Salivary Adiponectin and Albumin Levels on the Gingival Conditions of Patients Undergoing Bariatric Surgery: A Cohort Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Design and Participants
2.2. Ethical Aspects
2.3. Data Collect
2.4. Oral Clinical Evaluation
2.5. Saliva Collection and Storage
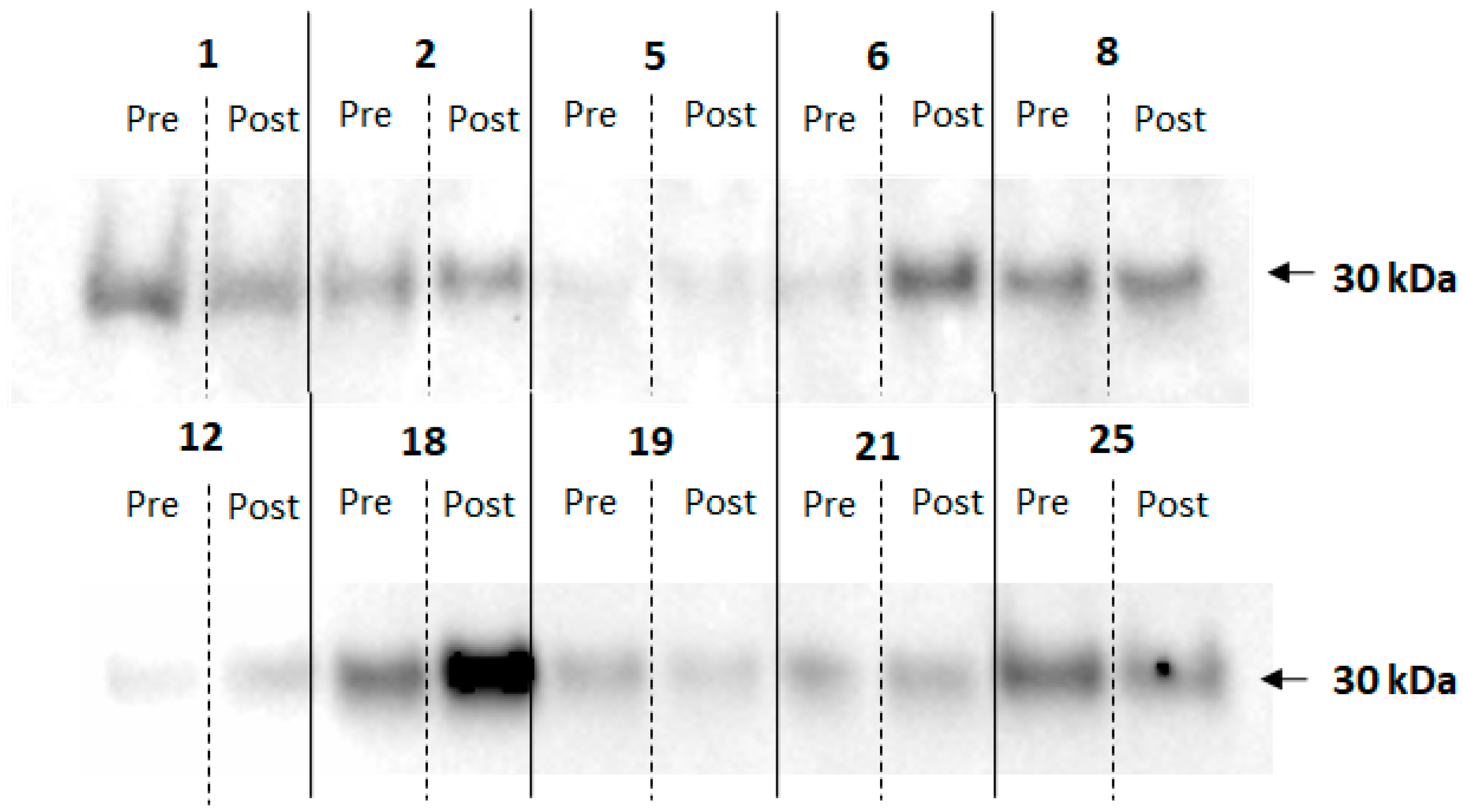
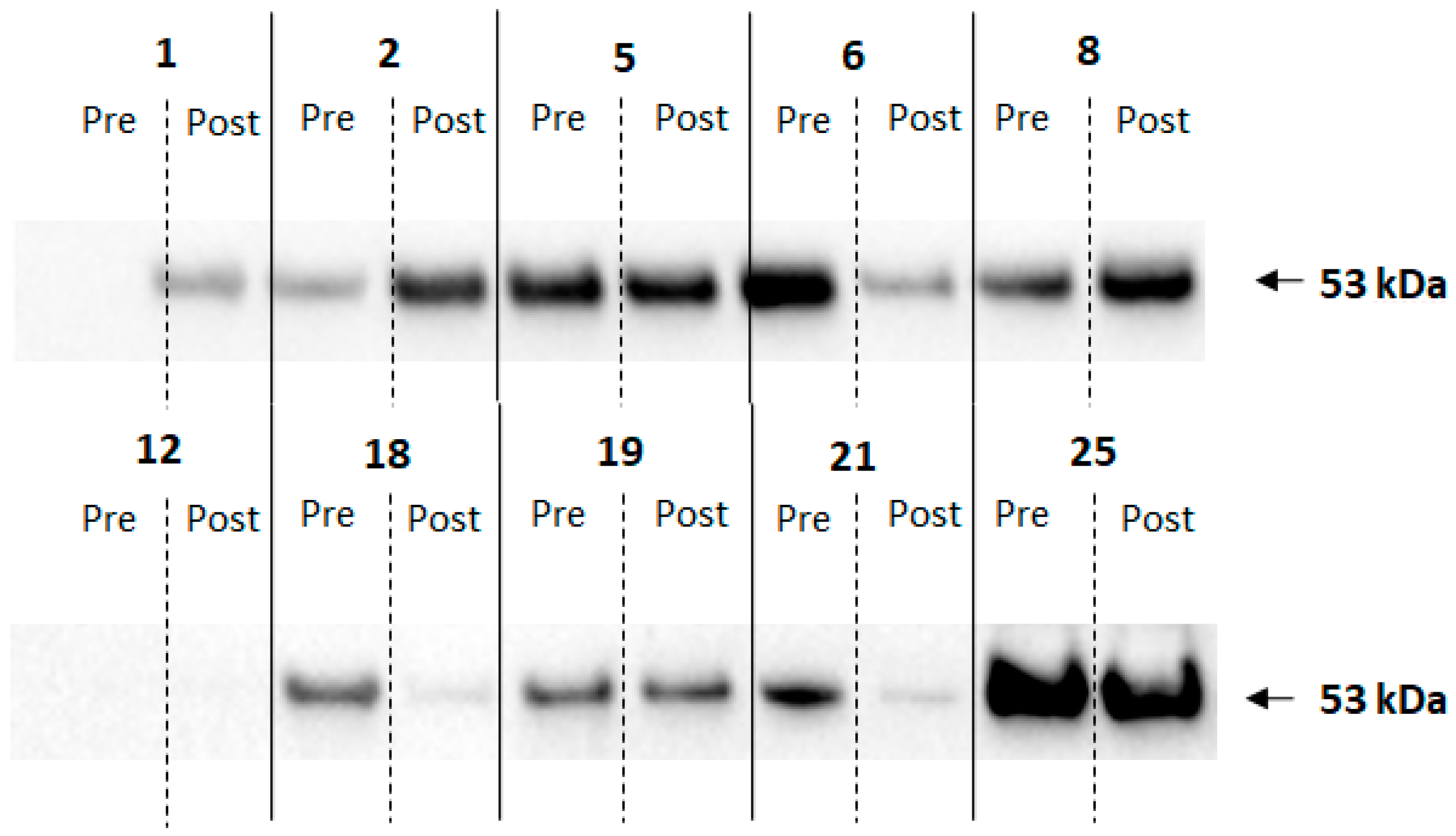
2.6. Electrophoresis and Immunoblotting
2.7. Enzyme Immunosorbent Assay (ELISA)
2.8. Statistical Analysis
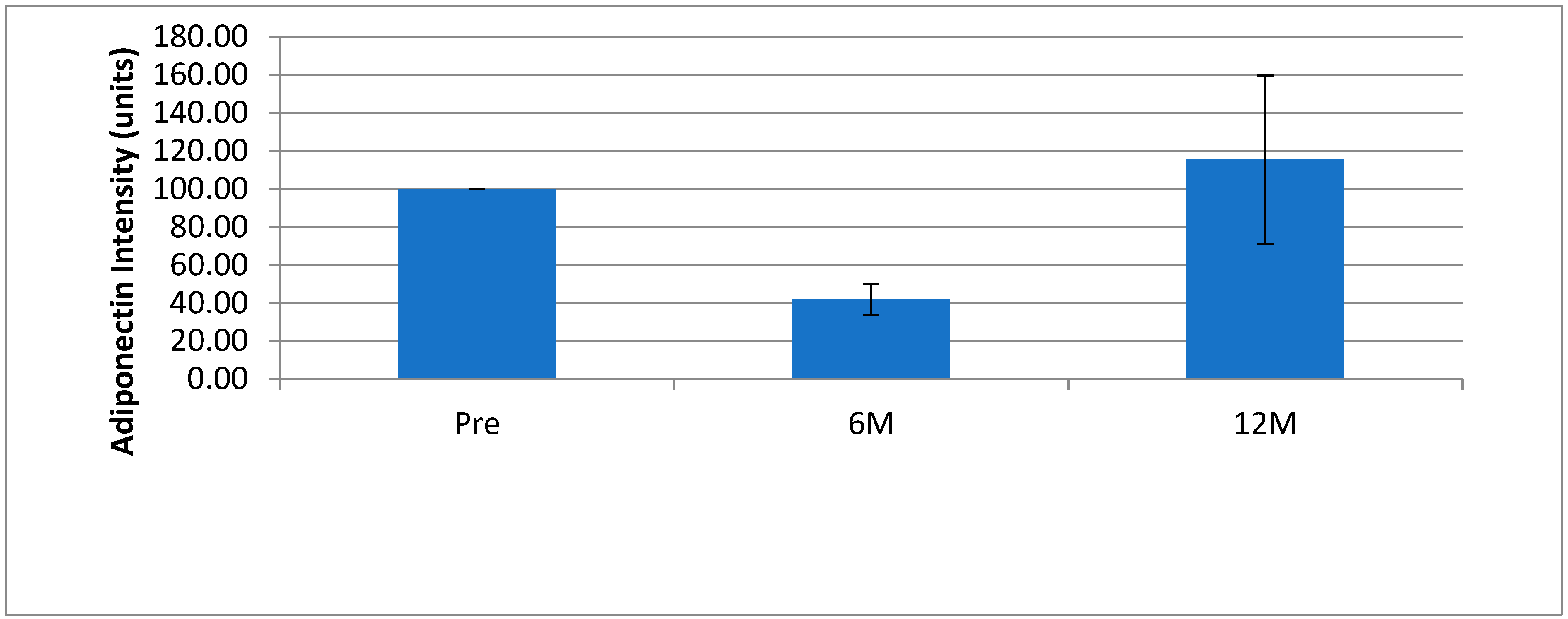
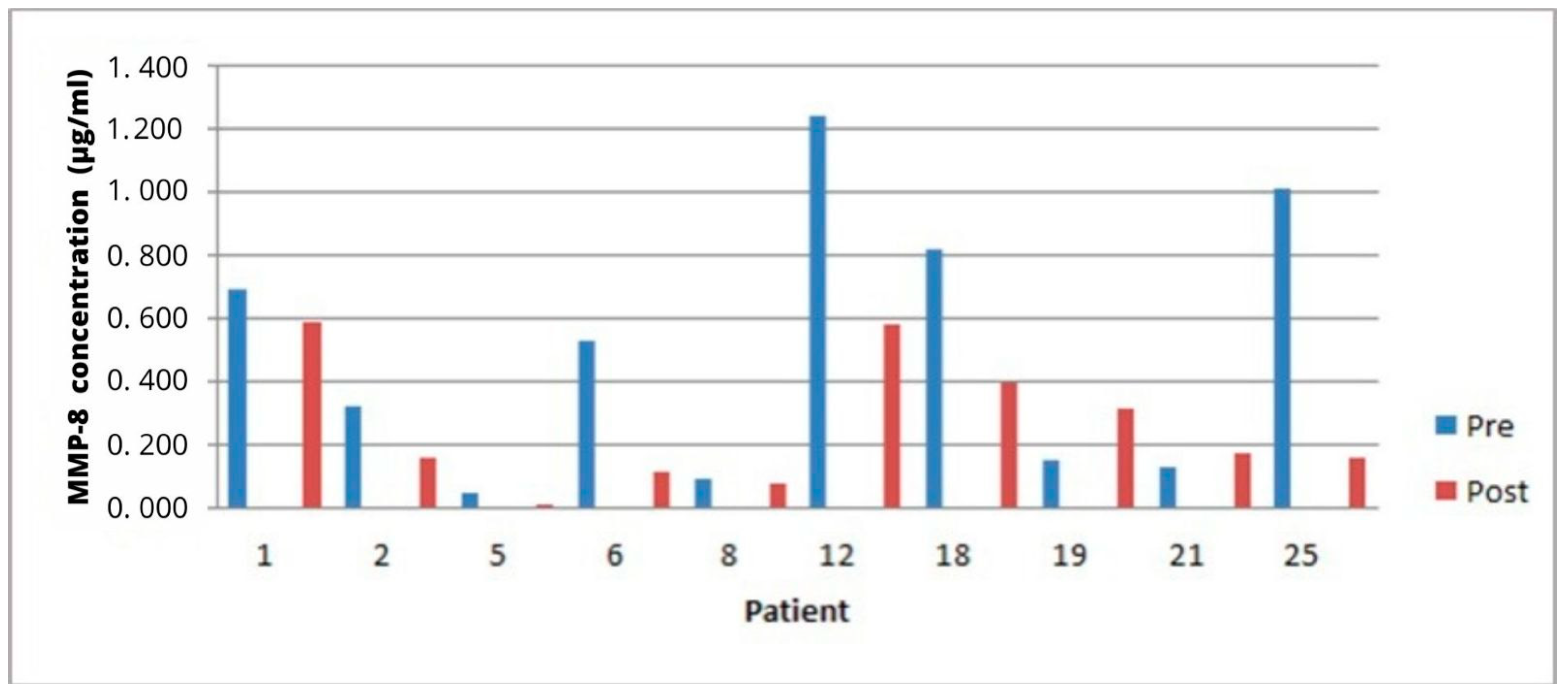
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abu-Shawish, G.; Betsy, J.; Anil, S. Is Obesity a Risk Factor for Periodontal Disease in Adults? A Systematic Review. Int. J. Environ. Res. Public Health 2022, 19, 12684. [Google Scholar] [CrossRef]
- Sales-Peres, S.H.; de Moura-Grec, P.G.; Yamashita, J.M.; Torres, E.A.; Dionísio, T.J.; Leite, C.V.; Sales-Peres, A.; Ceneviva, R. Periodontal status and pathogenic bacteria after gastric bypass: A cohort study. J. Clin. Periodontol. 2015, 42, 530–536. [Google Scholar] [CrossRef] [PubMed]
- Marsicano, J.A.; Sales-Peres, A.; Ceneviva, R.; Sales-Peres, S.H.C. Assessment of oral health status and salivary flow rate in obese patients after bariatric surgery. Eur. J. Dent. 2012, 6, 191–197. [Google Scholar]
- Antoniades, C.; Antonopoulos, A.S.; Tousoulis, D.; Stefanadis, C. Adiponectin: From obesity to cardiovascular disease. Obes. Rev. 2009, 10, 269–279. [Google Scholar] [CrossRef] [PubMed]
- Katsiougiannis, S.; Kapsogeorgou, E.K.; Manoussakis, M.N.; Skopouli, F.N. Salivary gland epithelial cells—A new source of the immunoregulatory hormone adiponectin. Arthritis Rheum. 2006, 54, 2295–2299. [Google Scholar] [CrossRef]
- Berner, H.S.; Lyngstadaas, S.P.; Spahr, A.; Monjo, M.; Thommesen, L.; Drevon, C.A.; Syversen, U.; Reseland, J.E. Adiponectin and its receptors are expressed in bone-forming cells. Bone 2004, 35, 842–849. [Google Scholar] [CrossRef]
- Pineiro, R.; Iglesias, M.J.; Gallego, R.; Raghay, K.; Eiras, S.; Rubio, J.; Diéguez, C.; Gualillo, O.; González-Juanatey, J.R.; Lago, F. Adiponectin is synthesized and secreted by human and murine cardiomyocytes. FEBS Lett. 2005, 579, 5163–5169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Waki, H.; Yamauchi, T.; Kamon, J.; Ito, Y.; Uchida, S.; Kita, S.; Hara, K.; Hada, Y.; Vasseur, F.; Froguel, P.; et al. Impaired multimerization of human adiponectin mutants associated with diabetes—Molecular structure and formation of adiponectin multimers. J. Biol. Chem. 2003, 278, 40352–40363. [Google Scholar] [CrossRef] [Green Version]
- Yamauchi, T.; Kamon, J.; Waki, H.; Terauchi, Y.; Kubota, N.; Hara, K.; Mori, Y.; Ide, T.; Murakami, K.; Tsuboyama-Kasaoka, N.; et al. The fat-derived hormone adiponectin reverses insulin resistance associated with lipoatrophy and obesity. Nat. Med. 2001, 7, 941–946. [Google Scholar] [CrossRef]
- Mangge, H.; Almer, G.; Truschnig-Wilders, M.; Schmidt, A.; Gasser, R.; Fuchs, D. Inflammation, Adiponectin, Obesity, and Cardiovascular Risk. Curr. Med. Chem. 2010, 17, 4511–4520. [Google Scholar] [CrossRef]
- Tu, Q.S.; Zhang, J.; Dong, L.Q.; Saunders, E.; Luo, E.; Tang, J.; Chen, J. Adiponectin inhibits osteoclastogenesis and bone resorption via APPL1-mediated suppression of Akt1. J. Biol. Chem. 2011, 286, 12542–12553. [Google Scholar] [CrossRef] [Green Version]
- Toda, M.; Morimoto, K. Comparison of saliva sampling methods for measuring salivary adiponectin levels. Scand. J. Clin. Lab. Investig. 2008, 68, 823–825. [Google Scholar] [CrossRef] [PubMed]
- Toda, M.; Tsukinoki, R.; Morimoto, K. Measurement of salivary adiponectin levels. Acta Diabetol. 2007, 44, 20–22. [Google Scholar] [CrossRef]
- Groschl, M.; Rauh, M.; Wagner, R.; Neuhuber, W.; Metzler, M.; Tamguney, G.; Zenk, J.; Schoof, E.; Dörr, H.G.; Blum, W.F.; et al. Identification of leptin in human saliva. J. Clin. Endocrinol. Metabol. 2001, 86, 5234–5239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Humphrey, S.P.; Williamson, R.T. A review of saliva: Normal composition, flow and function. J. Prosthet. Dent. 2001, 85, 162–169. [Google Scholar] [CrossRef]
- Xiao, H.; Wong, D.T. Proteomic analysis of microvesicles in human saliva by gel electrophoresis with liquid chromatography-mass spectrometry. Anal. Chim. Acta 2012, 723, 61–67. [Google Scholar] [CrossRef]
- Denny, P.; Hagen, F.K.; Hardt, M.; Liao, L.; Yan, W.; Arellanno, M.; Bassilian, S.; Bedi, G.S.; Boontheung, P.; Cociorva, D.; et al. Proteomes from human parotid and submandibular/sublingual gland salivas collected as ductal secretions. J. Proteome Res. 2008, 7, 1994–2006. [Google Scholar] [CrossRef] [Green Version]
- Kaufman, E.; Lamster, I.B. The diagnostic applications of saliva—A review. Crit. Rev. Oral Biol. Med. 2002, 13, 197–212. [Google Scholar] [CrossRef] [Green Version]
- Bartold, P.M.; Van Dyke, T.E. Periodontitis: A disruption of host-mediated microbial homeostasis. Unlearning learned concepts. Periodontol. 2000 2013, 62, 203–217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tonetti, M.S.; Greenwell, H.; Kornman, K.S. Staging and Grading of Periodontitis: Framework and Proposal of a New Classification and Case Definition. J. Clin. Periodontol. 2018, 45, S149–S161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kiili, M.; Cox, S.W.; Chen, H.W.; Wahlgren, J.; Maisi, P.; Eley, B.M.; Salo, T.; Sorsa, T. Collagenase-2 (MMP-8) and collagenase-3 (MMP-13) in adult periodontitis: Molecular forms and levels in gingival crevicular fluid and immunolocalization in gingival tissue. J. Clin. Periodontol. 2002, 29, 224–232. [Google Scholar] [CrossRef]
- Foratori-Junior, G.A.; Ventura, T.M.O.; Grizzo, L.T.; Carpenter, G.H.; Buzalaf, M.A.R.; Sales-Peres, S.H.C. Label-Free Quantitative Proteomic Analysis Reveals Inflammatory Pattern Associated with Obesity and Periodontitis in Pregnant Women. Metabolites 2022, 12, 1091. [Google Scholar] [CrossRef]
- Luchian, I.; Goriuc, A.; Sandu, D.; Covasa, M. The Role of Matrix Metalloproteinases (MMP-8, MMP-9, MMP-13) in Periodontal and Peri-Implant Pathological Processes. Int. J. Mol. Sci. 2022, 23, 1806. [Google Scholar] [CrossRef]
- Kshirsagar, A.V.; Craig, R.G.; Beck, J.D.; Moss, K.; Offenbacher, S.; Kotanko, P.; Falk, R.J. Severe periodontitis is associated with low serum albumin among patients on maintenance hemodialysis therapy. Clin. J. Am. Soc. Nephrol. 2007, 2, 239–244. [Google Scholar] [CrossRef] [Green Version]
- Yamashita, J.M.; moura-Grec, P.G.; Freitas, A.R.; Sales-Peres, A.; Groppo, F.C.; Ceneviva, R.; Sales-Peres, S.H.C. Assessment of Oral Conditions and Quality of Life in Morbid Obese and Normal Weight Individuals: A Cross-Sectional Study. PLoS ONE 2015, 10, e0129687. [Google Scholar]
- Kamel, E.G.; McNeill, G.; Van Wijk, M.C. Usefulness of anthropometry and DXA in predicting intra-abdominal fat in obese men and women. Obes. Res. 2000, 8, 36–42. [Google Scholar] [CrossRef]
- Sales-Peres, S.H.C.; Sales-Peres, M.C.; Ceneviva, R.; Bernabé, E. Weight loss after bariatric surgery and periodontal changes: A 12-month prospective study. Surg. Obes. Relat. Dis. 2017, 13, 637–642. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sales-Peres, S.H.; Groppo, F.C.; Rojas, L.V.; de CSales-Peres, M.; Sales-Peres, A. Periodontal status in morbidly obese patients with and without obstructive sleep apnea syndrome risk: A cross-sectional study. J. Periodontol. 2016, 87, 772–782. [Google Scholar] [CrossRef]
- Sorsa, T.; Mantyla, P.; Ronka, H.; Kallio, P.; Kallis, G.B.; Lundqvist, C.; Kinane, D.F.; Salo, T.; Golub, L.M.; Teronen, O.; et al. Scientific basis of a matrix metalloproteinase-8 specific chair-side test for monitoring periodontal and peri-implant health and disease. Ann. N. Y. Acad. Sci. 1999, 878, 130–140. [Google Scholar] [CrossRef] [PubMed]
- Nigro, E.; Piombino, P.; Scudiero, O.; Monaco, M.L.; Schettino, P.; Chambery, A.; Daniele, A. Evaluation of salivary adiponectin profile in obese patients. Peptides 2015, 63, 150–155. [Google Scholar] [CrossRef]
- Kardeşler, L.; Buduneli, N.; Cetinkalp, S.; Kinane, D.F. Adipokines and inflammatorymediators after initial periodontal treatment in patients with type 2 diabetesand chronic periodontitis. J. Periodontol. 2010, 81, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Balsan, G.A.; Vieira, J.L.; Oliveira, A.M.; Portal, V.L. Relationship between adiponectin, obesity and insulin resistance. Rev. Assoc. Med. Bras. (1992) 2015, 61, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Devanoorkar, A.; Dwarakanath, C.D.; Gundanavar, G.; Kathariya, R.; Patil, S.R. Evaluation of serum resistin levels in periodontal health and disease and effects of non-surgical periodontal therapy on its levels. Dis. Markers 2012, 32, 289–294. [Google Scholar] [CrossRef] [PubMed]
- Towbin, H.; Staehelin, T.; Gordon, J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: Procedure and some applications. Proc. Natl. Acad. Sci. USA 1979, 76, 4350–4354. [Google Scholar] [CrossRef] [Green Version]
- Arita, Y.; Kihara, S.; Ouchi, N.; Takahashi, M.; Maeda, K.; Miyagawa, J.; Hotta, K.; Shimomura, I.; Nakamura, T.; Miyaoka, K.; et al. Paradoxical decrease of a specific adipose protein, adiponectin, in obesity. Biochem. Biophys. Res. Commun. 1999, 257, 79–83. [Google Scholar] [CrossRef]
- Swarbrick, M.M.; Austrheim-Smith, I.T.; Stanhope, K.L.; Van Loan, M.D.; Ali, M.R.; Wolfe, B.; Havel, P.J. Circulating concentrations of high molecular weight adiponectin increase after Roux-en-Y gastric bypass surgery. Diabetologia 2006, 49, 2552–2558. [Google Scholar] [CrossRef] [Green Version]
- Carrasco, F.; Ruz, M.; Rojas, P.; Csendes, A.; Rebolledo, A.; Codoceo, J.; Inostroza, J.; Basfi-Fer, K.; Papapietro, K.; Rojas, J.; et al. Changes in bone mineral density, body composition and adiponectin levels in morbidly obese patients after bariatric surgery. Obes. Surg. 2009, 19, 41–46. [Google Scholar] [CrossRef]
- Tavares, L.T.R.; Saavedra-Silva, M.; López-Marcos, J.F.; Veiga, N.J.; Castilho, R.D.M.; Fernandes, G.V.D.O. Blood and Salivary Inflammatory Biomarkers Profile in Patients with Chronic Kidney Disease and Periodontal Disease: A Systematic Review. Diseases 2022, 10, 12. [Google Scholar] [CrossRef]
- Martin, W.P.; Docherty, N.G.; Le Roux, C.W. Impact of bariatric surgery on cardiovascular and renal complications of diabetes: Focus on clinical outcomes and putative mechanisms. Expert Rev. Endocrinol. Metab. 2018, 13, 251–262. [Google Scholar] [CrossRef]
- Prinsen, B.H.; Sain-van der Velden, M.G. Albumin renewal: Experimental approach and its application in kidney health and diseases. Clin. Chim. Acta. 2004, 347, 1–14. [Google Scholar] [CrossRef]
- Henskens, Y.M.; van der Velden, U.; Veerman, E.C.; Nieuw Amerongen, A.V. Protein, albumin and cystatin concentrations in the saliva of healthy subjects and patients with gingivitis or periodontitis. J. Periodontal. Res. 1993, 28, 43–48. [Google Scholar] [CrossRef] [PubMed]

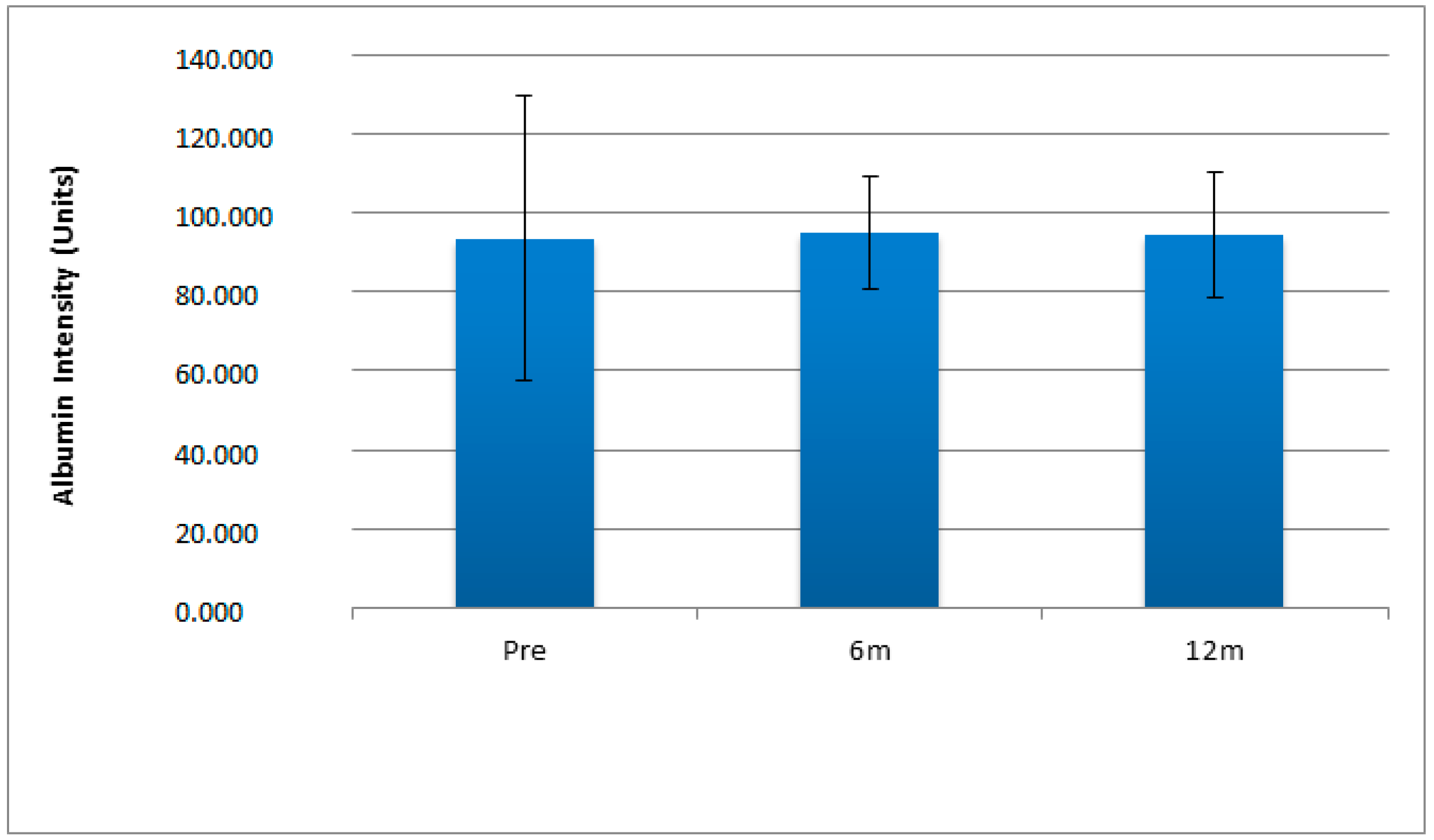
| Variables | Pre-Surgery | 6 Months Post-Surgery | 12 Months Post-Surgery |
|---|---|---|---|
| BMI Mean (SD) | 50.5 (7.76) a | 37.4 (6.07) b | 34.0 (5.47) b |
| Clinical Attachment Loss-CAL Mean (SD) | 1.88 (0.53) a | 2.17 (0.44) b | 2.07 (0.47) ab |
| Pocket Depth(mm)-PPD Mean (SD) | 1.84 (0.52) a | 2.09 (0.41) a | 2.02 (0.44) a |
| Variables | Pre-Treatment Mean ± SD | Post-Treatment Mean ± SD |
|---|---|---|
| Gingival Recession (mm) | 2.58 ± 0.2 a | 3.01 ± 0.29 b |
| Bleeding on Probing (BoP) (N. Teeth) | 18.01 ± 2.07 a | 12.5 ± 2.18 b |
| Pocket Depth (PD) (mm) | 3.21 ± 0.22 a | 2.90 ± 0.16 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sales-Peres, S.H.d.C.; Houghton, J.; Meira, G.d.F.; Moura-Grec, P.G.d.; Brienze, S.L.A.; Karim, B.A.; Carpenter, G.H. Salivary Adiponectin and Albumin Levels on the Gingival Conditions of Patients Undergoing Bariatric Surgery: A Cohort Study. Int. J. Environ. Res. Public Health 2023, 20, 5261. https://doi.org/10.3390/ijerph20075261
Sales-Peres SHdC, Houghton J, Meira GdF, Moura-Grec PGd, Brienze SLA, Karim BA, Carpenter GH. Salivary Adiponectin and Albumin Levels on the Gingival Conditions of Patients Undergoing Bariatric Surgery: A Cohort Study. International Journal of Environmental Research and Public Health. 2023; 20(7):5261. https://doi.org/10.3390/ijerph20075261
Chicago/Turabian StyleSales-Peres, Silvia Helena de Carvalho, Jack Houghton, Gabriela de Figueiredo Meira, Patrícia Garcia de Moura-Grec, Sergio Luis Aparecido Brienze, Belkais Abuuasha Karim, and Guy Howard Carpenter. 2023. "Salivary Adiponectin and Albumin Levels on the Gingival Conditions of Patients Undergoing Bariatric Surgery: A Cohort Study" International Journal of Environmental Research and Public Health 20, no. 7: 5261. https://doi.org/10.3390/ijerph20075261
APA StyleSales-Peres, S. H. d. C., Houghton, J., Meira, G. d. F., Moura-Grec, P. G. d., Brienze, S. L. A., Karim, B. A., & Carpenter, G. H. (2023). Salivary Adiponectin and Albumin Levels on the Gingival Conditions of Patients Undergoing Bariatric Surgery: A Cohort Study. International Journal of Environmental Research and Public Health, 20(7), 5261. https://doi.org/10.3390/ijerph20075261







