Medication Assessment in an Older Population during Acute Care Hospitalization and Its Effect on the Anticholinergic Burden: A Prospective Cohort Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Subjects
2.2. Sample Size
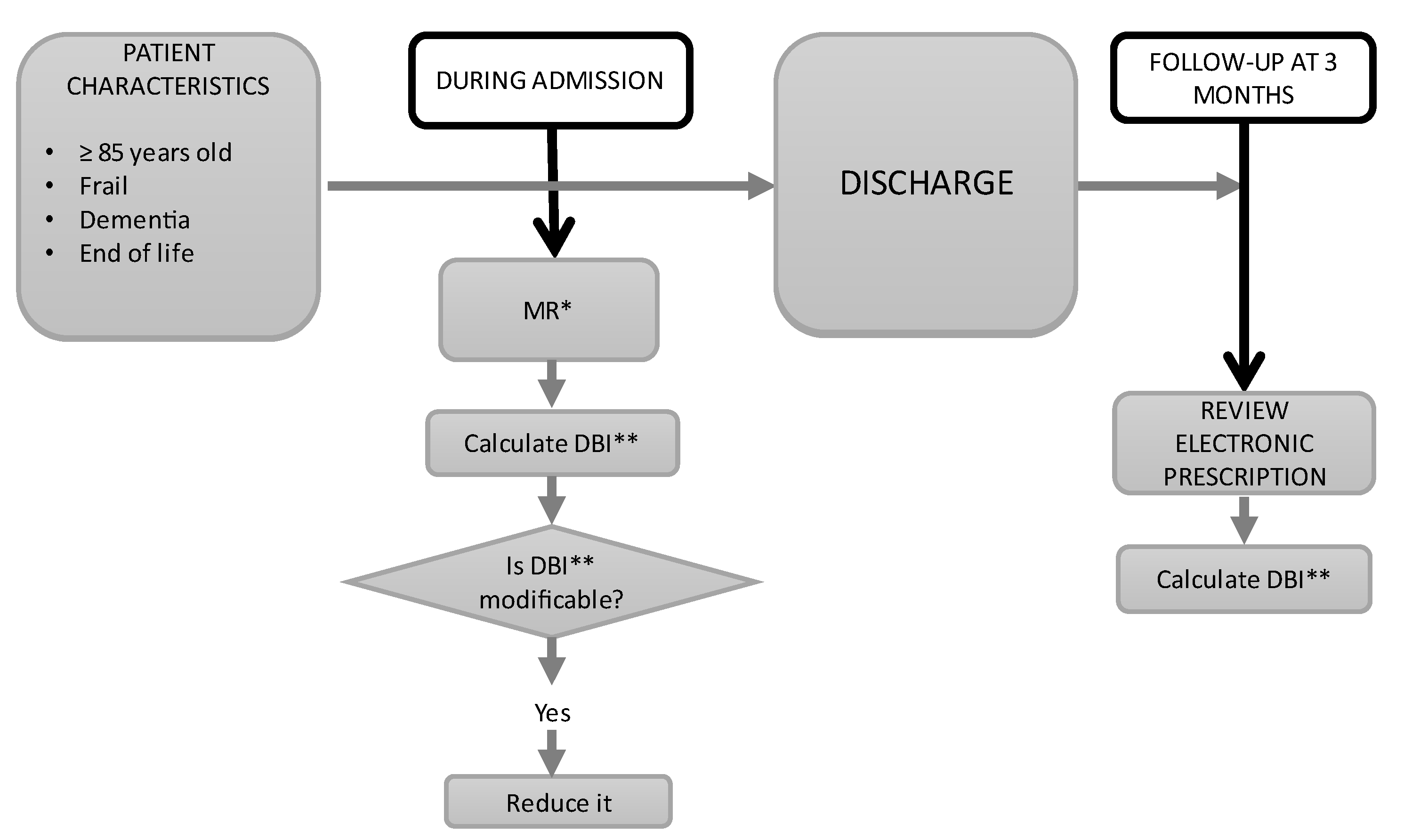
2.3. Pharmacological Assessment and Intervention
2.4. Outcome Measures
- (a)
- Sociodemographic data: age, gender, and place of residence;
- (b)
- Functional data (patient’s global health status): daily living activities quantified with the Barthel index [38]; various instrumental activities of daily living, such as handling finances, using a phone, and handling medications; and geriatric syndromes, such as falls, delirium, insomnia, dysphagia, constipation, urinary incontinence, and malnutrition [39,40];
- (c)
- Clinical data: comorbidities assessed using expanded diagnostic clusters with the Johns Hopkins University ACG System and Charlson Index [41] and cognitive assessment according to the Global Deterioration Scale—Functional Assessment Staging (GDS-FAST) for Alzheimer’s disease or the Clinical Dementia Rating scale in the case of other types of dementia [42];
- (d)
- Frailty: frailty was evaluated using the Frail-VIG index [36,43], which is based on a CGA and includes 25 items that assess functionality, cognition, social status, geriatric syndromes, and comorbidities. The Frail-VIG index enables the classification of patients into four groups according to their Frail-VIG scores: 0–0.19 (non-frail), 0.20–0.35 (mildly frail), 0.36–0.49 (moderately frail), and >0.50 (severely frail). In some analyses, the frailty variable was valued as a binary variable: non-frail (Frail-VIG score of 0–0.19) vs. frail (Frail-VIG score ≥ 0.20);
- (e)
- Complexity identification: chronic complex patients (CCPs) were defined as individuals in situations that included difficulties in their management and care, who needed to adopt specific individual plans owing to concurrent diseases, or who experienced difficulties in their utilization of healthcare services or in relation to their context according to Catalan Health Department criteria [44]. Identification of end-of-life (EOL) patients was undertaken using the NECPAL CCOMS-ICO© tool criteria [45]. These were patients who were considered to be in the final months or year of their life. The criteria used to identify them as EOL patients included: (a) identification as such by their primary care physician, (b) advanced disease criteria [45], or (c) Frail-VIG index > 0.50;
- (f)
- Therapeutic goals: in accordance with their baseline situation, patients were classified into groups with different goals: survival for patients with a robust baseline situation; functional for patients with an intermediate situation; and symptomatic for vulnerable patients (including end-of-life patients);
- (g)
- Pharmacological data: these data included the prevalence of polypharmacy (≥5 drugs) and severe polypharmacy (≥10 drugs) [20]. An MR was carried out to detect inappropriate prescriptions (IPs) among the most prevalent chronic conditions using current evidence and by applying the latest guidelines and recommendations issued by scientific societies [31,33,34]. Therapeutic complexity was qualified according to the Medication Regimen Complexity Index (MRCI) [46].
2.5. Ethical Considerations
2.6. Statistical Analysis
3. Results
3.1. Baseline Characteristics of the Study Population
3.2. Medication Review
3.3. Anticholinergic and Sedative Burden
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fried, L.P.; Tangen, C.M.; Walston, J.; Newman, A.B.; Hirsch, C.; Gottdiener, J.; Seeman, T.; Tracy, R.; Kop, W.J.; Burke, G.; et al. Frailty in older adults: Evidence for a phenotype. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2001, 56, M146–M156. [Google Scholar] [CrossRef]
- Espaulella-Ferrer, M.; Espaulella-Panicot, J.; Noell-Boix, R.; Casals-Zorita, M.; Ferrer-Sola, M.; Puigoriol-Juvanteny, E.; Cullell-Dalmau, M.; Otero-Viñas, M. Assessment of frailty in elderly patients attending a multidisciplinary wound care centre: A cohort study. BMC Geriatr. 2021, 21, 727. [Google Scholar] [CrossRef]
- Hilmer, S.; Gnjidic, D. Prescribing for frail older people. Aust. Prescr. 2017, 40, 174–178. [Google Scholar] [CrossRef]
- Herr, M.; Sirven, N.; Grondin, H.; Pichetti, S.; Sermet, C. Frailty, polypharmacy, and potentially inappropriate medications in old people: Findings in a representative sample of the French population. Eur. J. Clin. Pharmacol. 2017, 73, 1165–1172. [Google Scholar] [CrossRef]
- Hilmer, S.N.; Kirkpatrick, C.M.J. New Horizons in the impact of frailty on pharmacokinetics: Latest developments. Age Ageing 2021, 50, 1054–1063. [Google Scholar] [CrossRef]
- Landi, F.; Dell’Aquila, G.; Collamati, A.; Martone, A.M.; Zuliani, G.; Gasperini, B.; Eusebi, P.; Lattanzio, F.; Cherubini, A. Anticholinergic drug use and negative outcomes among the frail elderly population living in a nursing home. J. Am. Med. Dir. Assoc. 2014, 15, 825–829. [Google Scholar] [CrossRef]
- Landi, F.; Russo, A.; Liperoti, R.; Cesari, M.; Barillaro, C.; Pahor, M.; Bernabei, R.; Onder, G. Anticholinergic Drugs and Physical Function Among Frail Elderly Population. Clin. Pharmacol. Ther. 2006, 81, 235–241. [Google Scholar] [CrossRef]
- Collamati, A.; Martone, A.M.; Poscia, A.; Brandi, V.; Celi, M.; Marzetti, E.; Cherubini, A.; Landi, F. Anticholinergic drugs and negative outcomes in the older population: From biological plausibility to clinical evidence. Aging Clin. Exp. Res. 2016, 28, 25–35. [Google Scholar] [CrossRef]
- Grossi, C.M.; Richardson, K.; Savva, G.M.; Fox, C.; Arthur, A.; Loke, Y.K.; Steel, N.; Brayne, C.; Matthews, F.E.; Robinson, L.; et al. Increasing prevalence of anticholinergic medication use in older people in England over 20 years: Cognitive function and ageing study I and II. BMC Geriatr. 2020, 20, 267. [Google Scholar] [CrossRef]
- KRichardson, K.; Bennett, K.; Maidment, I.D.; Fox, C.; Smithard, D.; Kenny, R.A. Use of Medications with Anticholinergic Activity and Self-Reported Injurious Falls in Older Community-Dwelling Adults. J. Am. Geriatr. Soc. 2015, 63, 1561–1569. [Google Scholar] [CrossRef]
- Fox, C.; Smith, T.; Maidment, I.; Chan, W.-Y.; Bua, N.; Myint, P.K.; Boustani, M.; Kwok, C.S.; Glover, M.; Koopmans, I.; et al. Effect of medications with anti-cholinergic properties on cognitive function, delirium, physical function and mortality: A systematic review. Age Ageing 2014, 43, 604–615. [Google Scholar] [CrossRef]
- Gray, S.L.; Anderson, M.L.; Dublin, S.; Hanlon, J.T.; Hubbard, R.; Walker, R.; Yu, O.; Crane, P.K.; Larson, E.B. Cumulative use of strong anticholinergics and incident dementia: A prospective cohort study. AMA Intern. Med. 2015, 175, 401–407. [Google Scholar] [CrossRef]
- Gerretsen, P.; Pollock, B.G. Drugs with anticholinergic properties: A current perspective on use and safety. Expert Opin. Drug Saf. 2011, 10, 751–765. [Google Scholar] [CrossRef]
- Hilmer, S.N. A Drug Burden Index to Define the Functional Burden of Medications in Older People. Arch. Intern. Med. 2007, 167, 781–787. [Google Scholar] [CrossRef]
- Vetrano, D.L.; La Carpia, D.; Grande, G.; Casucci, P.; Bacelli, T.; Bernabei, R.; Onder, G. Anticholinergic Medication Burden and 5-Year Risk of Hospitalization and Death in Nursing Home Elderly Residents with Coronary Artery Disease. J. Am. Med. Dir. Assoc. 2016, 17, 1056–1059. [Google Scholar] [CrossRef]
- Palmer, R.M. The Acute Care for Elders unit model of care. Geriatrics 2018, 3, 59. [Google Scholar] [CrossRef]
- Griese-Mammen, N.; Hersberger, K.E.; Messerli, M.; Leikola, S.; Horvat, N.; van Mil, J.W.F.; Kos, M. PCNE definition of medication review: Reaching agreement. Int. J. Clin. Pharm. 2018, 40, 1199–1208. [Google Scholar] [CrossRef]
- Chowdhury, T.P.; Starr, R.; Brennan, M.; Knee, A.; Ehresman, M.; Velayutham, L.; Malanowski, A.J.; Courtney, H.-A.; Stefan, M.S. A Quality Improvement Initiative to Improve Medication Management in an Acute Care for Elders Program through Integration of a Clinical Pharmacist. J. Pharm. Pract. 2020, 33, 55–62. [Google Scholar] [CrossRef]
- Duerden, M.; Avery, T.; Payne, R. Polypharmacy and Medicines Optimisation: Making It Safe and Sound. London: The King’s Fund. 2013. Available online: https://www.kingsfund.org.uk/sites/default/files/field/field_publication_file/polypharmacy-and-medicines-optimisation-kingsfund-nov13.pdf (accessed on 4 December 2022).
- Gnjidic, D.; Hilmer, S.N.; Blyth, F.M.; Naganathan, V.; Waite, L.; Seibel, M.J.; McLachlan, A.J.; Cumming, R.G.; Handelsman, D.J.; Le Couteur, D.G. Polypharmacy cutoff and outcomes: Five or more medicines were used to identify community-dwelling older men at risk of different adverse outcomes. J. Clin. Epidemiol. 2012, 65, 989–995. [Google Scholar] [CrossRef]
- National Institute of Health and Care Excellence. Medicines Optimisation: The Safe and Effective Use of Medicines to Enable the Best Possible Outcomes; NICE Webside: London, UK, 2015; Available online: www.nice.org.uk/guidance/ng5 (accessed on 21 January 2022).
- Hernández-Rodríguez, M.Á.; Sempere-Verdú, E.; Vicens, C.; González-Rubio, F.; Miguel-García, F.; Palop-Larrea, V.; Orueta-Sánchez, R.; Esteban-Jiménez, Ó.; Sempere-Manuel, M.; Arroyo-Aniés, M.P.; et al. Evolution of polypharmacy in a spanish population (2005–2015): A database study. Pharmacoepidemiol. Drug Saf. 2020, 29, 433–443. [Google Scholar] [CrossRef]
- Spinewine, A.; Schmader, K.E.; Barber, N.; Hughes, C.; Lapane, K.L.; Swine, C.; Hanlon, J.T. Appropriate prescribing in elderly people: How well can it be measured and optimised? Lancet 2007, 370, 173–184. [Google Scholar] [CrossRef] [PubMed]
- Reallon, E.; Chavent, B.; Gervais, F.; Dauphinot, V.; Vernaudon, J.; Krolak-Salmon, P.; Mouchoux, C.; Novais, T. Medication exposure and frailty in older community-dwelling patients: A cross-sectional study. Int. J. Clin. Pharm. 2020, 42, 508–514. [Google Scholar] [CrossRef]
- Scott, I.A.; Hilmer, S.N.; Reeve, E.; Potter, K.; Le Couteur, D.; Rigby, D.; Gnjidic, D.; Del Mar, C.B.; Roughead, E.E.; Page, A.; et al. Reducing Inappropriate Polypharmacy: The Process of Deprescribing. JAMA Intern. Med. 2015, 175, 827–834. [Google Scholar] [CrossRef]
- Sevilla-Sanchez, D.; Molist-Brunet, N.; Amblàs-Novellas, J.; Roura-Poch, P.; Espaulella-Panicot, J.; Codina-Jané, C. Adverse drug events in patients with advanced chronic conditions who have a prognosis of limited life expectancy at hospital admission. Eur. J. Clin. Pharmacol. 2017, 73, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Zerah, L.; Henrard, S.; Thevelin, S.; Feller, M.; Meyer-Massetti, C.; Knol, W.; Wilting, I.; O’Mahony, D.; Crowley, E.; Dalleur, O.; et al. Performance of a trigger tool for detecting drug-related hospital admissions in older people: Analysis from the OPERAM trial. Age Ageing 2022, 51, afab196. [Google Scholar] [CrossRef] [PubMed]
- Heaton, J.; Britten, N.; Krska, J.; Reeve, J. Person-centred medicines optimisation policy in England: An agenda for research on polypharmacy. Prim. Health Care Res. Dev. 2016, 18, 24–34. [Google Scholar] [CrossRef]
- Dautzenberg, L.; Bretagne, L.; Koek, H.L.; Tsokani, S.; Zevgiti, S.; Rodondi, N.; Scholten, R.J.P.M.; Rutjes, A.W.; Di Nisio, M.; Raijmann, R.C.M.A.; et al. Medication review interventions to reduce hospital readmissions in older people. J. Am. Geriatr. Soc. 2021, 69, 1646–1658. [Google Scholar] [CrossRef]
- Sergi, G.; De Rui, M.; Sarti, S.; Manzato, E. Polypharmacy in the Elderly Can Comprehensive Geriatric Assessment Reduce Inappropriate Medication Use? Drugs Aging 2011, 28, 509–519. [Google Scholar] [CrossRef]
- Espaulella-Panicot, J.; Molist-Brunet, N.; Sevilla-Sánchez, D.; González-Bueno, J.; Amblàs-Novellas, J.; Solà-Bonada, N.; Codina-Jané, C. Patient-centred prescription model to improve adequate prescription and therapeutic adherence in patients with multiple disorders. Rev. Esp. Geriatr. Gerontol. 2017, 52, 278–281. [Google Scholar] [CrossRef]
- Molist-Brunet, N.; Sevilla-Sánchez, D.; Puigoriol-Juvanteny, E.; Espaulella-Ferrer, M.; Amblàs-Novellas, J.; Espaulella-Panicot, J. Factors associated with the detection of inappropriate prescriptions in older people: A prospective cohort. Int. J. Environ. Res. Public Health 2021, 18, 11310. [Google Scholar] [CrossRef]
- Brunet, N.M.; Panicot, J.E.; Sevilla-Sánchez, D.; Novellas, J.A.; Jané, C.C.; Roset, J.A.; Gómez-Batiste, X. A patient-centered prescription model assessing the appropriateness of chronic drug therapy in older patients at the end of life. Eur. Geriatr. Med. 2015, 6, 565–569. [Google Scholar] [CrossRef]
- Molist-Brunet, N.; Sevilla-Sánchez, D.; Puigoriol-Juvanteny, E.; Bajo-Peñas, L.; Cantizano-Baldo, I.; Cabanas-Collell, L.; Espaulella-Panicot, J. Individualized Medication Review in Older People with Multimorbidity: A Comparative Analysis between Patients Living at Home and in a Nursing Home. Int. J. Environ. Res. Public Health 2022, 19, 3423. [Google Scholar] [CrossRef] [PubMed]
- Molist-Brunet, N.; Sevilla-Sánchez, D.; Puigoriol-Juvanteny, E.; Barneto-Soto, M.; González-Bueno, J.; Espaulella-Panicot, J. Improving individualized prescription in patients with multimorbidity through medication review. BMC Geriatr. 2022, 22, 417. [Google Scholar] [CrossRef]
- Amblàs-Novellas, J.; Martori, J.C.; Espaulella, J.; Oller, R.; Molist-Brunet, N.; Inzitari, M.; Romero-Ortuno, R. Frail-VIG index: A concise frailty evaluation tool for rapid geriatric assessment. BMC Geriatr. 2018, 18, 29. [Google Scholar] [CrossRef] [PubMed]
- O’Mahony, D.; O’Connor, M.N. Pharmacotherapy at the end-of-life. Age Ageing 2011, 40, 419–422. [Google Scholar] [CrossRef]
- Sainsbury, A.; Seebass, G.; Bansal, A.; Young, J.B. Reliability of the Barthel Index when used with older people. Age Ageing 2005, 34, 228–232. [Google Scholar] [CrossRef]
- Inouye, S.K.; Studenski, S.; Tinetti, M.E.; Kuchel, G.A. Geriatric Syndromes: Clinical, Research, and Policy Implications of a Core Geriatric Concept. J. Am. Geriatr. Soc. 2007, 55, 780–791. [Google Scholar] [CrossRef]
- Lawton, M.P.; Brody, E.M. Assessment of Older People: Self-Maintaining and Instrumental Activities of Daily Living. J. Am. Med. Assoc. 1949, 139, 474. [Google Scholar] [CrossRef]
- Brusselaers, N.; Lagergren, J. The Charlson Comorbidity Index in Registrybased Research. Methods Inf. Med. 2017, 56, 401–406. [Google Scholar]
- Reisberg, B.; Ferris, S.H.; De Leon, M.J.; Crook, T. The Global Deterioration Scale for assessment of primary degenerative dementia. Am. J. Psychiatry 1982, 139, 1136–1139. [Google Scholar] [CrossRef]
- Amblàs-Novellas, J.; Martori, J.C.; Brunet, N.M.; Oller, R.; Gómez-Batiste, X.; Panicot, J.E. Índice frágil-VIG: Diseño y evaluación de un índice de fragilidad basado en la Valoración Integral Geriátrica. Rev. Esp. Geriatr. Gerontol. 2016, 52, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Santaeugènia, S.J.; Contel, J.C.; Vela, E.; Cleries, M.; Amil, P.; Melendo-Azuela, E.M.; Gil-Sánchez, E.; Mir, V.; Amblàs-Novellas, J. Characteristics and Service Utilization by Complex Chronic and Advanced Chronic Patients in Catalonia: A Retrospective Seven-Year Cohort-Based Study of an Implemented Chronic Care Program. Int. J. Environ. Res. Public Health 2021, 18, 9473. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Batiste, X.; Martínez-Muñoz, M.; Blay, C.; Amblàs, J.; Vila, L.; Costa, X.; Espaulella, J.; Espinosa, J.; Constante, C.; Mitchell, G.K. Prevalence and characteristics of patients with advanced chronic conditions in need of palliative care in the general population: A cross-sectional study. Palliat. Med. 2014, 28, 302–311. [Google Scholar] [CrossRef] [PubMed]
- George, J.; Phun, Y.T.; Bailey, M.J.; Kong, D.C.; Stewart, K. Development and validation of the medication regimen complexity index. Ann. Pharmacother. 2004, 38, 1369–1376. [Google Scholar] [CrossRef]
- Nwadiugwu, M.C. Frailty and the Risk of Polypharmacy in the Older Person: Enabling and Preventative Approaches. J. Aging Res. 2020, 2020, 1–6. [Google Scholar] [CrossRef]
- LaVan, A.H.; Gallagher, P. Predicting risk of adverse drug reactions in older adults. Ther. Adv. Drug Saf. 2016, 7, 11–22. [Google Scholar] [CrossRef]
- Castell, M.V.; Sánchez, M.; Julián, R.; Queipo, R.; Martín, S.; Otero, Á. Frailty prevalence and slow walking speed in persons age 65 and older: Implications for primary care. BMC Fam. Pract. 2013, 14, 86. [Google Scholar] [CrossRef]
- San-José, A.; Agustí, A.; Vidal, X.; Formiga, F.; Gómez-Hernández, M.; García, J.; López-Soto, A.; Ramírez-Duque, N.; Torres, O.H.; Barbé, J.; et al. Inappropriate prescribing to the oldest old patients admitted to hospital: Prevalence, most frequently used medicines, and associated factors. BMC Geriatr. 2015, 15, 42. [Google Scholar] [CrossRef]
- Ruiz, S.J.; Cevallos, V.; Baskaran, D.; Mintzer, M.J.; Ruiz, J.G. The cross-sectional association of frailty with past and current exposure to strong anticholinergic drugs. Aging Clin. Exp. Res. 2020, 33, 2283–2289. [Google Scholar] [CrossRef]
- Sourdet, S.; Lafont, C.; Rolland, Y.; Nourhashemi, F.; Andrieu, S.; Vellas, B. Preventable Iatrogenic Disability in Elderly Patients during Hospitalization. J. Am. Med. Dir. Assoc. 2015, 16, 674–681. [Google Scholar] [CrossRef]
- Porter, M. What is value in health care? N. Engl. J. Med. 2010, 363, 2477–2481. [Google Scholar] [CrossRef] [PubMed]
- Easter, J.C.; DeWalt, D.A. The Medication Optimization Value Proposition. North Carol. Med. J. 2017, 78, 168–172. [Google Scholar] [CrossRef] [PubMed]
- Masnoon, N.; Lo, S.; Hilmer, S. A stewardship program to facilitate anticholinergic and sedative medication deprescribing using the drug burden index in electronic medical records. Br. J. Clin. Pharmacol. 2023, 89, 687–698. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Ortiz, L.; Mompart-Penina, A.; Mias, M. Agència de Qualitat i Avaluació Sanitàries de Catalunya Anàlisi de les Defuncions Observades i Esperades Durant L’epidèmia de COVID-19 a Catalunya. 2020. Available online: https://84.88.27.52/handle/11351/5008 (accessed on 13 November 2022).

| Baseline Data | Total N = 150 | |
|---|---|---|
| Demographic Data | ||
| Age, mean (SD) | 89.3 (4.4) | |
| Gender, N (%) | Men | 57 (38%) |
| Women | 93 (62%) | |
| Origin, N (%) | Home | 112 (74.7%) |
| Nursing home | 38 (25.3%) | |
| Clinical, Functional, and Cognitive Data | ||
| Medication self-management * | 42 (37.5%) | |
| Barthel index (BI), mean (SD) | 59.8 (27.9) | |
| Barthel index | Independence: BI ≥ 95 | 26 (17.3%) |
| Mild dependence: BI 90–65 | 46 (30.7%) | |
| Moderate dependence: BI 60–25 | 59 (39.3%) | |
| Severe dependence: BI ≤ 20 | 19 (12.7%) | |
| Cognitive status | No dementia | 73 (48.7%) |
| Mild dementia | 22 (14.7%) | |
| Moderate dementia (from GDS 5 to GDS 6B) | 26 (17.3%) | |
| Advanced dementia (from GDS 6C) | 29 (19.3%) | |
| Geriatric syndromes (GSs), mean (SD) | 3.13 (1.6) | |
| Type of GS | Falls | 58 (38.7%) |
| Dysphagia | 28 (17.3%) | |
| Constipation | 55 (36.7%) | |
| Urinary incontinence | 64 (42.7%) | |
| Insomnia | 83 (55.3%) | |
| Malnutrition | 16 (10.7%) | |
| Delirium in the 6 months pre-admission | 37 (24.7%) | |
| Delirium during hospitalization | 68 (45.3%) | |
| Morbidities, mean (SD) | 5.76 (2.23) | |
| Morbidities (number) | 1–2 | 6 (4.0%) |
| 3–4 | 44 (29.3%) | |
| 5 or more | 100 (66.7%) | |
| Charlson Index, mean (SD) | 2.83 (2.19) | |
| Frailty index (FI), mean (SD) | 0.35 (0.15) | |
| Frailty index, degrees | No frailty (0–0.19) | 22 (14.6%) |
| Mild frailty (0.20–0.35) | 48 (32.0%) | |
| Moderate frailty (0.36–0.50) | 55 (36.7%) | |
| Severe frailty (>0.50) | 25 (16.7%) | |
| Complexity identification | None | 33 (22%) |
| Complex chronic patients (CCPs) | 85 (56.7%) | |
| Advanced chronic patients (ACPs)—end-of-life patients | 32 (21.3%) | |
| Therapeutic goal | Survival | 29 (19.3%) |
| Functionality | 79 (52.7%) | |
| Symptomatic control | 42 (28.0%) | |
| Baseline Pharmacological Data | ||
| Polypharmacy, mean (SD) | 8.7 (3.8) | |
| Polypharmacy, degrees | 0–4 medications | 25 (16.7%) |
| 5–9 medications | 60 (40.0%) | |
| 10 or more medications | 65 (43.3%) | |
| Medication Regimen Complexity Index (MRCI), mean (SD) | 32.7 (16.1) | |
| MRCI, degrees | Low complexity (0–19.99) | 32 (21.4%) |
| Moderate complexity (20–39.99) | 71 (47.3%) | |
| High complexity (40 or more) | 47 (31.3%) | |
| Drug burden index (DBI), mean (SD) | 1.04 (0.8) | |
| DBI (degrees) | Low DBI (0–0.99) | 76 (50.7%) |
| Moderate DBI (1–1.99) | 58 (38.7%) | |
| High DBI (2 or more) | 16 (10.7%) | |
| Inappropriate prescriptions (IP), mean (SD) | 3.37 (2.56) | |
| Number of IPs | 0 IP | 16 (10.7%) |
| 1 or more IPs | 134 (89.3%) | |
| 2 or more IPs | 113 (75.3%) | |
| 3 or more IPs | 92 (61.3%) | |
| Frailty Score | DBI Score | p Value * |
|---|---|---|
| Non-frail (0–0.19) | 0.36 ± 0.48 | |
| Mildly frail (0.2–0.35) | 0.75 ± 0.59 | <0.001 |
| Moderately frail (0.36–0.50) | 1.28 ± 0.73 | |
| Severely frail (>0.50) | 1.67 ± 0.81 |
| Frailty | |||
|---|---|---|---|
| Patient Characteristics | Non-Frail (IF-VIG: 0.19) vs. Frail (IF-VIG: ≥0.20) | ||
| UnivariateOR (95% CI) | Multivariate OR (95% CI) | ||
| Age | 0.93 (0.84–1.04) | 1.04 (0.87–1.25) | |
| Gender | Women | 1.00 | 1 |
| Men | 1.77 (0.65–4.81) | 7.37 (1.50–36.15) | |
| Origin | Home | 1 | na |
| Nursing home | 8.54 (1.11–65.81) | na | |
| Barthel index | 0.92 (0.88–0.95) | 0.90 (0.85–0.95) | |
| Barthel index | Independence: IB ≥ 95 | 1 | na |
| Mild dependence: IB 90–65 | 5.57 (1.83–16.95) | na | |
| Moderate dependence: IB 60–25 | 28.50 (5.72–142.01) | na | |
| Severe dependence: IB ≤ 20 | - | na | |
| Cognitive status | No dementia | 1 | na |
| Mild dementia | 8.48 (1.07–67.15) | na | |
| Moderate dementia (from GDS 5 to 6B) | - | na | |
| Advanced dementia (GDS 6C and above) | - | na | |
| Geriatric syndromes | 1.99 (1.37–2.90) | na | |
| Geriatric syndromes | 0 | 1 | na |
| 1–2 | 4.07 (0.61–26,98) | na | |
| 3 or more | 26.40 (3.56–195.71) | na | |
| Falls | No | 1 | na |
| Yes | 3.28 (1.05–10.26) | na | |
| Depressive syndrome | No | 1 | na |
| Yes | 10.65 (2.39–47.44) | na | |
| Insomnia | No | 1 | na |
| Yes | 4.03 (1.48–10.98) | na | |
| Morbidities | 1.50 (1.15–1.96) | na | |
| Morbidities | 1–2 | 1 | na |
| 3–4 | 0.48 (0.05–4.49) | na | |
| 5 or more | 2.30 (0.24–22.16) | na | |
| Chronicity | No chronicity | 1 | na |
| Chronic complex patients (CCPs) | 12.39 (4.23–36.30) | na | |
| End-of-life (EOL) patients | - | na | |
| Therapeutic goal | Survival | 1 | na |
| Functionality | 8.28 (2.95–23.25) | na | |
| Symptomatic control | - | na | |
| DBI | 7.81 (2.89–21.13) | 11.42 (2.77–47.15) | |
| Cognitive Status | DBI | ||
|---|---|---|---|
| 0–0.99 | 1–1.99 | ≥2 | |
| No dementia | 51 (69.9%) | 18 (24.7%) | 4 (5.5%) |
| Mild dementia | 9 (40.9%) | 13 (59.1%) | 0 (0%) |
| Moderate dementia (from GDS5 to GDS 6B) | 8 (30.8%) | 15 (57.7%) | 3 (11.5%) |
| Advanced dementia (GDS 6C and above) | 8 (27.6%) | 12 (41.4%) | 9 (31.0%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Espaulella-Ferrer, M.; Molist-Brunet, N.; Espaulella-Panicot, J.; Sevilla-Sánchez, D.; Puigoriol-Juvanteny, E.; Otero-Viñas, M. Medication Assessment in an Older Population during Acute Care Hospitalization and Its Effect on the Anticholinergic Burden: A Prospective Cohort Study. Int. J. Environ. Res. Public Health 2023, 20, 5322. https://doi.org/10.3390/ijerph20075322
Espaulella-Ferrer M, Molist-Brunet N, Espaulella-Panicot J, Sevilla-Sánchez D, Puigoriol-Juvanteny E, Otero-Viñas M. Medication Assessment in an Older Population during Acute Care Hospitalization and Its Effect on the Anticholinergic Burden: A Prospective Cohort Study. International Journal of Environmental Research and Public Health. 2023; 20(7):5322. https://doi.org/10.3390/ijerph20075322
Chicago/Turabian StyleEspaulella-Ferrer, Mariona, Nuria Molist-Brunet, Joan Espaulella-Panicot, Daniel Sevilla-Sánchez, Emma Puigoriol-Juvanteny, and Marta Otero-Viñas. 2023. "Medication Assessment in an Older Population during Acute Care Hospitalization and Its Effect on the Anticholinergic Burden: A Prospective Cohort Study" International Journal of Environmental Research and Public Health 20, no. 7: 5322. https://doi.org/10.3390/ijerph20075322
APA StyleEspaulella-Ferrer, M., Molist-Brunet, N., Espaulella-Panicot, J., Sevilla-Sánchez, D., Puigoriol-Juvanteny, E., & Otero-Viñas, M. (2023). Medication Assessment in an Older Population during Acute Care Hospitalization and Its Effect on the Anticholinergic Burden: A Prospective Cohort Study. International Journal of Environmental Research and Public Health, 20(7), 5322. https://doi.org/10.3390/ijerph20075322





