Effects of Non-Essential “Toxic” Trace Elements on Pregnancy Outcomes: A Narrative Overview of Recent Literature Syntheses
Abstract
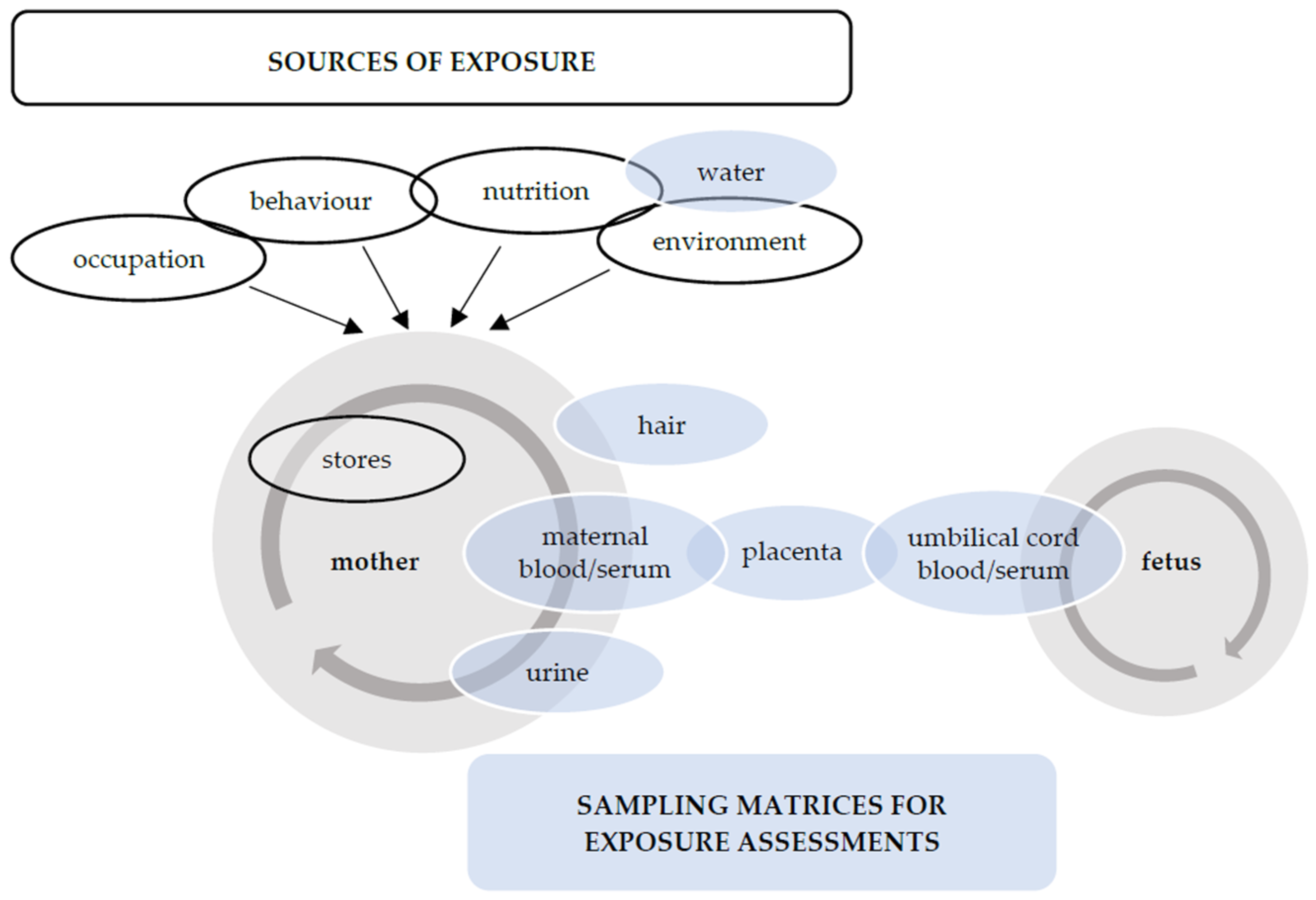
1. Introduction
2. Methods
3. Prenatal Exposure to Non-Essential (Toxic) Trace Elements
3.1. Cadmium
3.2. Lead
3.3. Arsenic
3.4. Mercury
4. Effects of Non-Essential Trace Elements on Pregnancy Outcomes
4.1. Pre-Eclampsia
4.2. Preterm Birth
| Exposure | Sample Type | Measure of Association | Heterogeneity | n | Reference | |
|---|---|---|---|---|---|---|
| Estimate (95% CI) | I2 | p | ||||
| Arsenic | maternal serum maternal urine | OR, 1.06 (0.92 to 1.21) | 57.7% | 0.037 | 6 | Wu et al., 2022 [56] |
| Cadmium | maternal serum maternal urine | OR, 1.33 (1.06 to 1.67) | 82.0% | 0.000 | 8 | Wu et al., 2022 [56] |
| Cadmium | maternal blood maternal urine | RR, 1.32 (1.05 to 1.67) | 90.0% | 0.000 | 5 | Amegah et al., 2021 [58] |
| Cadmium | maternal blood maternal serum maternal urine | OR, 1.32 (1.08 to 1.61) | 81.4% | 0.000 | 10 | Asefi et al., 2020 [59] |
| Lead | cord blood maternal serum maternal urine | OR, 1.39 (1.10 to 1.76) | 88.1% | 0.000 | 10 | Wu et al., 2022 [56] |
| Mercury | maternal serum maternal urine | OR, 1.09 (0.94 to 1.27) | 32.7% | 0.203 | 5 | Wu et al., 2022 [56] |
4.3. Prenatal Growth
4.3.1. Birth Weight
4.3.2. Birth Length and Head Circumference
5. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Stone, J.; Sutrave, P.; Gascoigne, E.; Givens, M.B.; Fry, R.C.; Manuck, T.A. Exposure to toxic metals and per- and polyfluoroalkyl substances and the risk of preeclampsia and preterm birth in the United States: A review. Am. J. Obstet. Gynecol. MFM 2021, 3, 100308. [Google Scholar] [CrossRef] [PubMed]
- Gajewska, K.; Błażewicz, A.; Laskowska, M.; Niziński, P.; Dymara-Konopka, W.; Komsta, Ł. Chemical elements and preeclampsia—An overview of current problems, challenges and significance of recent research. J. Trace Elem. Med. Biol. 2020, 59, 126468. [Google Scholar] [CrossRef]
- Khanam, R.; Kumar, I.; Oladapo-Shittu, O.; Twose, C.; Islam, A.A.; Biswal, S.S.; Raqib, R.; Baqui, A.H. Prenatal Environmental Metal Exposure and Preterm Birth: A Scoping Review. Int. J. Environ. Res. Public Health 2021, 18, 573. [Google Scholar] [CrossRef] [PubMed]
- McKeating, D.R.; Fisher, J.J.; Perkins, A.V. Elemental Metabolomics and Pregnancy Outcomes. Nutrients 2019, 11, 73. [Google Scholar] [CrossRef] [PubMed]
- Jaishankar, M.; Tseten, T.; Anbalagan, N.; Mathew, B.B.; Beeregowda, K.N. Toxicity, mechanism and health effects of some heavy metals. Interdiscip. Toxicol. 2014, 7, 60–72. [Google Scholar] [CrossRef]
- Tchounwou, P.B.; Yedjou, C.G.; Patlolla, A.K.; Sutton, D.J. Heavy metal toxicity and the environment. Exp. Suppl. 2012, 101, 133–164. [Google Scholar] [CrossRef]
- Iavicoli, I.; Fontana, L.; Bergamaschi, A. The effects of metals as endocrine disruptors. J. Toxicol. Environ. Health B Crit. Rev. 2009, 12, 206–223. [Google Scholar] [CrossRef]
- Rahman, A.; Kumarathasan, P.; Gomes, J. Infant and mother related outcomes from exposure to metals with endocrine disrupting properties during pregnancy. Sci. Total. Environ. 2016, 569–570, 1022–1031. [Google Scholar] [CrossRef]
- Rehman, K.; Fatima, F.; Waheed, I.; Akash, M.S.H. Prevalence of exposure of heavy metals and their impact on health consequences. J. Cell. Biochem. 2018, 119, 157–184. [Google Scholar] [CrossRef]
- Gundacker, C.; Hengstschläger, M. The role of the placenta in fetal exposure to heavy metals. Wien. Med. Wochenschr. 2012, 162, 201–206. [Google Scholar] [CrossRef]
- Esteban-Vasallo, M.D.; Aragonés, N.; Pollan, M.; López-Abente, G.; Perez-Gomez, B. Mercury, cadmium, and lead levels in human placenta: A systematic review. Environ. Health Perspect. 2012, 120, 1369–1377. [Google Scholar] [CrossRef]
- Faroon, O.; Ashizawa, A.; Wright, S.; Tucker, P.; Jenkins, K.; Ingerman, L.; Rudisill, C. Public Health Statement. In Toxicological Profile for Cadmium; Agency for Toxic Substances and Disease Registry (US): Atlanta, GA, USA, 2012; pp. 1–10. [Google Scholar]
- Wang, M.; Chen, Z.; Song, W.; Hong, D.; Huang, L.; Li, Y. A review on Cadmium Exposure in the Population and Intervention Strategies Against Cadmium Toxicity. Bull. Environ. Contam. Toxicol. 2021, 106, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Genchi, G.; Sinicropi, M.S.; Lauria, G.; Carocci, A.; Catalano, A. The Effects of Cadmium Toxicity. Int. J. Environ. Res. Public Health 2020, 17, 3782. [Google Scholar] [CrossRef]
- Singh, L.; Anand, M.; Singh, S.; Taneja, A. Environmental toxic metals in placenta and their effects on preterm delivery-current opinion. Drug Chem. Toxicol. 2020, 43, 531–538. [Google Scholar] [CrossRef]
- Nawrot, T.S.; Martens, D.S.; Hara, A.; Plusquin, M.; Vangronsveld, J.; Roels, H.A.; Staessen, J.A. Association of total cancer and lung cancer with environmental exposure to cadmium: The meta-analytical evidence. Cancer Causes Control 2015, 26, 1281–1288. [Google Scholar] [CrossRef]
- Satir, S. The relationship between oral cancer and cadmium: A review. Mol. Biol. Rep. 2022, 49, 2413–2419. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Xun, P.; Nishijo, M.; He, K. Cadmium exposure and risk of lung cancer: A meta-analysis of cohort and case-control studies among general and occupational populations. J. Expo. Sci. Environ. Epidemiol. 2016, 26, 437–444. [Google Scholar] [CrossRef] [PubMed]
- Filippini, T.; Torres, D.; Lopes, C.; Carvalho, C.; Moreira, P.; Naska, A.; Kasdagli, M.I.; Malavolti, M.; Orsini, N.; Vinceti, M. Cadmium exposure and risk of breast cancer: A dose-response meta-analysis of cohort studies. Environ. Int. 2020, 142, 105879. [Google Scholar] [CrossRef]
- Hartwig, A. Cadmium and cancer. Met. Ions Life Sci. 2013, 11, 491–507. [Google Scholar] [CrossRef]
- Tarhonska, K.; Lesicka, M.; Janasik, B.; Roszak, J.; Reszka, E.; Braun, M.; Kołacińska-Wow, A.; Jabłońska, E. Cadmium and breast cancer—Current state and research gaps in the underlying mechanisms. Toxicol. Lett. 2022, 361, 29–42. [Google Scholar] [CrossRef]
- Osman, K.; Akesson, A.; Berglund, M.; Bremme, K.; Schütz, A.; Ask, K.; Vahter, M. Toxic and essential elements in placentas of swedish women. Clin. Biochem. 2000, 33, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Geng, H.X.; Wang, L. Cadmium: Toxic effects on placental and embryonic development. Environ. Toxicol. Pharmacol. 2019, 67, 102–107. [Google Scholar] [CrossRef] [PubMed]
- Tekin, D.; Kayaaltı, Z.; Aliyev, V.; Söylemezoğlu, T. The effects of metallothionein 2A polymorphism on placental cadmium accumulation: Is metallothionein a modifiying factor in transfer of micronutrients to the fetus? J. Appl. Toxicol. 2012, 32, 270–275. [Google Scholar] [CrossRef]
- Sekovanić, A.; Jurasović, J.; Piasek, M.; Pašalić, D.; Orct, T.; Grgec, A.S.; Stasenko, S.; Čakanić, K.B.; Jazbec, A. Metallothionein 2A gene polymorphism and trace elements in mother-newborn pairs in the Croatian population. J. Trace Elem. Med. Biol. 2018, 45, 163–170. [Google Scholar] [CrossRef]
- Abadin, H.; Ashizawa, A.; Stevens, Y.W.; Llados, F.; Diamond, G.; Sage, G.; Citra, M.; Quinones, A.; Bosch, S.J.; Swarts, S.G. Public Health Statement. In Toxicological Profile for Lead; Agency for Toxic Substances and Disease Registry (US): Atlanta, GA, USA, 2007; pp. 1–9. [Google Scholar]
- Angrand, R.C.; Collins, G.; Landrigan, P.J.; Thomas, V.M. Relation of blood lead levels and lead in gasoline: An updated systematic review. Environ. Health 2022, 21, 138. [Google Scholar] [CrossRef]
- Pohl, H.R.; Ingber, S.Z.; Abadin, H.G. Historical View on Lead: Guidelines and Regulations. Met. Ions Life Sci. 2017, 17, 435–470. [Google Scholar] [CrossRef]
- Taylor, M.P.; Mould, S.A.; Kristensen, L.J.; Rouillon, M. Environmental arsenic, cadmium and lead dust emissions from metal mine operations: Implications for environmental management, monitoring and human health. Environ. Res. 2014, 135, 296–303. [Google Scholar] [CrossRef]
- Levin, R.; Zilli Vieira, C.L.; Rosenbaum, M.H.; Bischoff, K.; Mordarski, D.C.; Brown, M.J. The urban lead (Pb) burden in humans, animals and the natural environment. Environ. Res. 2021, 193, 110377. [Google Scholar] [CrossRef]
- Gidlow, D.A. Lead toxicity. Occup. Med. 2015, 65, 348–356. [Google Scholar] [CrossRef]
- Kumar, A.; Kumar, A.; Cabral-Pinto, M.; Chaturvedi, A.K.; Shabnam, A.A.; Subrahmanyam, G.; Mondal, R.; Gupta, D.K.; Malyan, S.K.; Kumar, S.S.; et al. Lead Toxicity: Health Hazards, Influence on Food Chain, and Sustainable Remediation Approaches. Int. J. Environ. Res. Public Health 2020, 17, 2179. [Google Scholar] [CrossRef]
- Ettinger, A.S.; Wengrovitz, A.M. Guidelines for the Identification and Management of Lead Exposure in Pregnant and Lactating Women; U.S. Department of Health and Human Services: Atlanta, GA, USA, 2010. [Google Scholar]
- Carlin, D.J.; Naujokas, M.F.; Bradham, K.D.; Cowden, J.; Heacock, M.; Henry, H.F.; Lee, J.S.; Thomas, D.J.; Thompson, C.; Tokar, E.J.; et al. Arsenic and Environmental Health: State of the Science and Future Research Opportunities. Environ. Health Perspect. 2016, 124, 890–899. [Google Scholar] [CrossRef]
- Ali, W.; Rasool, A.; Junaid, M.; Zhang, H. A comprehensive review on current status, mechanism, and possible sources of arsenic contamination in groundwater: A global perspective with prominence of Pakistan scenario. Environ. Geochem. Health 2019, 41, 737–760. [Google Scholar] [CrossRef]
- Jomova, K.; Jenisova, Z.; Feszterova, M.; Baros, S.; Liska, J.; Hudecova, D.; Rhodes, C.J.; Valko, M. Arsenic: Toxicity, oxidative stress and human disease. J. Appl. Toxicol. 2011, 31, 95–107. [Google Scholar] [CrossRef]
- Rahaman, M.S.; Rahman, M.M.; Mise, N.; Sikder, M.T.; Ichihara, G.; Uddin, M.K.; Kurasaki, M.; Ichihara, S. Environmental arsenic exposure and its contribution to human diseases, toxicity mechanism and management. Environ. Pollut. 2021, 289, 117940. [Google Scholar] [CrossRef]
- Martinez, V.D.; Lam, W.L. Health Effects Associated With Pre- and Perinatal Exposure to Arsenic. Front. Genet. 2021, 12, 664717. [Google Scholar] [CrossRef]
- Martínez-Castillo, M.; García-Montalvo, E.A.; Arellano-Mendoza, M.G.; Sánchez-Peña, L.D.C.; Soria Jasso, L.E.; Izquierdo-Vega, J.A.; Valenzuela, O.L.; Hernández-Zavala, A. Arsenic exposure and non-carcinogenic health effects. Hum. Exp. Toxicol. 2021, 40, S826–S850. [Google Scholar] [CrossRef]
- Palma-Lara, I.; Martínez-Castillo, M.; Quintana-Pérez, J.C.; Arellano-Mendoza, M.G.; Tamay-Cach, F.; Valenzuela-Limón, O.L.; García-Montalvo, E.A.; Hernández-Zavala, A. Arsenic exposure: A public health problem leading to several cancers. Regul. Toxicol. Pharmacol. 2020, 110, 104539. [Google Scholar] [CrossRef]
- Vahter, M. Effects of arsenic on maternal and fetal health. Annu. Rev. Nutr. 2009, 29, 381–399. [Google Scholar] [CrossRef]
- Clarkson, T.W. The three modern faces of mercury. Environ. Health Perspect. 2002, 110, 11–23. [Google Scholar] [CrossRef]
- Bernhoft, R.A. Mercury toxicity and treatment: A review of the literature. J. Environ. Public Health 2012, 2012, 460508. [Google Scholar] [CrossRef]
- Carocci, A.; Rovito, N.; Sinicropi, M.S.; Genchi, G. Mercury toxicity and neurodegenerative effects. Rev. Environ. Contam. Toxicol. 2014, 229, 1–18. [Google Scholar] [CrossRef]
- Vianna, A.D.S.; Matos, E.P.; Jesus, I.M.; Asmus, C.; Câmara, V.M. Human exposure to mercury and its hematological effects: A systematic review. Cad. Saude Publica 2019, 35, e00091618. [Google Scholar] [CrossRef]
- Bridges, C.C.; Zalups, R.K. Transport of Inorganic Mercury and Methylmercury in Target Tissues and Organs. J. Toxicol. Environ. Health B Crit. Rev. 2010, 13, 385–410. [Google Scholar] [CrossRef]
- American College of Obstetricians and Gynecologists; Task Force on Hypertension in Pregnancy. Executive Summary: Hypertension in Pregnancy. Report of the American College of Obstetricians and Gynecologists’ Task Force on Hypertension in Pregnancy. Obstet. Gynecol. 2013, 122, 1122–1131. [Google Scholar] [CrossRef]
- Rosen, E.M.; Muñoz, M.I.; McElrath, T.; Cantonwine, D.E.; Ferguson, K.K. Environmental contaminants and preeclampsia: A systematic literature review. J. Toxicol. Environ. Health B Crit. Rev. 2018, 21, 291–319. [Google Scholar] [CrossRef]
- Xiao, R.; Sorensen, T.K.; Williams, M.A.; Luthy, D.A. Influence of pre-eclampsia on fetal growth. J. Matern. Fetal Neonatal Med. 2003, 13, 157–162. [Google Scholar] [CrossRef]
- Poropat, A.E.; Laidlaw, M.A.S.; Lanphear, B.; Ball, A.; Mielke, H.W. Blood lead and preeclampsia: A meta-analysis and review of implications. Environ. Res. 2018, 160, 12–19. [Google Scholar] [CrossRef]
- Kahn, L.G.; Trasande, L. Environmental Toxicant Exposure and Hypertensive Disorders of Pregnancy: Recent Findings. Curr. Hypertens. Rep. 2018, 20, 87. [Google Scholar] [CrossRef]
- El-Badry, A.; Rezk, M.; El-Sayed, H. Mercury-induced Oxidative Stress May Adversely Affect Pregnancy Outcome among Dental Staff: A Cohort Study. Int. J. Occup. Environ. Med. 2018, 9, 113–119. [Google Scholar] [CrossRef]
- Committee on Understanding Premature Birth and Assuring Healthy Outcomes; Board on Health Sciences Policy; Institute of Medicine. Medical and Pregnancy Conditions Associated with Preterm Birth. In Preterm Birth: Causes, Consequences, and Prevention; Behrman, R.E., Butler, A.S., Eds.; National Academies Press (US): Washington, DC, USA, 2007; pp. 148–168. [Google Scholar]
- Preterm Birth. Available online: https://www.who.int/news-room/fact-sheets/detail/preterm-birth (accessed on 20 November 2022).
- Blencowe, H.; Cousens, S.; Oestergaard, M.Z.; Chou, D.; Moller, A.B.; Narwal, R.; Adler, A.; Vera Garcia, C.; Rohde, S.; Say, L.; et al. National, regional, and worldwide estimates of preterm birth rates in the year 2010 with time trends since 1990 for selected countries: A systematic analysis and implications. Lancet 2012, 379, 2162–2172. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, J.; Wei, Y.; Chen, J.; Kang, L.; Long, C.; Wu, S.; Shen, L.; Wei, G. Maternal exposure to endocrine disrupting chemicals (EDCs) and preterm birth: A systematic review, meta-analysis, and meta-regression analysis. Environ. Pollut. 2022, 292, 118264. [Google Scholar] [CrossRef]
- Osterman, M.; Hamilton, B.; Martin, J.A.; Driscoll, A.K.; Valenzuela, C.P. Births: Final Data for 2020. Natl. Vital. Stat. Rep. 2021, 70, 1–50. [Google Scholar]
- Amegah, A.K.; Sewor, C.; Jaakkola, J.J.K. Cadmium exposure and risk of adverse pregnancy and birth outcomes: A systematic review and dose-response meta-analysis of cohort and cohort-based case-control studies. J. Expo. Sci. Environ. Epidemiol. 2021, 31, 299–317. [Google Scholar] [CrossRef]
- Asefi, Y.; Gohari Mahmoudabad, A.; Habibian Sezavar, A.; Mirshahvaladi, S.; Abyadeh, M.; Abyareh, M. Association between maternal cadmium exposure and preterm birth: A meta-analysis. Int. J. Environ. Health Res. 2020, 32, 628–637. [Google Scholar] [CrossRef]
- Wai, K.M.; Mar, O.; Kosaka, S.; Umemura, M.; Watanabe, C. Prenatal Heavy Metal Exposure and Adverse Birth Outcomes in Myanmar: A Birth-Cohort Study. Int. J. Environ. Res. Public Health 2017, 14, 1339. [Google Scholar] [CrossRef]
- Flannery, B.M.; Schaefer, H.R.; Middleton, K.B. A scoping review of infant and children health effects associated with cadmium exposure. Regul. Toxicol. Pharmacol. 2022, 131, 105155. [Google Scholar] [CrossRef]
- Milton, A.H.; Hussain, S.; Akter, S.; Rahman, M.; Mouly, T.A.; Mitchell, K. A Review of the Effects of Chronic Arsenic Exposure on Adverse Pregnancy Outcomes. Int. J. Environ. Res. Public Health 2017, 14, 556. [Google Scholar] [CrossRef]
- Zhong, Q.; Cui, Y.; Wu, H.; Niu, Q.; Lu, X.; Wang, L.; Huang, F. Association of maternal arsenic exposure with birth size: A systematic review and meta-analysis. Environ. Toxicol. Pharmacol. 2019, 69, 129–136. [Google Scholar] [CrossRef]
- Khoshhali, M.; Rafiei, N.; Farajzadegan, Z.; Shoshtari-Yeganeh, B.; Kelishadi, R. Maternal Exposure to Cadmium and Fetal Growth: A Systematic Review and Meta-Analysis. Biol. Trace Elem. Res. 2020, 195, 9–19. [Google Scholar] [CrossRef]
- Huang, S.; Kuang, J.; Zhou, F.; Jia, Q.; Lu, Q.; Feng, C.; Yang, W.; Fan, G. The association between prenatal cadmium exposure and birth weight: A systematic review and meta-analysis of available evidence. Environ. Pollut. 2019, 251, 699–707. [Google Scholar] [CrossRef]
- Wang, D.; Fu, X.; Zhang, J.; Xu, C.; Hu, Q.; Lin, W. Association between blood lead level during pregnancy and birth weight: A meta-analysis. Am. J. Ind. Med. 2020, 63, 1085–1094. [Google Scholar] [CrossRef]
- Howe, C.G.; Nozadi, S.S.; Garcia, E.; O’Connor, T.G.; Starling, A.P.; Farzan, S.F.; Jackson, B.P.; Madan, J.C.; Alshawabkeh, A.N.; Cordero, J.F.; et al. Prenatal metal(loid) mixtures and birth weight for gestational age: A pooled analysis of three cohorts participating in the ECHO program. Environ. Int. 2022, 161, 107102. [Google Scholar] [CrossRef]
- Habibian Sezavar, A.; Abyareh, M.; Fahimi, R.; Nyasulu, P.S.; Abyadeh, M. The association between maternal cadmium exposure and small for gestational age: A systematic review and meta-analysis. Int. J. Environ. Health Res. 2021, 32, 1469–1477. [Google Scholar] [CrossRef]
- Gaudineau, A. Prévalence, facteurs de risque et morbi-mortalité materno-fœtale des troubles de la croissance fœtale. J. Gynecol. Obstet. Biol. Reprod. 2013, 42, 895–910. [Google Scholar] [CrossRef]
- Dack, K.; Fell, M.; Taylor, C.M.; Havdahl, A.; Lewis, S.J. Mercury and Prenatal Growth: A Systematic Review. Int. J. Environ. Res. Public Health 2021, 18, 7140. [Google Scholar] [CrossRef]
- Gulson, B.; Mizon, K.; Korsch, M.; Taylor, A. Revisiting mobilisation of skeletal lead during pregnancy based on monthly sampling and cord/maternal blood lead relationships confirm placental transfer of lead. Arch. Toxicol. 2016, 90, 805–816. [Google Scholar] [CrossRef]
- Gardner, R.M.; Nermell, B.; Kippler, M.; Grandér, M.; Li, L.; Ekström, E.C.; Rahman, A.; Lönnerdal, B.; Hoque, A.M.; Vahter, M. Arsenic methylation efficiency increases during the first trimester of pregnancy independent of folate status. Reprod. Toxicol. 2011, 31, 210–218. [Google Scholar] [CrossRef]
- Kales, S.N.; Huyck, K.L.; Goldman, R.H. Elevated urine arsenic: Un-speciated results lead to unnecessary concern and further evaluations. J. Anal. Toxicol. 2006, 30, 80–85. [Google Scholar] [CrossRef]
- Yim, G.; Wang, Y.; Howe, C.G.; Romano, M.E. Exposure to Metal Mixtures in Association with Cardiovascular Risk Factors and Outcomes: A Scoping Review. Toxics 2022, 10, 116. [Google Scholar] [CrossRef]
- Laine, J.E.; Ray, P.; Bodnar, W.; Cable, P.H.; Boggess, K.; Offenbacher, S.; Fry, R.C. Placental Cadmium Levels Are Associated with Increased Preeclampsia Risk. PLoS ONE 2015, 10, e0139341. [Google Scholar] [CrossRef]
- Kippler, M.; Hoque, A.M.; Raqib, R.; Ohrvik, H.; Ekström, E.C.; Vahter, M. Accumulation of cadmium in human placenta interacts with the transport of micronutrients to the fetus. Toxicol. Lett. 2010, 192, 162–168. [Google Scholar] [CrossRef] [PubMed]
| Outcome | Exposure | Sample Type | Measure of Association | Heterogeneity | n | Model | Reference | ||
|---|---|---|---|---|---|---|---|---|---|
| Effect Size | Effect Estimate (95% CI) | I2 | p | ||||||
| BW | Arsenic | maternal blood, maternal urine, maternal hair, drinking water | summary regression coefficient [g BW] in populations exposed to arsenic | −25.0 (−41.0 to −9.0) | 73.3% | 0.000 | 12 | REM | Zhong et al., 2019 [63] |
| BW | Cadmium | cord blood, maternal blood, maternal urine, placenta | Fisher-Z for the studies obtained from sensitivity analysis | −0.04 (−0.07 to −0.01) | 37.6% | 0.024 | 18 | FEM | Khoshhali et al., 2020 [64] |
| maternal blood, maternal urine, cord blood | summary-effect size: difference in BW [g] per 1 µg/L increment in cadmium | −42.11 (−69.03 to −15.18) | 64.6% | 0.006 | 8 | REM | Amegah et al., 2021 [58] | ||
| maternal blood | linear regression coefficient: difference in BW [g] associated with 50% increase in cadmium | −11.57 (−18.85 to −4.30) | 52.6% | 0.077 | 5 | REM | Huang et al., 2019 [65] | ||
| maternal urine | −6.15 (−10.81 to −1.49) | 0.0% | 0.507 | 5 | FEM | Huang et al., 2019 [65] | |||
| BW | Lead | cord blood | unadjusted standardized regression coefficients | −0.120 (−0.239 to −0.001) | 62.5% | 0.014 | 7 | REM | Wang et al., 2020 [66] |
| adjusted standardized regression coefficients | −0.017 (−0.045 to 0.012) | 0% | 0.838 | 4 | REM | Wang et al., 2020 [66] | |||
| maternal blood | unadjusted standardized regression coefficients | −0.094 (−0.157 to −0.030) | 40.0% | 0.101 | 9 | REM | Wang et al., 2020 [66] | ||
| adjusted standardized regression coefficients | −0.037 (−0.073 to −0.002) | 67.1% | 0.028 | 4 | REM | Wang et al., 2020 [66] | |||
| BW for GA | Cadmium | maternal urine | SD difference in BW for GA for a 25th to 75th percentile change in cadmium | 0.02 (−0.08 to 0.13) | - | - | 3 | FEM | Howe et al., 2022 [67] |
| BW for GA | Mercury | maternal urine | SD difference in BW for GA for a 25th to 75th percentile change in mercury | −0.09 (−0.20 to 0.03) | - | - | 3 | FEM | Howe et al., 2022 [67] |
| LBW | Cadmium | maternal blood | odds ratio | 1.13 (0.74 to 1.72) | 0% | 0.629 | 2 | FEM | Huang et al., 2019 [65] |
| maternal blood, maternal urine | relative risk | 1.21 (1.02 to 1.43) | 43.0% | 0.135 | 5 | REM | Amegah et al., 2021 [58] | ||
| maternal urine | odds ratio | 1.12 (1.03 to 1.22) | 6.9% | 0.341 | 2 | FEM | Huang et al., 2019 [65] | ||
| SGA | Cadmium | maternal blood | odds ratio | 1.31 (1.16 to 1.47) | 9% | 0.358 | 6 | FEM | Habibian et al., 2021 [68] |
| maternal blood, maternal urine | relative risk | 1.10 (0.96 to 1.27) | 0% | 0.464 | 3 | REM | Amegah et al., 2021 [58] | ||
| Outcome | Exposure | Sample Type | Measure of Association | Heterogeneity | n | Model | Reference | ||
|---|---|---|---|---|---|---|---|---|---|
| Effect Size | Effect Estimate (95% CI) | I2 | p | ||||||
| BL | Arsenic | maternal blood, maternal urine | summary regression coefficient [cm] | −0.12 (−0.17 to −0.07) | 0.0% | 0.917 | 5 | FEM | Zhong et al., 2019 [63] |
| BL | Cadmium | maternal blood, maternal urine, cord blood | Fisher-Z | −0.03 (−0.07 to 0.01) | 62.7% | 0.001 | 11 | REM | Khoshhali et al., 2020 [64] |
| maternal blood, maternal urine, cord blood | Fisher-Z, result of sensitivity analysis | −0.01 (−0.04 to 0.02) | 41.1% | 0.054 | 10 | REM | Khoshhali et al., 2020 [64] | ||
| maternal blood, maternal urine, cord blood | summary-effect size: difference [cm] per 1 µg/L increment in blood/urine cadmium levels | 0.00 (−0.08 to 0.09) | 14.9% | 0.318 | 4 | REM | Amegah et al., 2021 [58] | ||
| HC | Arsenic | maternal blood, maternal urine | summary regression coefficient [cm] | −0.12 (−0.24 to −0.01) | 59.6% | 0.030 | 6 | REM | Zhong et al., 2019 [63] |
| HC | Cadmium | maternal blood, maternal urine, cord blood | Fisher-Z | −0.04 (−0.09 to 0.01) | 61.3% | 0.003 | 8 | REM | Khoshhali et al., 2020 [64] |
| Fisher-Z, result of sensitivity analysis | −0.02 (−0.06 to 0.02) | 45.2% | 0.051 | 7 | REM | Khoshhali et al., 2020 [64] | |||
| maternal blood, maternal urine, cord blood | summary-effect size: difference [cm] per 1 µg/L increment in blood/urine cadmium levels | −0.11 (−0.18 to −0.03) | 0.0% | 0.802 | 4 | REM | Amegah et al., 2021 [58] | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dettwiler, M.; Flynn, A.C.; Rigutto-Farebrother, J. Effects of Non-Essential “Toxic” Trace Elements on Pregnancy Outcomes: A Narrative Overview of Recent Literature Syntheses. Int. J. Environ. Res. Public Health 2023, 20, 5536. https://doi.org/10.3390/ijerph20085536
Dettwiler M, Flynn AC, Rigutto-Farebrother J. Effects of Non-Essential “Toxic” Trace Elements on Pregnancy Outcomes: A Narrative Overview of Recent Literature Syntheses. International Journal of Environmental Research and Public Health. 2023; 20(8):5536. https://doi.org/10.3390/ijerph20085536
Chicago/Turabian StyleDettwiler, Maria, Angela C. Flynn, and Jessica Rigutto-Farebrother. 2023. "Effects of Non-Essential “Toxic” Trace Elements on Pregnancy Outcomes: A Narrative Overview of Recent Literature Syntheses" International Journal of Environmental Research and Public Health 20, no. 8: 5536. https://doi.org/10.3390/ijerph20085536
APA StyleDettwiler, M., Flynn, A. C., & Rigutto-Farebrother, J. (2023). Effects of Non-Essential “Toxic” Trace Elements on Pregnancy Outcomes: A Narrative Overview of Recent Literature Syntheses. International Journal of Environmental Research and Public Health, 20(8), 5536. https://doi.org/10.3390/ijerph20085536






