Effectiveness of a Self-Decontaminating Coating Containing Usnic Acid in Reducing Environmental Microbial Load in Tertiary-Care Hospitals
Abstract
1. Introduction
2. Material and Methods
2.1. Setting
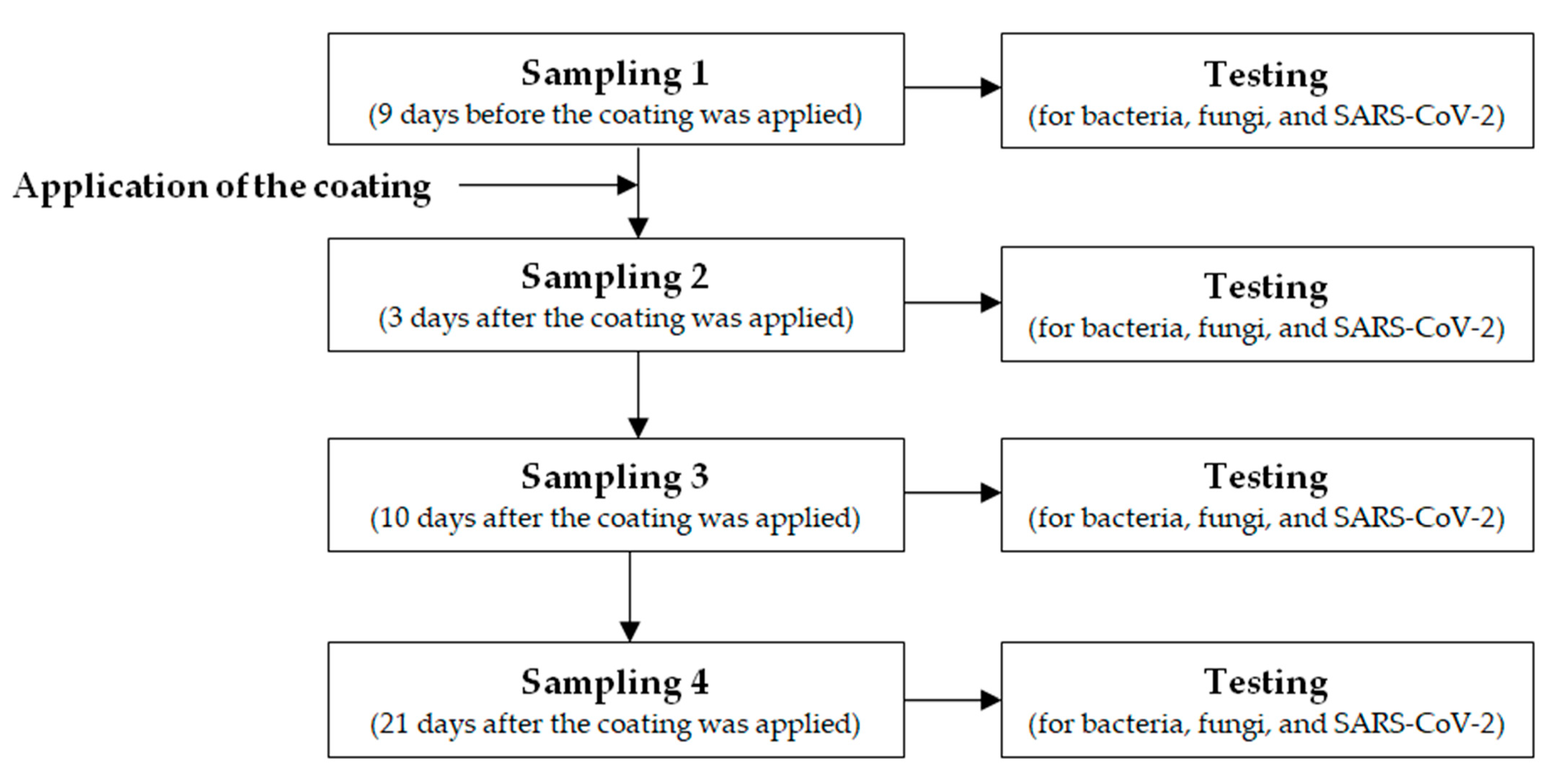
2.2. Application of the Coating
2.3. Environmental Investigation
2.4. Laboratory Testing
2.5. Statistical Analysis
3. Results
3.1. Sampling Phase 1
3.2. Sampling Phase 2
3.3. Sampling Phase 3
3.4. Sampling Phase 4
3.5. Antimicrobial Effectiveness of the Coating
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. No Time to Wait: Securing the Future from Drug-Resistant Infections Report to the Secretary-General of the United Nations.2019. Available online: https://www.who.int/publications/i/item/no-time-to-wait-securing-the-future-from-drug-resistant-infections (accessed on 30 March 2023).
- European Centre for Disease Prevention and Control. Point Prevalence Survey of Healthcare-Associated Infections and Antimicrobial Use in European Acute Care Hospitals. Technical Document. Available online: https://www.ecdc.europa.eu/sites/portal/files/media/en/publications/Publications/PPS-HAI-antimicrobial-use-EU-acute-care-hospitals-V5-3.pdf (accessed on 30 March 2023).
- Maltezou, H.C.; Kontopidou, F.; Dedoukou, X.; Katerelos, P.; Gourgoulis, G.M.; Tsonou, P.; Maragos, A.; Gargalianos, P.; Gikas, A.; Gogos, C.; et al. Action Plan to combat infections due to carbapenem-resistant, Gram-negative pathogens in acute-care hospitals in Greece. J. Glob. Antimicrob. Resist. 2014, 2, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Papanikolopoulou, A.; Maltezou, H.C.; Gargalianos-Kakolyris, P.; Michou, I.; Kalofissoudis, Y.; Moussas, N.; Pantazis, N.; Kotteas, E.; Syrigos, K.N.; Pantos, C.; et al. Central line-associated bloodstream infections, multidrug-resistant bacteremias and infection control interventions: A six-year time-series analysis in a tertiary-care hospital in Greece. J. Hosp. Infect. 2022, 23, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Hota, B. Contamination, disinfection, and cross-colonization: Are hospital surfaces reservoirs for nosocomial infection? Clin. Infect. Dis. 2004, 39, 1182–1189. [Google Scholar] [PubMed]
- Querido, M.M.; Aguiar, L.; Neves, P.; Pereira, C.C.; Teixera, J.P. Self-disinfecting surfaces and infection control. Colloids Surf. B Biointerfaces 2019, 178, 8–21. [Google Scholar] [CrossRef] [PubMed]
- Maltezou, H.C. Metallo-beta-lactamases in Gram-negative bacteria: Introducing the era of pan-resistance? Int. J. Antimicrob. Agents 2009, 33, 405.e1–405.e7. [Google Scholar] [CrossRef] [PubMed]
- Volling, C.; Thomas, S.; Johnstone, J.; Maltezou, H.C.; Mertz, D.; Stuart, R.; Jamal, A.; Kandel, C.; Ahangari, N.; Coleman, B.; et al. Development of a tool to assess evidence for causality in studies implicating sink drains as a reservoir for hospital-acquiredgammaproteobacterial infection. J. Hosp. Infect. 2020, 106, 454–464. [Google Scholar] [CrossRef]
- Dancer, S.J.; Adams, C.E.; Smith, J.; Pichon, B.; Kearns, A.; Morrison, D. Tracking Staphylococcus aureus in the intensive care unit using whole-genome sequencing. J. Hosp. Infect. 2019, 103, 13–20. [Google Scholar] [CrossRef]
- Yan, Z.; Zhou, Y.; Du, M.; Bai, Y.; Liu, B.; Gong, M.; Song, H.; Tong, Y.; Liu, Y. Prospective investigation of carbapenem-resistant Klebsiella pneumoniae transmission among the staff, environment and patients in five major intensive care units, Beijing. J. Hosp. Infect. 2019, 101, 150–157. [Google Scholar] [CrossRef]
- Moore, G.; Cookson, B.; Gordon, N.C.; Jackson, R.; Kearns, A.; Singleton, J.; Smyth, D.; Wilson, A. Whole-genome sequencing in hierarchy with pulsedfield gel electrophoresis: The utility of this approach to establish possible sources of MRSA cross-transmission. J. Hosp. Infect. 2015, 90, 38–45. [Google Scholar] [CrossRef]
- Vella, F.; Senia, P.; Ceccarelli, M.; Vitale, E.; Maltezou, H.C.; Taibi, R.; Lleshi, A.; Rullo, E.V.; Pellicanò, G.F.; Rapisarda, V.; et al. Transmission mode associated with coronavirus disease 2019: A review. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 7889–7904. [Google Scholar]
- Kim, T.; Jin, C.E.; Sung, H.; Koo, B.; Park, J.; Kim, S.M.; Kim, J.; Chong, Y.; Lee, S.-O.; Choi, S.-H.; et al. Molecular epidemiology and environmental contamination during an outbreak of parainfluenza virus 3 in a haematology ward. J. Hosp. Infect. 2017, 97, 403–413. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Otter, J.A.; Price, J.R.; Cimpeanu, C.; Garcia, D.M.; Kinross, J.; Boshier, P.R.; Mason, S.; Bolt, F.; Holmes, A.H.; et al. Investigating SARS-CoV-2 surface and air contamination in an acute healthcare setting during the peak of the COVID-19 pandemic in London. Clin. Infect. Dis. 2021, 73, e1870–e1877. [Google Scholar] [CrossRef] [PubMed]
- Moore, G.; Rickard, H.; Stevenson, D.; Aranega-Bou, P.; Pitman, J.; Crook, A.; Davies, K.; Spencer, A.; Burton, C.; Easterbrook, L.; et al. Detection of SARS-CoV-2 within the healthcare environment: A multi-centre study conducted during the first wave of the COVID-19 outbreak in England. J. Hosp. Infect. 2021, 108, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Van Doremalen, N.; Bushmaker, T.; Morris, D.H.; Holbrook, M.G.; Gamble, A.; Williamson, B.N.; Tamin, A.; Harcourt, J.L.; Thornburg, N.J.; Gerber, S.I.; et al. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N. Engl. J. Med. 2020, 382, 1564–1567. [Google Scholar] [CrossRef]
- Assadian, O.; Harbarth, S.; Vos, M.; Knobloch, J.K.; Asensio, A.; Widmer, A.F. Practical recommendations for routine cleaning and disinfection procedures in healthcare institutions: A narrative review. J. Hosp. Infect. 2021, 113, 104–114. [Google Scholar] [CrossRef]
- Choi, H.; Chatterjee, P.; Coppin, J.D.; Martel, J.A.; Hwang, M.; Jinadatha, C.; Sharma, V.K. Current understanding of the surface contamination and contact transmission of SARS-CoV-2 in healthcare settings. Environ. Chem. Lett. 2021, 19, 1935–1944. [Google Scholar] [CrossRef]
- Francolini, I.; Taresco, V.; Crisante, F.; Martinelli, A.; D’Ilario, L.; Piozzi, A. Water soluble usnicacid-polyacrylamide complexes with enhanced antimicrobial activity against Staphylococcus epidermidis. Int. J. Mol. Sci. 2013, 14, 7356–7369. [Google Scholar] [CrossRef]
- Gupta, V.K.; Verma, S.; Gupta, S.; Singh, A.; Pal, A.; Srivastava, S.K.; Singh, S.C.; Darokar, M.P. Membrane-damaging potential of natural L-(-)-usnic acid in Staphylococcus aureus. Eur. J. Clin. Microbiol. Infect. Dis. 2012, 31, 3375–3383. [Google Scholar] [CrossRef]
- Kartsev, V.; Lichitsky, B.; Geronikaki, A.; Petrou, A.; Smiljikovic, M.; Kostc, M.; Radanovic, O.; Soković, M. Design, synthesis and antimicrobial activity of usnic acid derivatives. Med. Chem. Commun. 2018, 9, 870. [Google Scholar] [CrossRef]
- Nithyanand, P.; BeemaShafreen, R.M.; Muthamil, S.; Karutha Pandian, S. Usnicacid inhibits biofilm formation and virulent morphological traits of Candida albicans. Microbiol. Res. 2015, 179, 20–28. [Google Scholar] [CrossRef]
- Pompilio, A.; Riviello, A.; Crocetta, V.; Di Giuseppe, F.; Pomponio, S.; Sulpizio, M.; Di Ilio, C.; Angelucci, S.; Barone, L.; Di Giulio, A.; et al. Evaluation of antibacterial and antibiofilm mechanisms by usnic acid against methicillin-resistant Staphylococcus aureus. Future Microbiol. 2016, 11, 1315–1338. [Google Scholar] [CrossRef]
- Tozatti, M.G.; Ferreira, D.S.; Flauzino, L.G.; MoraesTda, S.; Martins, C.H.; Groppo, M.; Márcio, L.; Silvaa, A.E.; Januárioa, A.H.; Paulettia, P.M.; et al. Activity of the lichen Usneasteineri and its major metabolites against Gram-positive, multidrug-resistant bacteria. Nat. Prod. Commun. 2016, 11, 493–496. [Google Scholar]
- Elo, H.; Matikainen, J.; Pelttari, E. Potent activity of the lichen antibiotic (+)-usnic acid against clinical isolates of vancomycin-resistant enterococci and methicillin-resistant Staphylococcus aureus. Naturwissenschaften 2007, 94, 465–468. [Google Scholar] [CrossRef]
- Pires, R.H.; Lucarini, R.; Mendes-Giannini, M.J. Effect of usnic acid on Candida orthopsilosis and C. parapsilosis. Antimicrob. Agents Chemother. 2012, 56, 595–597. [Google Scholar] [CrossRef]
- Shtro, A.A.; Zarubaev, V.V.; Luzina, O.A.; Sokolov, D.N.; Salakhutdinov, N.F. Derivatives of usnicacid inhibit broad range of influenza viruses and protect mice from lethal influenza infection. Antivir. Chem. Chemother. 2015, 24, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Sokolov, D.N.; Zarubaev, V.V.; Shtro, A.A.; Polovinka, M.P.; Luzina, O.A.; Komarova, N.I.; Salakhutdinov, N.F.; Kiselev, O.I. Anti-viral activity of (−)- and (+)-usnic acids and their derivatives against influenza virus A(H1N1)2009. Bioorg. Med. Chem. Lett. 2012, 22, 7060–7064. [Google Scholar] [CrossRef]
- Vestatis. Risultati e Certificazioni. Available online: https://www.vestatis.com/it/efficacia-certificata.html (accessed on 30 March 2023). (In Italian).
- Brauge, T.; Barre, L.; Leleu, G.; André, S.; Denis, C.; Hanin, A.; Frémaux, B.; Guilbaud, M.; Herry, J.-M.; Oulahal, N.; et al. European survey and evaluation of sampling methods recommended by the standard EN ISO 18593 for the detection of Listeria monocytogenes and Pseudomonas fluorescens on industrial surfaces. FEMS Microbiol. Lett. 2020, 367, fnaa057. [Google Scholar] [CrossRef]
- Corman, V.M.; Landt, O.; Kaiser, M.; Molenkamp, R.; Meijer, A.; Chu, D.K.W.; Bleicker, T.; Brünink, S.; Schneider, J.; Schmidt, M.L.; et al. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Eurosurveillance 2020, 25, 2000045. [Google Scholar] [CrossRef]
- Alcoba-Florez, J.; Gil-Campesino, H.; García-Martínez de Artola, D.; Díez-Gil, O.; Valenzuela-Fernández, A.; González-Montelongo, R.; Ciuffreda, L.; Flores, C. Increasing SARS-CoV-2 RT-qPCR testing capacity by sample pooling. Int. J. Infect. Dis. 2021, 103, 19–22. [Google Scholar] [CrossRef] [PubMed]
- Tamini, A.H.; Carlino, S.; Gerba, C.P. Long-term efficacy of a self-disinfecting coating in an intensive care unit. Am. J. Infect. Control 2014, 42, 1178–1181. [Google Scholar] [CrossRef] [PubMed]
- Francolini, I.; Norris, P.; Piozzi, A.; Donelli, G.; Stoodley, P. Usnic acid, a natural antimicrobial agent able to inhibit bacterial biofilm formation on polymer surfaces. Antimicrob. Agents Chemother. 2004, 48, 4360–4365. [Google Scholar] [CrossRef] [PubMed]
- Martinelli, A.; Bakry, A.; D’Ilario, L.; Francolini, I.; Piozzi, A.; Taresco, V. Release behavior and antibiofilm activity of usnicacid-loaded carboxylated poly(L-lactide) microparticles. Eur. J. Pharm. Biopharm. 2014, 88, 415–423. [Google Scholar] [CrossRef] [PubMed]
- Pandit, S.; Rahimi, S.; Derouiche, A.; Boulaoued, A.; Mijakovic, I. Sustained release of usnicacid from graphene coatings ensures long term antibiofilm protection. Sci. Rep. 2021, 11, 9956. [Google Scholar] [CrossRef] [PubMed]
- Maltezou, H.C.; Horefti, E.; Papamichalopoulos, N.; Tseroni, M.; Ioannidis, A.; Angelakis, E.; Chatzipanagiotou, S. Antimicrobial effectiveness of an usnic-acid-containing self-decontaminating coating on underground metro surfaces in Athens. Microorganisms 2022, 10, 2233. [Google Scholar] [CrossRef]
- Fusco, F.M.; Schilling, S.; De Iaco, G.; Brodt, H.R.; Brouqui, P.; Maltezou, H.C.; Bannister, B.; Gottschalk, R.; Thomson, G.; Puro, V.; et al. Infection control management of patients with suspected highly infectious diseases in emergency departments: Data from a survey in 41 facilities in 14 European countries. BMC Infect. Dis. 2012, 12, 27. [Google Scholar] [CrossRef]
- Maltezou, H.C.; Tseroni, M.; Daflos, C.; Anastassopoulou, C.; Vasilogiannakopoulos, A.; Daligarou, O.; Panagiotou, M.; Botsa, E.; Spanakis, N.; Lourida, A.; et al. Environmental testing for SARS-CoV-2 in three tertiary-care hospitals during the peak of the third COVID-19 wave. Am. J. Infect. Control 2021, 49, 1435–1437. [Google Scholar] [CrossRef] [PubMed]
- Ben-Samuel, A.; Brosh-Nissimov, T.; Glinert, I.; Bar-David, E.; Sittner, A.; Poni, R.; Cohen, R.; Achdout, H.; Tamir, H.; Yahalom-Ronen, Y.; et al. Detection and infectivity potential of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) environmental contamination in isolation units and quarantine facilities. Clin. Microbiol. Infect. 2020, 6, 1658–1662. [Google Scholar] [CrossRef]
- Liu, Y.; Li, T.; Deng, Y.; Liu, S.; Zhang, D.; Li, H.; Wang, X.; Jia, L.; Han, J.; Bei, Z.; et al. Stability of SARS-CoV-2 on environmental surfaces and in human excreta. J. Hosp. Infect. 2021, 107, 105–107. [Google Scholar] [CrossRef]
- Geng, Y.; Wang, Y. Stability and transmissibility of SARS-CoV-2 in the environment. J. Med. Virol. 2022, 95, e28103. [Google Scholar] [CrossRef]
| Sampling Site | Sampling 1 * | Sampling 2 * | Sampling 3 * | Sampling 4 * |
|---|---|---|---|---|
| Emergency Department | 12/15 | 0/15 | 0/15 | 0/15 |
| COVID-19 patients’ rooms | 6/8 | 0/8 | 0/8 | 0/8 |
| Intensive Care Unit | 12/20 | 2/20 | 2/20 | 1/20 |
| Radiology and CT Department | 10/12 | 2/12 | 0/12 | 0/12 |
| Stairs and elevators | 13/14 | 0/14 | 1/14 | 0/14 |
| Sampling Site | Sampling 1 * | Sampling 2 * | Sampling 3 * | Sampling 4 * |
|---|---|---|---|---|
| Emergency Department | 3/15 | 0/15 | 0/15 | 0/15 |
| COVID-19 patients’ rooms | 0/8 | 0/8 | 0/8 | 0/8 |
| Intensive Care Unit | 3/20 | 0/20 | 0/20 | 0/20 |
| Radiology and CT Department | 1/12 | 0/12 | 0/12 | 0/12 |
| Stairs and elevators | 2/14 | 0/14 | 0/14 | 0/14 |
| Hospital A | Sampling 1 * | Sampling 2 * | Sampling 3 * | Sampling 4 * |
|---|---|---|---|---|
| Emergency Department | 0/15 | 0/15 | 0/15 | 0/15 |
| COVID-19 patients’ rooms | 1/8 | 0/8 | 0/8 | 0/8 |
| Intensive Care Unit | 0/20 | 0/20 | 0/20 | 0/20 |
| Radiology and CT Department | 1/12 | 0/12 | 0/12 | 0/12 |
| Stairs and elevators | 0/14 | 0/14 | 1/14 | 0/14 |
| Hospital B | Sampling 1 * | Sampling 2 * | Sampling 3 * | Sampling 4 * |
| Emergency Department | 3/19 | 0/19 | 0/19 | 0/19 |
| COVID-19 patients’ rooms | 2/6 | 0/6 | 0/6 | 0/6 |
| Intensive Care Unit | 0/20 | 0/20 | 0/20 | 0/20 |
| Radiology and CT Department | 1/11 | 0/11 | 0/11 | 0/11 |
| Stairs and elevators | 2/14 | 0/14 | 0/14 | 0/14 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maltezou, H.C.; Papamichalopoulos, N.; Horefti, E.; Tseroni, M.; Karapanou, A.; Gamaletsou, M.N.; Veneti, L.; Ioannidis, A.; Panagiotou, M.; Dimitroulia, E.; et al. Effectiveness of a Self-Decontaminating Coating Containing Usnic Acid in Reducing Environmental Microbial Load in Tertiary-Care Hospitals. Int. J. Environ. Res. Public Health 2023, 20, 5434. https://doi.org/10.3390/ijerph20085434
Maltezou HC, Papamichalopoulos N, Horefti E, Tseroni M, Karapanou A, Gamaletsou MN, Veneti L, Ioannidis A, Panagiotou M, Dimitroulia E, et al. Effectiveness of a Self-Decontaminating Coating Containing Usnic Acid in Reducing Environmental Microbial Load in Tertiary-Care Hospitals. International Journal of Environmental Research and Public Health. 2023; 20(8):5434. https://doi.org/10.3390/ijerph20085434
Chicago/Turabian StyleMaltezou, Helena C., Nikolaos Papamichalopoulos, Elina Horefti, Maria Tseroni, Amalia Karapanou, Maria N. Gamaletsou, Lamprini Veneti, Anastasios Ioannidis, Marina Panagiotou, Evangelia Dimitroulia, and et al. 2023. "Effectiveness of a Self-Decontaminating Coating Containing Usnic Acid in Reducing Environmental Microbial Load in Tertiary-Care Hospitals" International Journal of Environmental Research and Public Health 20, no. 8: 5434. https://doi.org/10.3390/ijerph20085434
APA StyleMaltezou, H. C., Papamichalopoulos, N., Horefti, E., Tseroni, M., Karapanou, A., Gamaletsou, M. N., Veneti, L., Ioannidis, A., Panagiotou, M., Dimitroulia, E., Vasilogiannakopoulos, A., Angelakis, E., Chatzipanagiotou, S., & Sipsas, N. V. (2023). Effectiveness of a Self-Decontaminating Coating Containing Usnic Acid in Reducing Environmental Microbial Load in Tertiary-Care Hospitals. International Journal of Environmental Research and Public Health, 20(8), 5434. https://doi.org/10.3390/ijerph20085434







