NHANES 2011–2014 Reveals Decreased Cognitive Performance in U.S. Older Adults with Metabolic Syndrome Combinations
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
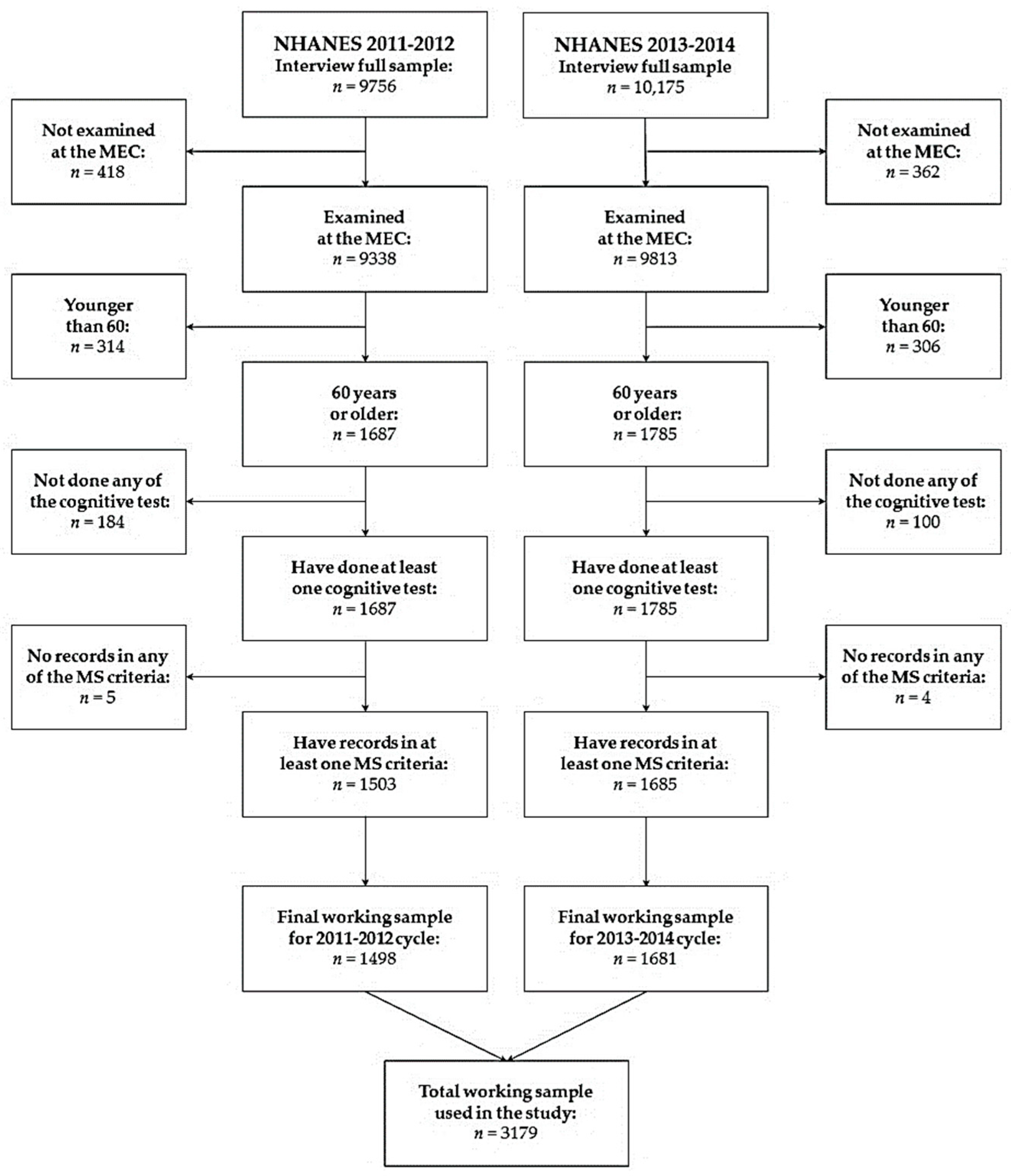
2.2. Participants
2.3. Ethical Aspect
2.4. Cognitive Assessments
2.4.1. Consortium to Establish a Registry for Alzheimer’s Disease
2.4.2. Animal Fluency Test
2.4.3. Digit Symbol Substitution Test
2.5. Metabolic Syndrome Diagnosis
2.6. Dependent Variables, Predictors, Covariates, and Missing Data
2.7. Data Analysis
3. Results
3.1. Sociodemographic Characteristics of the Participants
3.2. Metabolic Syndrome and Its Components
3.3. Cognitive Performance
3.4. Relationship between Metabolic Syndrome and Cognitive Performance
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- El-Tallawy, H.N.; Saadeldin, H.M.; Ezzeldin, A.M.; Tohamy, A.M.; Eltellawy, S.; Bathalath, A.M.; Shehab, M.M. Genetic, Clinical, and Biochemical Aspects of Patients with Alzheimer Disease. Egypt. J. Neurol. Psychiatry Neurosurg. 2022, 58, 24. [Google Scholar] [CrossRef]
- Johnson, S.C.; Koscik, R.L.; Jonaitis, E.M.; Clark, L.R.; Mueller, K.D.; Berman, S.E.; Bendlin, B.B.; Engelman, C.D.; Okonkwo, O.C.; Hogan, K.J.; et al. The Wisconsin Registry for Alzheimer’s Prevention: A Review of Findings and Current Directions. Alzheimers Dement. 2018, 10, 130–142. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Gu, B.J.; Masters, C.L.; Wang, Y.-J. A Systemic View of Alzheimer’s Disease—Insights from Amyloid-β Metabolism beyond the Brain. Nat. Rev. Neurol. 2017, 13, 612–623. [Google Scholar] [CrossRef] [PubMed]
- Bennett, D.A.; Wilson, R.S.; Boyle, P.A.; Buchman, A.S.; Schneider, J.A. Relation of Neuropathology to Cognition in Persons without Cognitive Impairment. Ann. Neurol. 2012, 72, 599–609. [Google Scholar] [CrossRef] [PubMed]
- Katzman, R.; Terry, R.; DeTeresa, R.; Brown, T.; Davies, P.; Fuld, P.; Renbing, X.; Peck, A. Clinical, Pathological, and Neurochemical Changes in Dementia: A Subgroup with Preserved Mental Status and Numerous Neocortical Plaques. Ann. Neurol. 1988, 23, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Jansen, W.J.; Ossenkoppele, R.; Knol, D.L.; Tijms, B.M.; Scheltens, P.; Verhey, F.R.J.; Visser, P.J.; Amyloid Biomarker Study Group; Aalten, P.; Aarsland, D.; et al. Prevalence of Cerebral Amyloid Pathology in Persons without Dementia: A Meta-Analysis. JAMA 2015, 313, 1924–1938. [Google Scholar] [CrossRef]
- Bennett, D.A. Mixed Pathologies and Neural Reserve: Implications of Complexity for Alzheimer Disease Drug Discovery. PLoS Med. 2017, 14, e1002256. [Google Scholar] [CrossRef]
- Stern, Y. What Is Cognitive Reserve? Theory and Research Application of the Reserve Concept. J. Int. Neuropsychol. Soc. 2002, 8, 448–460. [Google Scholar] [CrossRef]
- Atamna, H.; Tenore, A.; Lui, F.; Dhahbi, J.M. Organ Reserve, Excess Metabolic Capacity, and Aging. Biogerontology 2018, 19, 171–184. [Google Scholar] [CrossRef]
- Wang, S.; Qin, L. Homeostatic Medicine: A Strategy for Exploring Health and Disease. Curr. Med. 2022, 1, 16. [Google Scholar] [CrossRef]
- Iliodromiti, S.; Iglesias Sanchez, C.; Messow, C.-M.; Cruz, M.; Garcia Velasco, J.; Nelson, S.M. Excessive Age-Related Decline in Functional Ovarian Reserve in Infertile Women: Prospective Cohort of 15,500 Women. J. Clin. Endocrinol. Metab. 2016, 101, 3548–3554. [Google Scholar] [CrossRef]
- Polverino, A.; Sorrentino, P.; Pesoli, M.; Mandolesi, L. Nutrition and Cognition across the Lifetime: An Overview on Epigenetic Mechanisms. AIMS Neurosci. 2021, 8, 448–476. [Google Scholar] [CrossRef]
- Rodgers, G.P.; Collins, F.S. Precision Nutrition—The Answer to “What to Eat to Stay Healthy”. JAMA 2020, 324, 735–736. [Google Scholar] [CrossRef]
- Prokopidis, K.; Giannos, P.; Ispoglou, T.; Witard, O.C.; Isanejad, M. Dietary Fiber Intake Is Associated with Cognitive Function in Older Adults: Data from the National Health and Nutrition Examination Survey. Am. J. Med. 2022, 135, E257–E262. [Google Scholar] [CrossRef]
- Mao, X.-Y.; Yin, X.-X.; Guan, Q.-W.; Xia, Q.-X.; Yang, N.; Zhou, H.-H.; Liu, Z.-Q.; Jin, W.-L. Dietary Nutrition for Neurological Disease Therapy: Current Status and Future Directions. Pharmacol. Ther. 2021, 226, 107861. [Google Scholar] [CrossRef]
- Foret, J.T.; Oleson, S.; Hickson, B.; Valek, S.; Tanaka, H.; Haley, A.P. Metabolic Syndrome and Cognitive Function in Midlife. Arch. Clin. Neuropsychol. 2021, 36, 897–907. [Google Scholar] [CrossRef]
- Wooten, T.; Ferland, T.; Poole, V.; Milberg, W.; McGlinchey, R.; DeGutis, J.; Esterman, M.; Leritz, E. Metabolic Risk in Older Adults Is Associated with Impaired Sustained Attention. Neuropsychology 2019, 33, 947–955. [Google Scholar] [CrossRef]
- Frazier, D.T.; Bettcher, B.M.; Dutt, S.; Patel, N.; Mungas, D.; Miller, J.; Green, R.; Kramer, J.H. Relationship between Insulin-Resistance Processing Speed and Specific Executive Function Profiles in Neurologically Intact Older Adults. J. Int. Neuropsychol. Soc. 2015, 21, 622–628. [Google Scholar] [CrossRef]
- Bahchevanov, K.M.; Dzhambov, A.M.; Chompalov, K.A.; Massaldjieva, R.I.; Atanassova, P.A.; Mitkov, M.D. Contribution of Components of Metabolic Syndrome to Cognitive Performance in Middle-Aged Adults. Arch. Clin. Neuropsychol. 2021, 36, 498–506. [Google Scholar] [CrossRef]
- Mone, P.; Gambardella, J.; Lombardi, A.; Pansini, A.; De Gennaro, S.; Leo, A.L.; Famiglietti, M.; Marro, A.; Morgante, M.; Frullone, S.; et al. Correlation of Physical and Cognitive Impairment in Diabetic and Hypertensive Frail Older Adults. Cardiovasc. Diabetol. 2022, 21, 10. [Google Scholar] [CrossRef]
- Mundell, N.L.; Sethi, P.; Anstey, K.J.; Macpherson, H.; Dunstan, D.W.; Fraser, S.F.; Daly, R.M. The Influence of Adiposity on the Interactions between Strength, Physical Function and Cognition among Older Adults in the Australian Diabetes, Obesity, and Lifestyle (AusDiab) Study. BMC Geriatr. 2022, 22, 357. [Google Scholar] [CrossRef] [PubMed]
- An, Y.; Zhang, X.; Wang, Y.; Wang, Y.; Liu, W.; Wang, T.; Qin, Z.; Xiao, R. Longitudinal and Nonlinear Relations of Dietary and Serum Cholesterol in Midlife with Cognitive Decline: Results from EMCOA Study. Mol. Neurodegener. 2019, 14, 51. [Google Scholar] [CrossRef] [PubMed]
- Ihle, A.; Gouveia, É.R.; Gouveia, B.R.; Freitas, D.L.; Jurema, J.; Tinôco, M.A.; Kliegel, M. High-Density Lipoprotein Cholesterol Level Relates to Working Memory, Immediate and Delayed Cued Recall in Brazilian Older Adults: The Role of Cognitive Reserve. Dement. Geriatr. Cogn. Disord. 2017, 44, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Parthasarathy, V.; Frazier, D.T.; Bettcher, B.M.; Jastrzab, L.; Chao, L.; Reed, B.; Mungas, D.; Weiner, M.; DeCarli, C.; Chui, H.; et al. Triglycerides Are Negatively Correlated with Cognitive Function in Nondemented Aging Adults. Neuropsychology 2017, 31, 682–688. [Google Scholar] [CrossRef] [PubMed]
- Mizuhara, R.; Mitaki, S.; Takamura, M.; Abe, S.; Onoda, K.; Yamaguchi, S.; Nagai, A. Pulse Pressure Is Associated with Cognitive Performance in Japanese Non-Demented Population: A Cross-Sectional Study. BMC Neurol. 2022, 22, 137. [Google Scholar] [CrossRef]
- Ogawa, E.F.; Leritz, E.; McGlinchey, R.; Milberg, W.; Bean, J.F. Metabolic Syndrome, and Physical Performance: The Moderating Role of Cognition among Middle-to-Older-Aged Adults. J. Int. Neuropsychol. Soc. 2021, 27, 172–180. [Google Scholar] [CrossRef]
- González, H.M.; Tarraf, W.; Vásquez, P.; Sanderlin, A.H.; Rosenberg, N.I.; Davis, S.; Rodríguez, C.J.; Gallo, L.C.; Thyagarajan, B.; Daviglus, M.; et al. Metabolic Syndrome and Neurocognition among Diverse Middle-Aged and Older Hispanics/Latinos: HCHS/SOL Results. Diabetes Care 2018, 41, 1501–1509. [Google Scholar] [CrossRef]
- Kosyreva, A.M.; Sentyabreva, A.V.; Tsvetkov, I.S.; Makarova, O.V. Alzheimer’s Disease and Inflammaging. Brain Sci. 2022, 12, 1237. [Google Scholar] [CrossRef]
- Newcombe, E.A.; Camats-Perna, J.; Silva, M.L.; Valmas, N.; Huat, T.J.; Medeiros, R. Inflammation: The Link between Comorbidities, Genetics, and Alzheimer’s Disease. J. Neuroinflamm. 2018, 15, 276. [Google Scholar] [CrossRef]
- Gierach, M.; Rasmus, A.; Orłowska, E. Verbal Fluency in Metabolic Syndrome. Brain Sci. 2022, 12, 255. [Google Scholar] [CrossRef]
- Halikas, A.; Gibas, K.J. AMPK Induced Memory Improvements in the Diabetic Population: A Case Study. Diabetes Metab. Syndr. 2018, 12, 1141–1146. [Google Scholar] [CrossRef]
- Pal, K.; Mukadam, N.; Petersen, I.; Cooper, C. Mild Cognitive Impairment and Progression to Dementia in People with Diabetes, Prediabetes and Metabolic Syndrome: A Systematic Review and Meta-Analysis. Soc. Psychiatry Psychiatr. Epidemiol. 2018, 53, 1149–1160. [Google Scholar] [CrossRef]
- Yates, K.F.; Sweat, V.; Yau, P.L.; Turchiano, M.M.; Convit, A. Impact of Metabolic Syndrome on Cognition and Brain: A Selected Review of the Literature. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 2060–2067. [Google Scholar] [CrossRef]
- Karlsson, I.K.; Ploner, A.; Song, C.; Gatz, M.; Pedersen, N.L.; Hägg, S. Genetic Susceptibility to Cardiovascular Disease and Risk of Dementia. Transl. Psychiatry 2017, 7, e1142. [Google Scholar] [CrossRef]
- Atti, A.R.; Valente, S.; Iodice, A.; Caramella, I.; Ferrari, B.; Albert, U.; Mandelli, L.; De Ronchi, D. Metabolic Syndrome, Mild Cognitive Impairment, and Dementia: A Meta–Analysis of Longitudinal Studies. Am. J. Geriatr. Psychiatry 2019, 27, 625–637. [Google Scholar] [CrossRef]
- Solfrizzi, V.; Scafato, E.; Capurso, C.; D’Introno, A.; Colacicco, A.M.; Frisardi, V.; Vendemiale, G.; Baldereschi, M.; Crepaldi, G.; Di Carlo, A.; et al. Metabolic Syndrome, Mild Cognitive Impairment, and Progression to Dementia. The Italian Longitudinal Study on Aging. Neurobiol. Aging 2011, 32, 1932–1941. [Google Scholar] [CrossRef]
- Botchway, B.O.; Okoye, F.C.; Chen, Y.; Arthur, W.E.; Fang, M. Alzheimer Disease: Recent Updates on Apolipoprotein E and Gut Microbiome Mediation of Oxidative Stress, and Prospective Interventional Agents. Aging Dis. 2022, 13, 87–102. [Google Scholar] [CrossRef]
- Murray, E.R.; Kemp, M.; Nguyen, T.T. The Microbiota-Gut-Brain Axis in Alzheimer’s Disease: A Review of Taxonomic Alterations and Potential Avenues for Interventions. Arch. Clin. Neuropsychol. 2022, 37, 595–607. [Google Scholar] [CrossRef]
- Thu Thuy Nguyen, V.; Endres, K. Targeting Gut Microbiota to Alleviate Neuroinflammation in Alzheimer’s Disease. Adv. Drug Deliv. Rev. 2022, 188, 114418. [Google Scholar] [CrossRef]
- Kheirvari, M.; Lacy, V.A.; Goudarzi, H.; RabieNezhad Ganji, N.; Kamali Ardekani, M.; Anbara, T. The Changes in Cognitive Function following Bariatric Surgery Considering the Function of Gut Microbiome. Obes. Pillars 2022, 3, 100020. [Google Scholar] [CrossRef]
- Handley, J.D.; Williams, D.M.; Caplin, S.; Stephens, J.W.; Barry, J. Changes in Cognitive Function following Bariatric Surgery: A Systematic Review. Obes. Surg. 2016, 26, 2530–2537. [Google Scholar] [CrossRef] [PubMed]
- Collden, H.; Hagberg, T.M.; Landin, A.; Norlen, A.-K.; Ryberg, H.; Wu, J.; Gustafsson, K.L.; Grahnemo, L.; Nilsson, K.; Sjogren, K.; et al. Origins of Progesterone in Male Mice. In Endocrine Abstracts; Bioscientifica: Bristol, UK, 2022; Volume 81. [Google Scholar] [CrossRef]
- Tahmi, M.; Palta, P.; Luchsinger, J.A. Metabolic Syndrome, and Cognitive Function. Curr. Cardiol. Rep. 2021, 23, 180. [Google Scholar] [CrossRef] [PubMed]
- Kordestani-Moghadam, P.; Assari, S.; Nouriyengejeh, S.; Mohammadipour, F.; Pourabbasi, A. Cognitive Impairments and Associated Structural Brain Changes in Metabolic Syndrome and Implications of Neurocognitive Intervention. J. Obes. Metab. Syndr. 2020, 29, 174–179. [Google Scholar] [CrossRef] [PubMed]
- NHANES—National Health and Nutrition Examination Survey Homepage. Available online: https://www.cdc.gov/nchs/nhanes/index.htm (accessed on 2 July 2022).
- Johnson, C.L.; Dohrmann, S.M.; Burt, V.L.; Mohadjer, L.K. National Health, and Nutrition Examination Survey: Sample Design, 2011–2014. In Vital and Health Statistics. Series 2, Data Evaluation and Methods Research; U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Health Statistics: Hyattsville, MD, USA, 2014; No. 162; pp. 1–33. [Google Scholar]
- Brody, D.J.; Kramarow, E.A.; Taylor, C.A.; McGuire, L.C. Cognitive Performance in Adults Aged 60 and Over: National Health and Nutrition Examination Survey, 2011–2014. Natl. Health Stat. Rep. 2019, 126, 23. [Google Scholar]
- Morris, J.C.; Heyman, A.; Mohs, R.C.; Hughes, J.P.; van Belle, G.; Fillenbaum, G.; Mellits, E.D.; Clark, C. The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD). Part I. Clinical and Neuropsychological Assessment of Alzheimer’s Disease. Neurology 1989, 39, 1159–1165. [Google Scholar] [CrossRef]
- Fillenbaum, G.G.; van Belle, G.; Morris, J.C.; Mohs, R.C.; Mirra, S.S.; Davis, P.C.; Tariot, P.N.; Silverman, J.M.; Clark, C.M.; Welsh-Bohmer, K.A.; et al. Consortium to Establish a Registry for Alzheimer’s Disease (CERAD): The First Twenty Years. Alzheimer’s Dement. 2008, 4, 96–109. [Google Scholar] [CrossRef]
- Henry, J.D.; Crawford, J.R. A Meta-Analytic Review of Verbal Fluency Performance following Focal Cortical Lesions. Neuropsychology 2004, 18, 284–295. [Google Scholar] [CrossRef]
- Henry, J.D.; Crawford, J.R.; Phillips, L.H. Verbal Fluency Performance in Dementia of the Alzheimer’s Type: A Meta-Analysis. Neuropsychologia 2004, 42, 1212–1222. [Google Scholar] [CrossRef]
- Jaeger, J. Digit Symbol Substitution Test: The Case for Sensitivity Over Specificity in Neuropsychological Testing. J. Clin. Psychopharmacol. 2018, 38, 513–519. [Google Scholar] [CrossRef]
- Alberti, K.G.M.M.; Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z.; Cleeman, J.I.; Donato, K.A.; Fruchart, J.-C.; James, W.P.T.; Loria, C.M.; Smith, S.C.; et al. A Joint Interim Statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 2009, 120, 1640–1645. [Google Scholar] [CrossRef]
- NHANES Survey Methods and Analytic Guidelines. Available online: https://wwwn.cdc.gov/nchs/nhanes/analyticguidelines.aspx (accessed on 13 July 2022).
- National Center for Health Statistics (U.S.) (Ed.) National Center for Health Statistics Data Presentation Standards for Proportions; Vital and Health Statistics. Series 2, Data Evaluation and Methods Research; U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Health Statistics: Hyattsville, MD, USA, 2017.
- Gao, H.; Wang, K.; Ahmadizar, F.; Zhuang, J.; Jiang, Y.; Zhang, L.; Gu, J.; Zhao, W.; Xia, Z. Associations of Changes in Late-Life Blood Pressure with Cognitive Impairment among Older Population in China. BMC Geriatr. 2021, 21, 536. [Google Scholar] [CrossRef]
- Pedditzi, E.; Peters, R.; Beckett, N. The Risk of Overweight/Obesity in Mid-Life and Late Life for the Development of Dementia: A Systematic Review and Meta-Analysis of Longitudinal Studies. Age Ageing 2016, 45, 14–21. [Google Scholar] [CrossRef]
- Zhang, T.; Shaw, M.; Cherbuin, N. Association between Type 2 Diabetes Mellitus and Brain Atrophy: A Meta-Analysis. Diabetes Metab. J. 2022, 46, 781–802. [Google Scholar] [CrossRef]
- Goughari, A.S.; Mazhari, S.; Pourrahimi, A.M.; Sadeghi, M.M.; Nakhaee, N. Associations between Components of Metabolic Syndrome and Cognition in Patients with Schizophrenia. J. Psychiatr. Pract. 2015, 21, 190–197. [Google Scholar] [CrossRef]
- Liu, C.-L.; Lin, M.-H.; Peng, L.-N.; Chen, L.-K.; Su, C.-T.; Liu, L.-K.; Chen, L.-Y. Late-Life Metabolic Syndrome Prevents Cognitive Decline among Older Men Aged 75 Years and over: One-Year Prospective Cohort Study. J. Nutr. Health Aging 2013, 17, 523–526. [Google Scholar] [CrossRef]
- Martinez-Miller, E.E.; Kohl, H.W.; Barlow, C.E.; Willis, B.L.; DeFina, L.F. Metabolic Syndrome and Cognitive Impairment among High Socioeconomic, Nondemented Older US Adults. J. Am. Geriatr. Soc. 2019, 67, 1437–1443. [Google Scholar] [CrossRef]
- Feinkohl, I.; Janke, J.; Hadzidiakos, D.; Slooter, A.; Winterer, G.; Spies, C.; Pischon, T. Associations of the Metabolic Syndrome and Its Components with Cognitive Impairment in Older Adults. BMC Geriatr. 2019, 19, 77. [Google Scholar] [CrossRef]
- Harrison, S.L.; Stephan, B.C.M.; Siervo, M.; Granic, A.; Davies, K.; Wesnes, K.A.; Kirkwood, T.B.L.; Robinson, L.; Jagger, C. Is There an Association between Metabolic Syndrome and Cognitive Function in Very Old Adults? The Newcastle 85+ Study. J. Am. Geriatr. Soc. 2015, 63, 667–675. [Google Scholar] [CrossRef]
- Gross, T.J.; Araújo, R.B.; Vale, F.A.C.; Bassani, M.; Maciel, C.D. Dependence between Cognitive Impairment and Metabolic Syndrome Applied to a Brazilian Elderly Dataset. Artif. Intell. Med. 2018, 90, 53–60. [Google Scholar] [CrossRef]
- Guicciardi, M.; Crisafulli, A.; Doneddu, A.; Fadda, D.; Lecis, R. Effects of Metabolic Syndrome on Cognitive Performance of Adults During Exercise. Front. Psychol. 2019, 10, 1845. [Google Scholar] [CrossRef]
- Kim, S.-H.; Kim, M.; Ahn, Y.-B.; Lim, H.-K.; Kang, S.-G.; Cho, J.; Park, S.-J.; Song, S.-W. Effect of Dance Exercise on Cognitive Function in Elderly Patients with Metabolic Syndrome: A Pilot Study. J. Sports. Sci. Med. 2011, 10, 671–678. [Google Scholar] [PubMed]
- Yaffe, K.; Haan, M.; Blackwell, T.; Cherkasova, E.; Whitmer, R.A.; West, N. Metabolic Syndrome and Cognitive Decline in Elderly Latinos: Findings from the Sacramento Area Latino Study of Aging Study. J. Am. Geriatr. Soc. 2007, 55, 758–762. [Google Scholar] [CrossRef] [PubMed]
- Hishikawa, N.; Fukui, Y.; Sato, K.; Kono, S.; Yamashita, T.; Ohta, Y.; Deguchi, K.; Abe, K. Cognitive and Affective Functions in Alzheimer’s Disease Patients with Metabolic Syndrome. Eur. J. Neurol. 2016, 23, 339–345. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.Y.; Lee, S.J.; Kim, K.M.; Yun, Y.M.; Song, B.M.; Kim, J.E.; Kim, H.C.; Rhee, Y.; Youm, Y.; Kim, C.O. Association of Metabolic Syndrome and 25-Hydroxyvitamin D with Cognitive Impairment among Elderly Koreans. Geriatr. Gerontol. Int. 2017, 17, 1069–1075. [Google Scholar] [CrossRef] [PubMed]
- Mehra, A.; Suri, V.; Kumari, S.; Avasthi, A.; Grover, S. Association of Mild Cognitive Impairment and Metabolic Syndrome in Patients with Hypertension. Asian J. Psychiatr. 2020, 53, 102185. [Google Scholar] [CrossRef]
- Oh, H.-M.; Kim, S.-H.; Kang, S.-G.; Park, S.-J.; Song, S.-W. The Relationship between Metabolic Syndrome and Cognitive Function. Korean J. Fam. Med. 2011, 32, 358–366. [Google Scholar] [CrossRef]
- Schmitt, L.O.; Gaspar, J.M. Obesity-Induced Brain Neuroinflammatory and Mitochondrial Changes. Metabolites 2023, 13, 86. [Google Scholar] [CrossRef]
- Marcos, J.L.; Olivares-Barraza, R.; Ceballo, K.; Wastavino, M.; Ortiz, V.; Riquelme, J.; Martínez-Pinto, J.; Muñoz, P.; Cruz, G.; Sotomayor-Zárate, R. Obesogenic Diet-Induced Neuroinflammation: A Pathological Link between Hedonic and Homeostatic Control of Food Intake. Int. J. Mol. Sci. 2023, 24, 1468. [Google Scholar] [CrossRef]
- Jin, Y.; Wu, R.; Li, L.; Shen, L.; Gu, Y.; Sun, C. Exosomes from Inflamed Macrophages Promote the Progression of Parkinson’s Disease by Inducing Neuroinflammation. Mol. Neurobiol. 2023, 60, 1914–1928. [Google Scholar] [CrossRef]
- Hata, M.; Andriessen, E.M.M.A.; Hata, M.; Diaz-Marin, R.; Fournier, F.; Crespo-Garcia, S.; Blot, G.; Juneau, R.; Pilon, F.; Dejda, A.; et al. Past History of Obesity Triggers Persistent Epigenetic Changes in Innate Immunity and Exacerbates Neuroinflammation. Science 2023, 379, 45–62. [Google Scholar] [CrossRef]
- Yang, X.; Xu, Y.; Gao, W.; Wang, L.; Zhao, X.; Liu, G.; Fan, K.; Liu, S.; Hao, H.; Qu, S.; et al. Hyperinsulinemia-Induced Microglial Mitochondrial Dynamic and Metabolic Alterations Lead to Neuroinflammation In Vivo and in Vitro. Front. Neurosci. 2022, 16, 1036872. [Google Scholar] [CrossRef]
- Salas-Venegas, V.; Flores-Torres, R.P.; Rodríguez-Cortés, Y.M.; Rodríguez-Retana, D.; Ramírez-Carreto, R.J.; Concepción-Carrillo, L.E.; Pérez-Flores, L.J.; Alarcón-Aguilar, A.; López-Díazguerrero, N.E.; Gómez-González, B.; et al. The Obese Brain: Mechanisms of Systemic and Local Inflammation, and Interventions to Reverse the Cognitive Deficit. Front. Integr. Neurosci. 2022, 16, 798995. [Google Scholar] [CrossRef]
- Zingale, V.D.; D’Angiolini, S.; Chiricosta, L.; Calcaterra, V.; Selvaggio, G.G.O.; Zuccotti, G.; Destro, F.; Pelizzo, G.; Mazzon, E. Does Childhood Obesity Trigger Neuroinflammation? Biomedicines 2022, 10, 1953. [Google Scholar] [CrossRef]
- Neniskyte, U.; Neher, J.J.; Brown, G.C. Neuronal Death Induced by Nanomolar Amyloid β Is Mediated by Primary Phagocytosis of Neurons by Microglia. J. Biol. Chem. 2011, 286, 39904–39913. [Google Scholar] [CrossRef]
- Cavalieri, M.; Ropele, S.; Petrovic, K.; Pluta-Fuerst, A.; Homayoon, N.; Enzinger, C.; Grazer, A.; Katschnig, P.; Schwingenschuh, P.; Berghold, A.; et al. Metabolic Syndrome, Brain Magnetic Resonance Imaging, and Cognition. Diabetes Care 2010, 33, 2489–2495. [Google Scholar] [CrossRef]
| Characteristic | Categories | Unweighted | Weighted | ||||
|---|---|---|---|---|---|---|---|
| n | % | % | SE | LCL a | UCL a | ||
| Gender | Males | 1542 | 48.51 | 45.44 | 0.97 | 43.45 | 47.44 |
| Females b | 1637 | 51.49 | 54.56 | 0.97 | 52.56 | 56.55 | |
| Education level | Up to 12th grade | 837 | 27.49 | 16.91 | 1.50 | 13.95 | 20.21 |
| High school graduate | 731 | 23.02 | 21.97 | 1.37 | 19.22 | 24.92 | |
| Some college or AA degree | 867 | 27.30 | 31.13 | 1.34 | 28.42 | 33.95 | |
| College graduate or above b | 705 | 22.20 | 29.99 | 1.98 | 25.98 | 34.24 | |
| Race | Mexican-American | 291 | 9.15 | 3.68 | 0.80 | 2.21 | 5.71 |
| Other Hispanic | 326 | 10.25 | 3.77 | 0.68 | 2.50 | 5.44 | |
| Non-Hispanic White b | 1467 | 46.15 | 78.40 | 1.94 | 74.13 | 82.27 | |
| Non-Hispanic Black | 778 | 24.47 | 8.91 | 1.24 | 6.54 | 11.80 | |
| Non-Hispanic Asian | 271 | 8.52 | 3.45 | 0.47 | 2.55 | 4.56 | |
| Other race and multi-racial | 46 | 1.45 | 1.78 | 0.51 | 0.88 | 3.19 | |
| Marital status | Single or never married | 188 | 5.92 | 4.41 | 0.46 | 3.51 | 5.46 |
| Divorced or separated | 530 | 16.69 | 13.75 | 0.64 | 12.47 | 15.11 | |
| Widowed | 659 | 20.76 | 17.50 | 0.86 | 15.77 | 19.33 | |
| Married or living with partner b | 1798 | 56.63 | 64.34 | 1.12 | 62.00 | 66.64 | |
| Annual household income c | $0–$14,999 | 459 | 16.00 | 17.10 | 1.13 | 14.84 | 19.55 |
| $15,000–$34,999 | 870 | 30.33 | 29.57 | 1.10 | 27.35 | 31.87 | |
| $35,000–$64,999 | 653 | 22.77 | 23.42 | 1.39 | 20.63 | 26.39 | |
| $65,000 and over | 886 | 30.89 | 29.91 | 1.14 | 27.58 | 32.31 | |
| Annual family income c | $0–$14,999 | 549 | 18.86 | 19.67 | 1.27 | 17.12 | 22.41 |
| $15,000–$34,999 | 872 | 29.96 | 29.47 | 1.12 | 27.20 | 31.82 | |
| $35,000–$64,999 | 656 | 22.54 | 23.21 | 1.53 | 20.14 | 26.51 | |
| $65,000 and over | 834 | 28.65 | 27.65 | 1.25 | 25.12 | 30.29 | |
| Medical History Variables | Categories | Unweighted | Weighted | ||||
|---|---|---|---|---|---|---|---|
| n | % | % | SE | LCL a | UCL a | ||
| Self-reported general health condition | Poor | 164 | 5.26 | 3.62 | 0.42 | 2.81 | 4.57 |
| Good or fair | 1981 | 63.60 | 55.64 | 1.47 | 52.58 | 58.67 | |
| Excellent or very good b | 970 | 31.14 | 40.74 | 1.50 | 37.66 | 43.88 | |
| Difficulties in thinking or remembering | Yes | 497 | 15.64 | 13.56 | 0.74 | 12.08 | 15.14 |
| No b | 2680 | 84.36 | 86.44 | 0.74 | 84.86 | 87.92 | |
| Ever told you have a heart disease | Yes | 287 | 9.09 | 9.36 | 0.85 | 7.69 | 11.25 |
| No b | 2871 | 90.91 | 90.64 | 0.85 | 88.75 | 92.31 | |
| Ever told you had a stroke | Yes | 242 | 7.63 | 6.83 | 0.53 | 5.79 | 7.99 |
| No b | 2931 | 92.37 | 93.17 | 0.53 | 92.01 | 94.21 | |
| Ever told you have diabetes | Yes | 763 | 25.17 | 20.59 | 0.84 | 18.89 | 22.37 |
| No b | 2268 | 74.83 | 79.41 | 0.84 | 77.63 | 81.11 | |
| Ever told you have high blood pressure two or more times | Yes | 1672 | 83.85 | 84.30 | 1.07 | 81.99 | 86.42 |
| No b | 322 | 16.15 | 15.70 | 1.07 | 13.58 | 18.01 | |
| Have smoked at least 100 cigarettes in life | Yes | 1600 | 50.38 | 50.35 | 1.48 | 47.29 | 53.41 |
| No b | 1576 | 49.62 | 49.65 | 1.48 | 46.59 | 52.71 | |
| Taking medication for high glucose levels c | Yes | 620 | 56.93 | 54.68 | 2.41 | 49.63 | 59.67 |
| No b | 469 | 43.07 | 45.32 | 2.41 | 40.34 | 50.37 | |
| Taking medication for high cholesterol levels c | Yes | 1353 | 85.36 | 86.86 | 0.99 | 84.70 | 88.83 |
| No b | 232 | 14.64 | 13.14 | 0.99 | 11.17 | 15.30 | |
| Taking medication for high blood pressure c | Yes | 1782 | 93.59 | 93.86 | 0.82 | 91.95 | 95.44 |
| No b | 122 | 6.41 | 6.14 | 0.82 | 4.56 | 8.05 | |
| Combinations | Unweighted | Weighted | ||||
|---|---|---|---|---|---|---|
| n | % | % | SE | LCL a | UCL a | |
| Classical combinations | ||||||
| Completely healthy (no criteria) b | 74 | 2.33 | 6.31 | 1.00 | 4.42 | 8.68 |
| Unhealthy (one or two criteria) | 2023 | 63.64 | 46.91 | 2.89 | 40.90 | 52.98 |
| Metabolic syndrome (three or more criteria) | 912 | 28.69 | 45.93 | 2.90 | 39.90 | 52.03 |
| Unable to define c | 170 | 5.35 | 0.87 d | 0.38 | 0.26 | 2.08 |
| Three-criteria combinations | 647 | 20.35 | 28.25 | 1.68 | 24.85 | 31.83 |
| AO + TRI + HDL | 15 | 0.47 | 0.97 d | 0.46 | 0.27 | 2.47 |
| AO + TRI + HBP | 21 | 0.66 | 1.86 | 0.54 | 0.92 | 3.35 |
| AO + TRI + GLY | 50 | 1.57 | 3.92 | 0.82 | 2.42 | 5.95 |
| AO + HDL + HBP | 225 | 7.08 | 0.90 d | 0.31 | 0.38 | 1.80 |
| AO + HDL + GLY | 63 | 1.98 | 3.61 | 0.61 | 2.47 | 5.09 |
| AO + HBP + GLY | 236 | 7.42 | 15.42 | 1.34 | 12.77 | 18.37 |
| TRI + HDL + HBP | 6 | 0.19 | 0.21 d | 0.16 | 0.01 | 0.85 |
| TRI + HDL + GLY | 8 | 0.25 | 0.56 d | 0.31 | 0.12 | 1.62 |
| TRI + HBP + GLY | 11 | 0.35 | 0.44 d | 0.16 | 0.16 | 0.95 |
| HDL + HBP + GLY | 12 | 0.38 | 0.36d | 0.13 | 0.12 | 0.84 |
| Four-criteria combinations | 196 | 6.17 | 13.34 | 1.75 | 9.95 | 17.36 |
| AO + TRI + HDL + HBP | 11 | 0.35 | 0.58 d | 0.21 | 0.22 | 1.21 |
| AO + TRI + HDL + GLY | 65 | 2.04 | 5.20 | 1.15 | 3.09 | 8.13 |
| AO + TRI + HBP + GLY | 68 | 2.14 | 4.82 | 0.85 | 3.22 | 6.88 |
| AO + HDL + HBP + GLY | 47 | 1.48 | 2.38 | 0.49 | 1.49 | 3.59 |
| TRI + HDL + HBP + GLY | 5 | 0.16 | 0.37d | 0.20 | 0.08 | 1.07 |
| Five-criteria combination | 69 | 2.17 | 4.34 | 0.87 | 2.72 | 6.52 |
| AO + TRI + HDL + HBP + GLY | 69 | 2.17 | 4.34 | 0.87 | 2.72 | 6.52 |
| Combinations of particular interest | ||||||
| Only combinations with AO | 870 | 27.37 | 43.99 | 3.03 | 37.71 | 50.41 |
| Only combinations without AO | 42 | 1.32 | 1.96 | 0.45 | 1.12 | 3.09 |
| Only combinations with GLY | 634 | 19.94 | 41.40 | 2.64 | 35.97 | 47.00 |
| Only combinations without GLY | 278 | 8.74 | 4.52 | 0.65 | 3.29 | 6.05 |
| Cognitive Test | Weighted Scores | Raw Scores Percentiles | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| M (SE) a | 95% CI | 1st | 5th | 10th | 25th | 50th | 75th | 90th | 95th | 99th | |
| CERAD–IR | 19.70 (0.21) | [19.26, 20.13] | 9 | 12 | 14 | 17 | 20 | 23 | 25 | 26 | 28 |
| CERAD–DR | 6.20 (0.09) | [6.02, 6.39] | 0 | 2 | 3 | 5 | 6 | 8 | 9 | 10 | 10 |
| AFT | 17.71 (0.13) | [17.44, 17.97] | 6 | 9 | 11 | 14 | 18 | 21 | 25 | 27 | 29 |
| DSST | 51.46 (0.54) | [50.35, 52.56] | 13 | 23 | 29 | 41 | 53 | 64 | 73 | 78 | 85 |
| MetS Combination as Independent Variables | Beta (SE) | MetS Combination | Metabolically-Healthy b | ||
|---|---|---|---|---|---|
| M c (SE) | 95% CI | M c (SE) | 95% CI | ||
| Dependent variable: CERAD Immediate Recall Test (CERAD–IR) | |||||
| Combinations with three or more criteria | −1.27 * (0.51) | 19.03 (0.64) | [17.72, 20.34] | 20.29 (0.79) | [18.69, 21.90] |
| Combinations with three criteria | −1.47 * (0.56) | 18.43 (1.00) | [16.40, 20.47] | 19.90 (1.12) | [17.63, 22.18] |
| Combinations with abdominal obesity | −1.24 * (0.51) | 19.13 (0.65) | [17.81, 20.45] | 20.36 (0.76) | [18.81, 21.92] |
| Combinations with hyperglycemia | −1.12 * (0.52) | 19.32 (0.68) | [17.94, 20.71] | 20.44 (0.89) | [18.63, 22.26] |
| Dependent variable: CERAD Delayed Recall Test (CERAD–DR) | |||||
| Combinations with three or more criteria | −0.57 * (0.26) | 6.00 (0.33) | [5.34, 6.67] | 6.57 (0.43) | [5.70, 7.45] |
| Combinations with three criteria | −0.68 * (0.29) | 5.31 (0.51) | [4.27, 6.35] | 5.99 (0.64) | [4.68, 7.30] |
| Combinations with abdominal obesity | −0.58 * (0.26) | 6.08 (0.33) | [5.41, 6.74] | 6.66 (0.41) | [5.82, 7.50] |
| Dependent variable: digit symbol substitution test (DSST) | |||||
| Combinations with four criteria | 4.58 * (2.11) | 42.40 (2.70) | [36.87, 47.93] | 37.81 (2.62) | [32.43, 43.20] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Díaz-Camargo, E.; Hernández-Lalinde, J.; Sánchez-Rubio, M.; Chaparro-Suárez, Y.; Álvarez-Caicedo, L.; Fierro-Zarate, A.; Gravini-Donado, M.; García-Pacheco, H.; Rojas-Quintero, J.; Bermúdez, V. NHANES 2011–2014 Reveals Decreased Cognitive Performance in U.S. Older Adults with Metabolic Syndrome Combinations. Int. J. Environ. Res. Public Health 2023, 20, 5257. https://doi.org/10.3390/ijerph20075257
Díaz-Camargo E, Hernández-Lalinde J, Sánchez-Rubio M, Chaparro-Suárez Y, Álvarez-Caicedo L, Fierro-Zarate A, Gravini-Donado M, García-Pacheco H, Rojas-Quintero J, Bermúdez V. NHANES 2011–2014 Reveals Decreased Cognitive Performance in U.S. Older Adults with Metabolic Syndrome Combinations. International Journal of Environmental Research and Public Health. 2023; 20(7):5257. https://doi.org/10.3390/ijerph20075257
Chicago/Turabian StyleDíaz-Camargo, Edgar, Juan Hernández-Lalinde, María Sánchez-Rubio, Yudy Chaparro-Suárez, Liseth Álvarez-Caicedo, Alexandra Fierro-Zarate, Marbel Gravini-Donado, Henry García-Pacheco, Joselyn Rojas-Quintero, and Valmore Bermúdez. 2023. "NHANES 2011–2014 Reveals Decreased Cognitive Performance in U.S. Older Adults with Metabolic Syndrome Combinations" International Journal of Environmental Research and Public Health 20, no. 7: 5257. https://doi.org/10.3390/ijerph20075257
APA StyleDíaz-Camargo, E., Hernández-Lalinde, J., Sánchez-Rubio, M., Chaparro-Suárez, Y., Álvarez-Caicedo, L., Fierro-Zarate, A., Gravini-Donado, M., García-Pacheco, H., Rojas-Quintero, J., & Bermúdez, V. (2023). NHANES 2011–2014 Reveals Decreased Cognitive Performance in U.S. Older Adults with Metabolic Syndrome Combinations. International Journal of Environmental Research and Public Health, 20(7), 5257. https://doi.org/10.3390/ijerph20075257





