foodborne diseases in China: Lessons from laboratory-based surveillance since 2011. Front. Med. 2017, 12, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Green, M.S. The Male Predominance in the Incidence of Infectious Diseases in Children: A Postulated Explanation for Disparities in the Literature. Leuk. Res. 1992, 21, 381–386. [Google Scholar] [CrossRef] [PubMed]
- Peer, V.; Schwartz, N.; Green, M.S. Sex Differences in Salmonellosis Incidence Rates—An Eight-Country National Data-Pooled Analysis. J. Clin. Med. 2021, 10, 5767. [Google Scholar] [CrossRef]
- Quinlan, J.J. Foodborne Illness Incidence Rates and Food Safety Risks for Populations of Low Socioeconomic Status and Minority Race/Ethnicity: A Review of the Literature. Int. J. Environ. Res. Public Health 2013, 10, 3634–3652. [Google Scholar] [CrossRef]
- Sodagari, H.R.; Wang, P.; Robertson, I.; Habib, I.; Sahibzada, S. Non-Typhoidal Salmonella at the Human-Food-of-Animal-Origin Interface in Australia. Animals 2020, 10, 1192. [Google Scholar] [CrossRef]
- Sher, A.A.; Mustafa, B.E.; Grady, S.C.; Gardiner, J.C.; Saeed, A.M. Outbreaks of foodborne Salmonella enteritidis in the United States between 1990 and 2015: An analysis of epidemiological and spatial-temporal trends. Int. J. Infect. Dis. 2021, 105, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Cohen, E.; Davidovich, M.; Rokney, A.; Valinsky, L.; Rahav, G.; Gal-Mor, O. Emergence of new variants of antibiotic resistance genomic islands among multidrug-resistant Salmonella enterica in poultry. Environ. Microbiol. 2019, 22, 413–432. [Google Scholar] [CrossRef]
- Antunes, P.; Mourão, J.; Campos, J.; Peixe, L. Salmonellosis: The role of poultry meat. Clin. Microbiol. Infect. 2016, 22, 110–121. [Google Scholar] [CrossRef]
- Cohen, E.; Kriger, O.; Amit, S.; Davidovich, M.; Rahav, G.; Gal-Mor, O. The emergence of a multidrug resistant Salmonella Muenchen in Israel is associated with horizontal acquisition of the epidemic pESI plasmid. Clin. Microbiol. Infect. 2022, 28, 1499.e7–1499.e14. [Google Scholar] [CrossRef] [PubMed]
- Arnold, K.; Lim, S.; Rakler, T.; Rovira, A.; Satuchne, C.; Yechezkel, E.; Wiseman, A.; Pima, Y.; Yakunin, E.; Rokney, A.; et al. Using genetic markers for detection and subtyping of the emerging Salmonella enterica subspecies enterica serotype Muenchen. Poult. Sci. 2022, 101. [Google Scholar] [CrossRef]
- Tzani, M.; Mandilara, G.; Dias, J.G.; Sideroglou, T.; Chrysostomou, A.; Mellou, K. Impact of Salmonella Control Programmes in Poultry on Human Salmonellosis Burden in Greece. Antibiotics 2021, 10, 121. [Google Scholar] [CrossRef] [PubMed]
- Ziv, T.; Heymann, A.D.; Azuri, J.; Leshno, M.; Cohen, D. Assessment of the underestimation of childhood diarrhoeal disease burden in Israel. Epidemiol. Infect. 2010, 139, 1379–1387. [Google Scholar] [CrossRef]
| Variable | Category | N | % |
|---|---|---|---|
| Sentinel laboratory reporting the isolation of Salmonella spp. | Haemek Medical Center | 784 | 6.7 |
| Clalit Haifa HMO | 2727 | 23.4 | |
| Maccabi Dan District HMO | 1288 | 11.1 | |
| Soroka Medical Center | 2323 | 20.0 | |
| Sheba Medical Center | 291 | 2.5 | |
| Hadassah Medical Centers | 540 | 4.6 | |
| Meuhedet HMO | 2824 | 24.3 | |
| Clalit Petah-Tikva HMO | 868 | 7.5 | |
| Total | 11,645 | 100.0 | |
| Age group (years) | 0–4 | 6450 | 55.5 |
| 5–14 | 1528 | 13.1 | |
| 15–19 | 441 | 3.8 | |
| 20–24 | 431 | 3.7 | |
| 25–34 | 556 | 4.8 | |
| 35–44 | 402 | 3.5 | |
| 45–54 | 397 | 3.4 | |
| 55–64 | 498 | 4.3 | |
| 65+ | 922 | 7.9 | |
| Gender | Male | 6036 | 52.1 |
| Female | 5557 | 47.9 | |
| Birth Country | Israel | 10,154 | 88.8 |
| Other | 1279 | 11.2 | |
| Population group | Jews and Others | 9263 | 83.2 |
| Arabs | 1867 | 16.8 | |
| District | Jerusalem | 2621 | 22.8 |
| North | 2034 | 17.7 | |
| Haifa | 1488 | 12.9 | |
| Central | 998 | 8.7 | |
| Tel-Aviv | 1234 | 10.7 | |
| South | 2380 | 20.7 | |
| Judea and Samaria | 749 | 6.5 | |
| Socioeconomic rank | Mean ± standard deviation | 10,164 | 4.6 ± 2.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bassal, R.; Davidovich-Cohen, M.; Yakunin, E.; Rokney, A.; Ken-Dror, S.; Strauss, M.; Wolf, T.; Sagi, O.; Amit, S.; Moran-Gilad, J.; et al. Trends in the Epidemiology of Non-Typhoidal Salmonellosis in Israel between 2010 and 2021. Int. J. Environ. Res. Public Health 2023, 20, 5626. https://doi.org/10.3390/ijerph20095626
Bassal R, Davidovich-Cohen M, Yakunin E, Rokney A, Ken-Dror S, Strauss M, Wolf T, Sagi O, Amit S, Moran-Gilad J, et al. Trends in the Epidemiology of Non-Typhoidal Salmonellosis in Israel between 2010 and 2021. International Journal of Environmental Research and Public Health. 2023; 20(9):5626. https://doi.org/10.3390/ijerph20095626
Chicago/Turabian StyleBassal, Ravit, Maya Davidovich-Cohen, Eugenia Yakunin, Assaf Rokney, Shifra Ken-Dror, Merav Strauss, Tamar Wolf, Orli Sagi, Sharon Amit, Jacob Moran-Gilad, and et al. 2023. "Trends in the Epidemiology of Non-Typhoidal Salmonellosis in Israel between 2010 and 2021" International Journal of Environmental Research and Public Health 20, no. 9: 5626. https://doi.org/10.3390/ijerph20095626
APA StyleBassal, R., Davidovich-Cohen, M., Yakunin, E., Rokney, A., Ken-Dror, S., Strauss, M., Wolf, T., Sagi, O., Amit, S., Moran-Gilad, J., Treygerman, O., Karyo, R., Keinan-Boker, L., & Cohen, D. (2023). Trends in the Epidemiology of Non-Typhoidal Salmonellosis in Israel between 2010 and 2021. International Journal of Environmental Research and Public Health, 20(9), 5626. https://doi.org/10.3390/ijerph20095626






