Estimating Excess Mortality Due to Prostate, Breast, and Uterus Cancer during the COVID-19 Pandemic in Peru: A Time Series Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Ethical Aspects
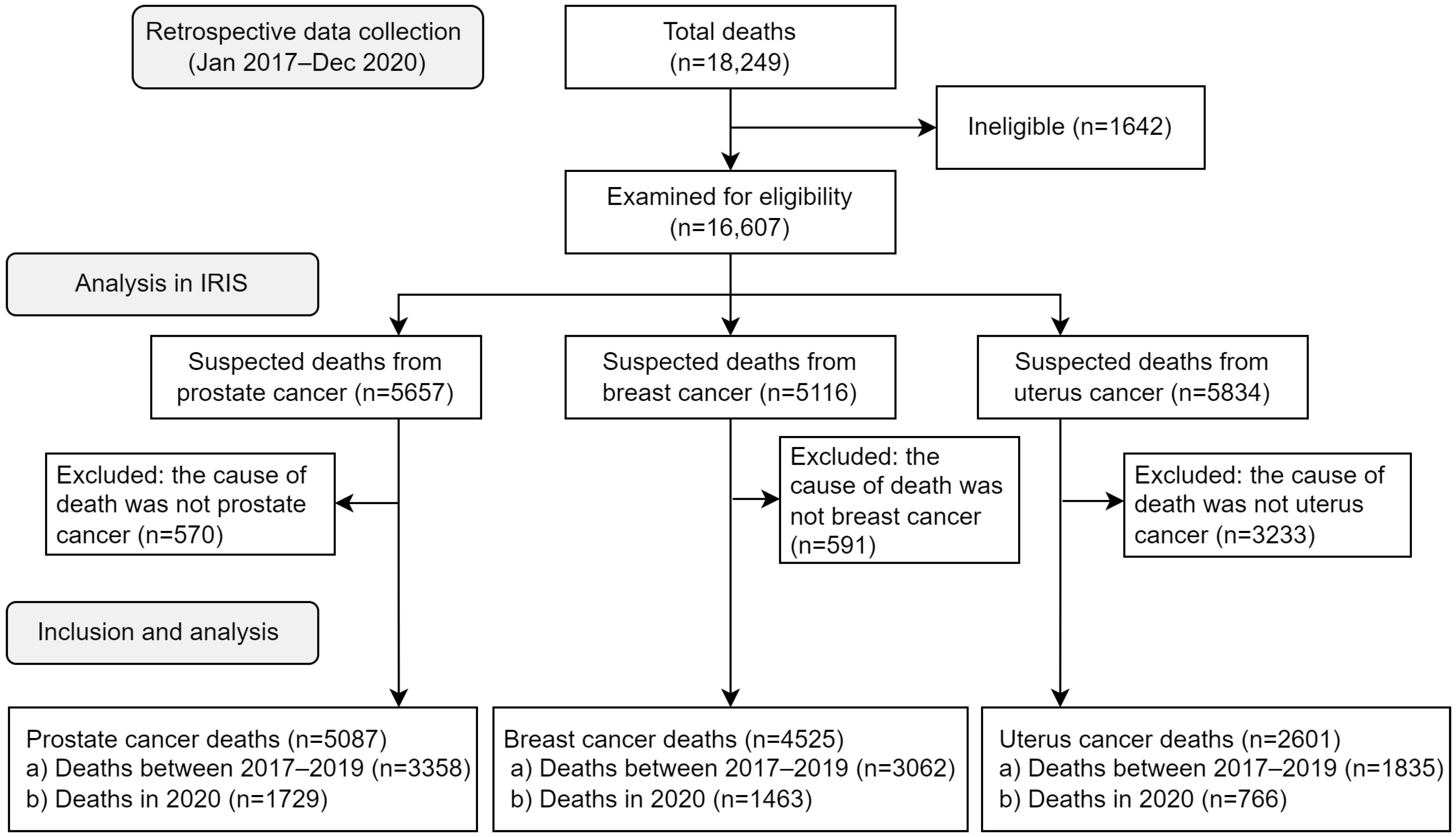
2.3. Population Study
2.4. Use of IRIS Software for Automatic Coding and Selection of the Underlying Cause of Death
2.5. Statistical Analysis
3. Results
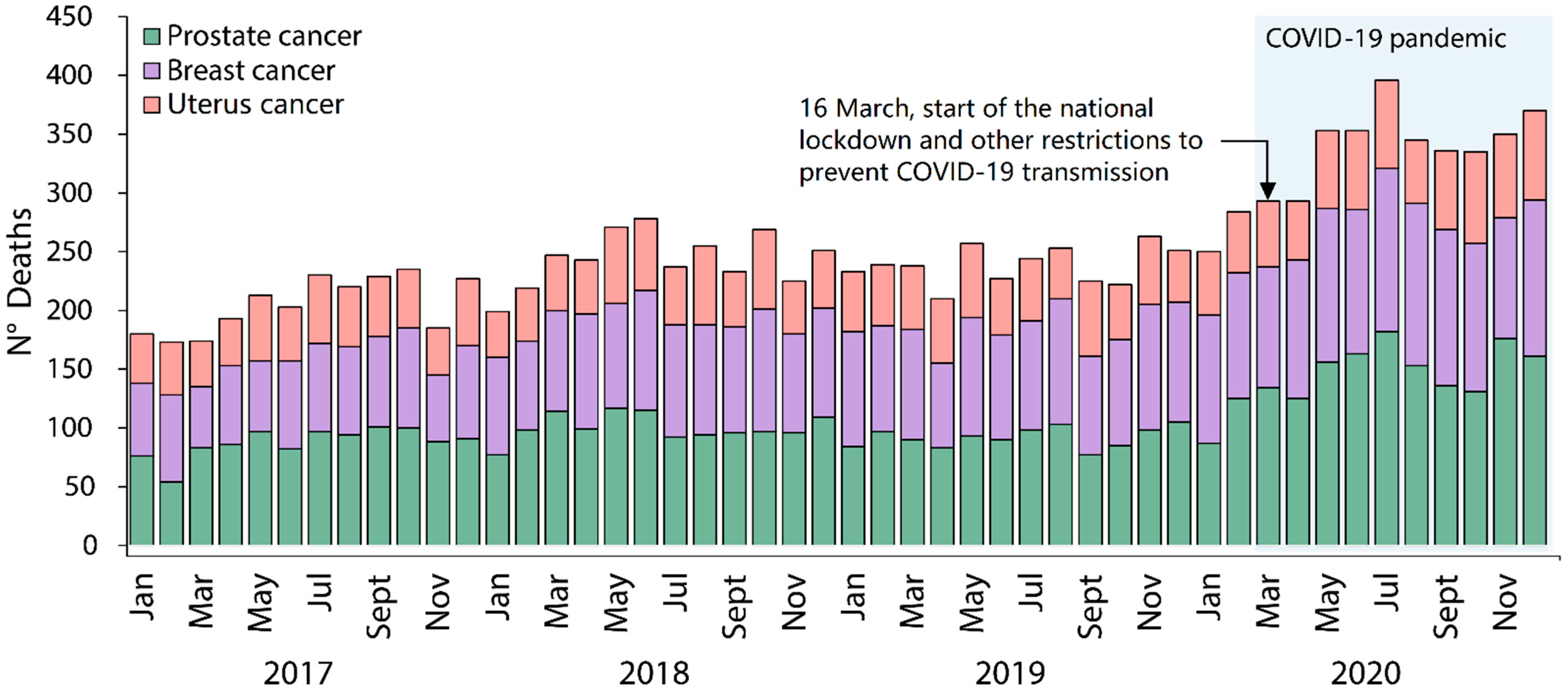
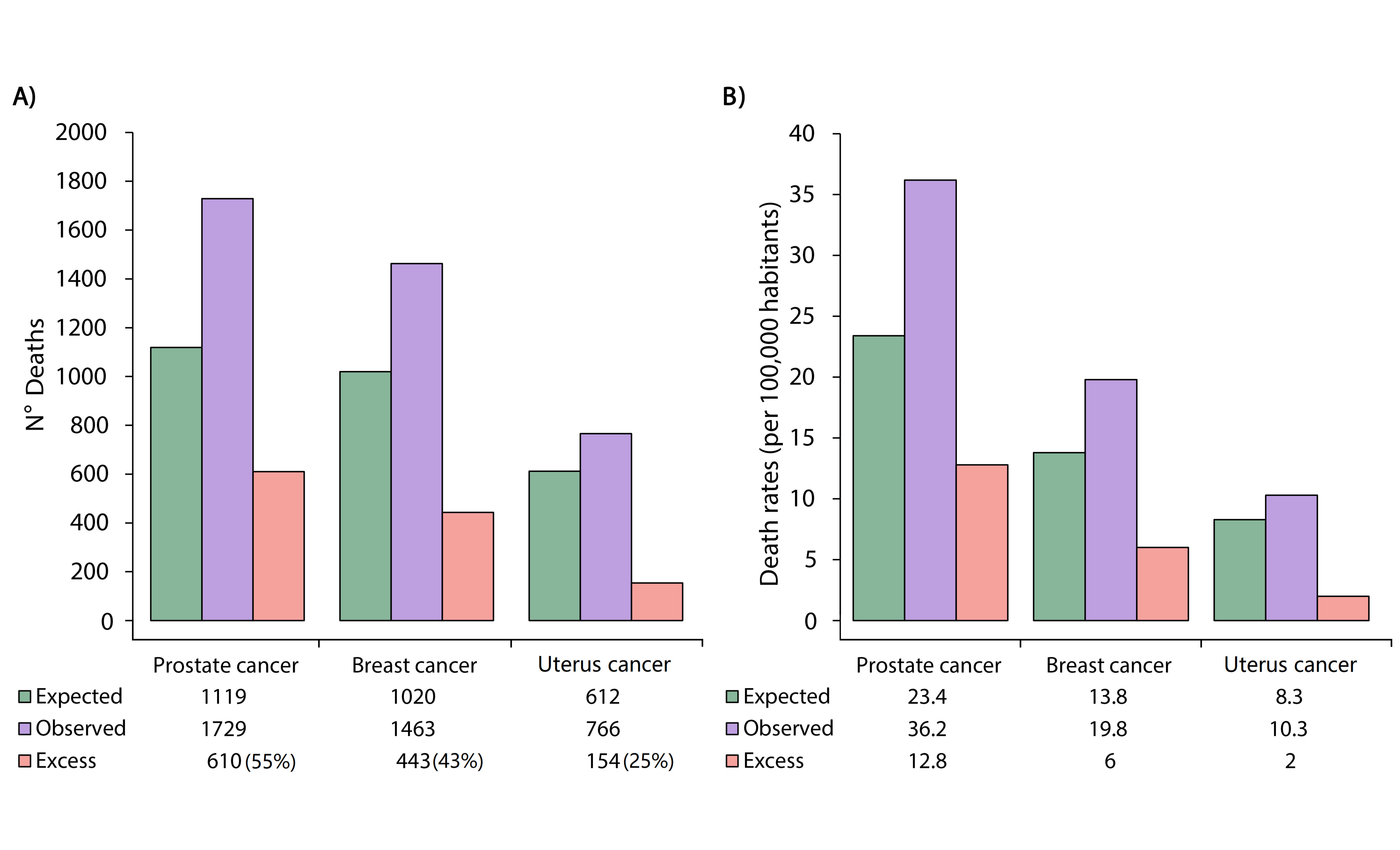
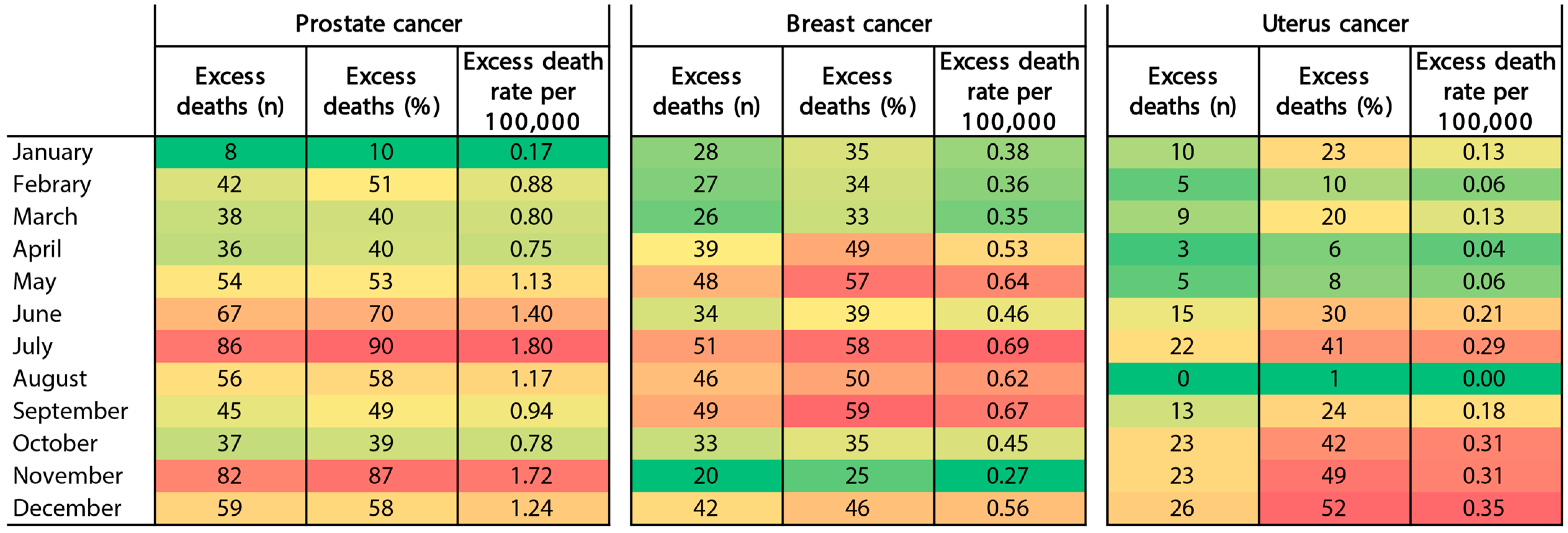
3.1. Excess Deaths and Excess Death Rates by Cancer Type
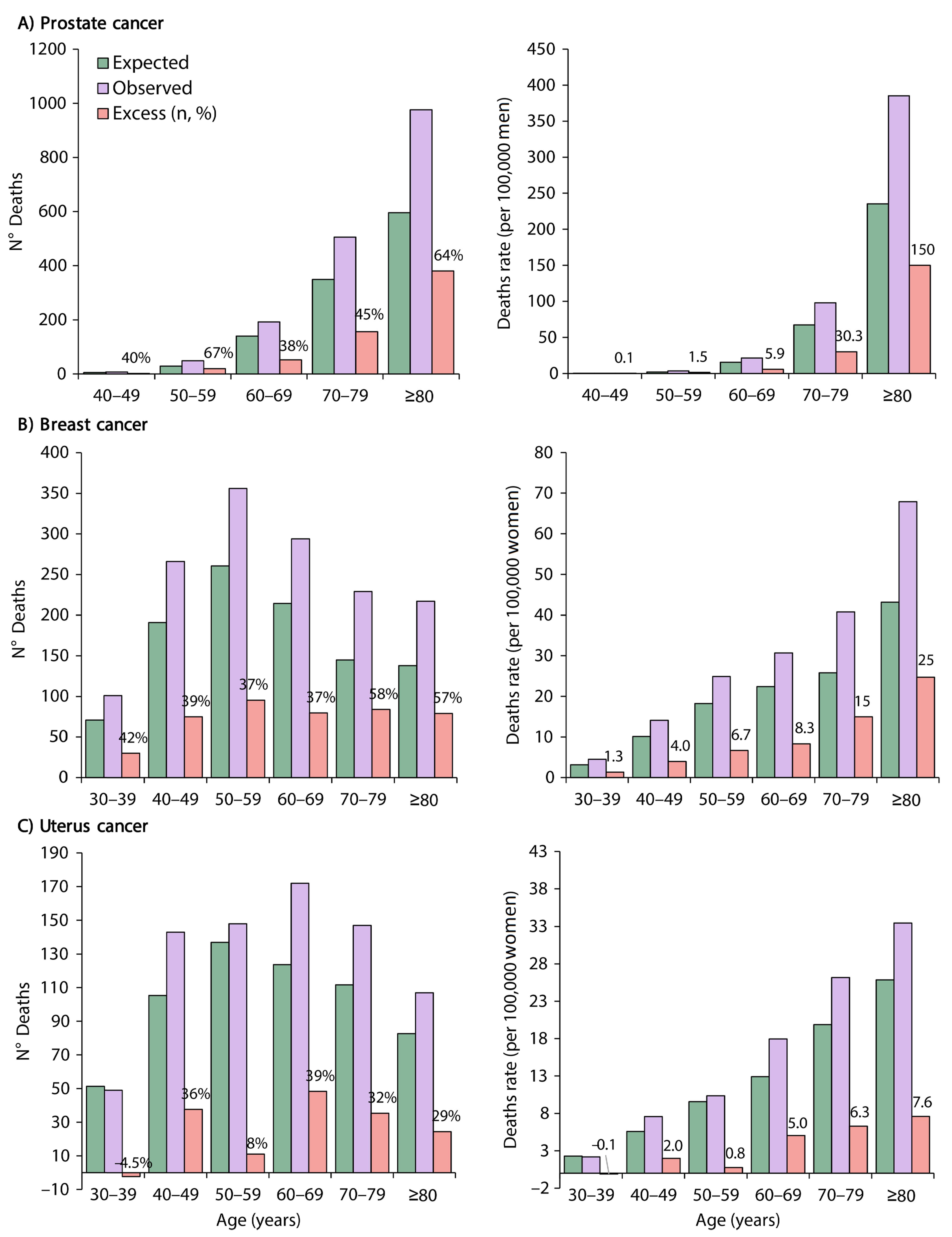
3.2. Excess Deaths and Excess Death Rates by Cancer Type and Age
3.3. ORs for Observed Deaths vs. Expected Deaths by Cancer Type and Age
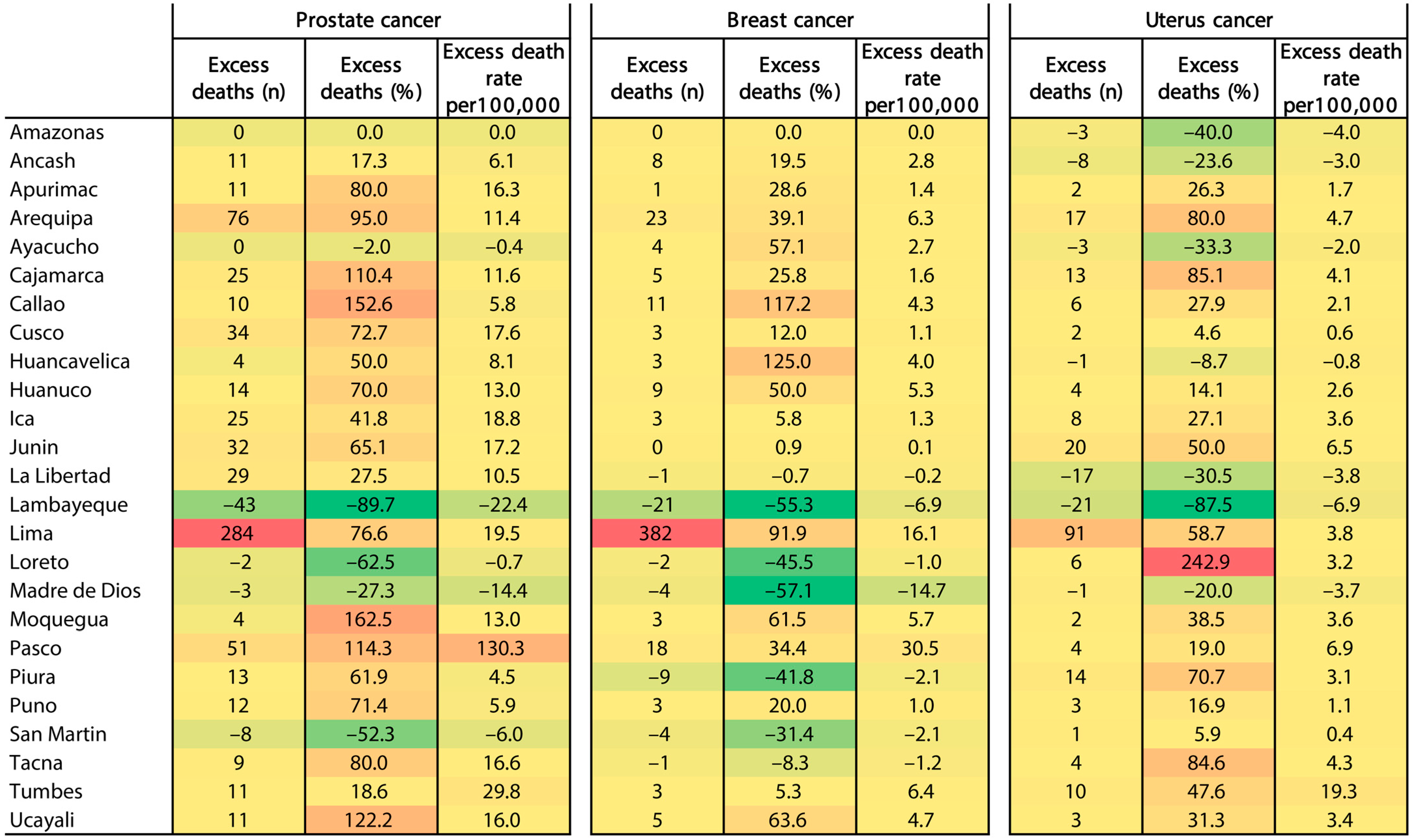
3.4. Excess Deaths and Excess Death Rates by Cancer Type and Region
4. Discussion
4.1. Potential Explanations and Implications
4.2. Limitations
4.3. Implications for Public Health
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Item No | Recommendation | |
|---|---|---|
| Title and abstract | 1 | (a) Indicate the study’s design with a commonly used term in the title or the abstract |
| (b) Provide in the abstract an informative and balanced summary of what was done and what was found | ||
| Introduction | ||
| Background/rationale | 1 | Explain the scientific background and rationale for the investigation being reported |
| Objectives | 2 | State specific objectives, including any prespecified hypotheses |
| Methods | ||
| Study design | 2 | Present key elements of study design early in the paper |
| Setting | 2 | Describe the setting, locations, and relevant dates, including periods of recruitment, exposure, follow-up, and data collection |
| Participants | 2–3 | (a) Cohort study—Give the eligibility criteria, and the sources and methods of selection of participants. Describe methods of follow-up Case-control study—Give the eligibility criteria, and the sources and methods of case ascertainment and control selection. Give the rationale for the choice of cases and controls Cross-sectional study—Give the eligibility criteria, and the sources and methods of selection of participants |
| (b) Cohort study—For matched studies, give matching criteria and number of exposed and unexposed Case-control study—For matched studies, give matching criteria and the number of controls per case | ||
| Variables | 3 | Clearly define all outcomes, exposures, predictors, potential confounders, and effect modifiers. Give diagnostic criteria, if applicable |
| Data sources/measurement | 3 * | For each variable of interest, give sources of data and details of methods of assessment (measurement). Describe comparability of assessment methods if there is more than one group |
| Bias | NA | Describe any efforts to address potential sources of bias |
| Study size | NA | Explain how the study size was arrived at |
| Quantitative variables | 3 | Explain how quantitative variables were handled in the analyses. If applicable, describe which groupings were chosen and why |
| Statistical methods | 3 | (a) Describe all statistical methods, including those used to control for confounding |
| (b) Describe any methods used to examine subgroups and interactions | ||
| (c) Explain how missing data were addressed | ||
| (d) Cohort study—If applicable, explain how loss to follow-up was addressed Case-control study—If applicable, explain how matching of cases and controls was addressed Cross-sectional study—If applicable, describe analytical methods taking account of sampling strategy | ||
| (e) Describe any sensitivity analyses | ||
| Results | ||
| Participants | 4 * | (a) Report numbers of individuals at each stage of study—e.g., numbers potentially eligible, examined for eligibility, confirmed eligible, included in the study, completing follow-up, and analyzed |
| (b) Give reasons for non-participation at each stage | ||
| (c) Consider use of a flow diagram | ||
| Descriptive data | 4 * | (a) Give characteristics of study participants (e.g., demographic, clinical, social) and information on exposures and potential confounders |
| (b) Indicate number of participants with missing data for each variable of interest | ||
| (c) Cohort study—Summarize follow-up time (e.g., average and total amount) | ||
| Outcome data | 4–8 * | Cohort study—Report numbers of outcome events or summary measures over time |
| Case-control study—Report numbers in each exposure category or summary measures of exposure | ||
| Cross-sectional study—Report numbers of outcome events or summary measures | ||
| Main results | 4–8 | (a) Give unadjusted estimates and, if applicable, confounder-adjusted estimates and their precision (e.g., 95% confidence interval). Make clear which confounders were adjusted for and why they were included |
| (b) Report category boundaries when continuous variables were categorized | ||
| (c) If relevant, consider translating estimates of relative risk into absolute risk for a meaningful time period | ||
| Other analyses | 4–8 | Report other analyses done—e.g., analyses of subgroups and interactions and sensitivity analyses |
| Discussion | ||
| Key results | 8–10 | Summarize key results with reference to study objectives |
| Limitations | 10 | Discuss limitations of the study, taking into account sources of potential bias or imprecision. Discuss both direction and magnitude of any potential bias |
| Interpretation | 8–10 | Give a cautious overall interpretation of results considering objectives, limitations, multiplicity of analyses, results from similar studies, and other relevant evidence |
| Generalizability | 8–10 | Discuss the generalizability (external validity) of the study results |
| Other information | ||
| Funding | 11 | Give the source of funding and the role of the funders for the present study and, if applicable, for the original study on which the present article is based |
References
- COVID-19 Excess Mortality Collaborators. Estimating excess mortality due to the COVID-19 pandemic: A systematic analysis of COVID-19-related mortality, 2020–2021. Lancet 2022, 399, 1513–1536. [Google Scholar] [CrossRef]
- Beaney, T.; Clarke, J.M.; Jain, V.; Golestaneh, A.K.; Lyons, G.; Salman, D.; Majeed, A. Excess mortality: The gold standard in measuring the impact of COVID-19 worldwide? J. R. Soc. Med. 2020, 113, 329–334. [Google Scholar] [CrossRef] [PubMed]
- Kontopantelis, E.; Mamas, M.A.; Webb, R.T.; Castro, A.; Rutter, M.K.; Gale, C.P.; Ashcroft, D.M.; Pierce, M.; Abel, K.M.; Price, G.; et al. Excess deaths from COVID-19 and other causes by region, neighbourhood deprivation level and place of death during the first 30 weeks of the pandemic in England and Wales: A retrospective registry study. Lancet Reg. Health Eur. 2021, 7, 100144. [Google Scholar] [CrossRef] [PubMed]
- Maringe, C.; Spicer, J.; Morris, M.; Purushotham, A.; Nolte, E.; Sullivan, R.; Rachet, B.; Aggarwal, A. The impact of the COVID-19 pandemic on cancer deaths due to delays in diagnosis in England, UK: A national, population-based, modelling study. Lancet Oncol. 2020, 21, 1023–1034. [Google Scholar] [CrossRef]
- Silversmit, G.; Verdoodt, F.; Van Damme, N.; De Schutter, H.; Van Eycken, L. Excess Mortality in a Nationwide Cohort of Cancer Patients during the Initial Phase of the COVID-19 Pandemic in Belgium. Cancer Epidemiol. Biomark. Prev. 2021, 30, 1615–1619. [Google Scholar] [CrossRef]
- Gedeborg, R.; Styrke, J.; Loeb, S.; Garmo, H.; Stattin, P. Androgen deprivation therapy and excess mortality in men with prostate cancer during the initial phase of the COVID-19 pandemic. PLoS ONE 2021, 16, e0255966. [Google Scholar] [CrossRef] [PubMed]
- Alagoz, O.; Lowry, K.P.; Kurian, A.W.; Mandelblatt, J.S.; Ergun, M.A.; Huang, H.; Lee, S.J.; Schechter, C.B.; Tosteson, A.N.A.; Miglioretti, D.L.; et al. From the CISNET Breast Working Group. Impact of the COVID-19 Pandemic on Breast Cancer Mortality in the US: Estimates From Collaborative Simulation Modeling. J. Natl. Cancer Inst. 2021, 113, 1484–1494. [Google Scholar] [CrossRef] [PubMed]
- Gheorghe, A.; Maringe, C.; Spice, J.; Purushotham, A.; Chalkidou, K.; Rachet, B.; Sullivan, R.; Aggarwal, A. Economic impact of avoidable cancer deaths caused by diagnostic delay during the COVID-19 pandemic: A national population-based modelling study in England, UK. Eur. J. Cancer 2021, 152, 233–242. [Google Scholar] [CrossRef]
- Vela-Ruiz, J.M.; Ramos, W.; Cruz-Vargas, J.A. Desafíos en la atención de los pacientes con cáncer durante la pandemia COVID-19 [Cancer care challenges during COVID -19 pandemic]. Rev. Peru Med. Exp. Salud Publica 2020, 37, 580–581. [Google Scholar] [CrossRef]
- Huaroto-Landeo, C.; Kon-Liao, K.; Falcon Pacheco, G.M.; Ticse, R. Description of surgical activity and mortality of oncological surgeries at the National Institute of Neoplastic Diseases (INEN) during the SARS-CoV-2 pandemic. Rev. Peru Med. Exp. Salud Publica 2022, 39, 120–121. [Google Scholar] [CrossRef]
- Vidaurre, T.; Enriquez-Vera, D.; Bertani, S. Excess mortality in patients with cancer during the COVID-19 pandemic in Peru: An analysis of death registry data. Lancet Oncol. 2022, 23, S28. [Google Scholar] [CrossRef]
- Federal Institute for Drugs and Medical Devices. Iris Software [Computer Program]. Available online: https://www.bfarm.de/EN/Code-systems/Collaboration-and-projects/Iris-Institute/Iris-software/_node.html (accessed on 21 June 2022).
- Vandenbroucke, J.P.; von Elm, E.; Altman, D.G.; Gøtzsche, P.C.; Mulrow, C.D.; Pocock, S.J.; Poole, C.; Schlesselman, J.J.; Egger, M.; STROBE Initiative. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): Explanation and elaboration. PLoS Med. 2007, 4, e297. [Google Scholar] [CrossRef]
- Peruvian Ministry of Health (MINSA). COVID-19 Deaths. National System of Deaths (SINADEF); MINSA: Lima, Peru, 2021; Available online: https://www.datosabiertos.gob.pe/dataset/fallecidos-por-covid-19-ministerio-de-salud-minsa (accessed on 26 December 2021).
- Wolrd Health Organization (WHO). International Statistical Classification of Diseases and Related Health Problems 10th Revision; ICD-10 Version: 2019; WHO: Geneva, Switzerland, 2019; Available online: https://icd.who.int/browse10/2019/en#/ (accessed on 21 June 2022).
- DIMDI Medical Knowledge. IRIS Software. Available online: https://www.dimdi.de/dynamic/en/klassi/irisinstitute/downloadcenter/manuals/user-guide/ (accessed on 21 June 2022).
- Federal Institute for Drugs and Medical Devices. Iris Institure. Iris Version 5.8.1 [Computer Program]. Available online: https://www.bfarm.de/EN/Code-systems/Collaboration-and-projects/Iris-Institute/Iris-downloads/_node.html (accessed on 21 June 2022).
- Mathieu, E.; Ritchie, H.; Rodés-Guirao, L.; Appel, C.; Giattino, C.; Ortiz-Ospina, E.; Hasell, J.; Macdonald, B.; Beltekian, D.; Roser, M. (2020)—“Coronavirus Pandemic (COVID-19)”. Excess Mortality during the Coronavirus Pandemic (COVID-19). Available online: https://ourworldindata.org/coronavirus (accessed on 10 January 2022).
- National Institute of Statistics and Informatics (INEI). Peruvian Population; INEI: Lima, Peru, 2020; Available online: https://www.inei.gob.pe/estadisticas/indice-tematico/population-estimates-and-projections/ (accessed on 30 March 2022).
- The Nordic Cochrane Centre Review Manager (RevMan) [Computer Program]. Version 5.3. Copenhagen, The Cochrane Collaboration. 2014. Available online: https://training.cochrane.org/online-learning/core-software/revman/revman-5-download (accessed on 5 November 2022).
- Fallara, G.; Sandin, F.; Styrke, J.; Carlsson, S.; Lissbrant, I.F.; Ahlgren, J.; Bratt, O.; Lambe, M.; Stattin, P. Prostate cancer diagnosis, staging, and treatment in Sweden during the first phase of the COVID-19 pandemic. Scand. J. Urol. 2021, 55, 184–191. [Google Scholar] [CrossRef] [PubMed]
- Blay, J.Y.; Boucher, S.; Le Vu, B.; Cropet, C.; Chabaud, S.; Perol, D.; Barranger, E.; Campone, M.; Conroy, T.; Coutant, C.; et al. Delayed care for patients with newly diagnosed cancer due to COVID-19 and estimated impact on cancer mortality in France. ESMO Open 2021, 6, 100134. [Google Scholar] [CrossRef] [PubMed]
- Klaassen, Z.; Stock, S.; Waller, J.; De Hoedt, A.; Freedland, S.J. Association of the COVID-19 Pandemic with Rates of Prostate Cancer Biopsies and Diagnoses in Black vs. White US Veterans. JAMA Oncol. 2022, 8, 914–918. [Google Scholar] [CrossRef] [PubMed]
- Valdiviezo, N.; Alcarraz, C.; Castro, D.; Salas, R.; Begazo-Mollo, V.; Galvez-Villanueva, M.; Aguirre, L.M.; Garcia-León, E.; Quispe-Santivañez, I.; Cornejo-Raymundo, C.; et al. Oncological Care During First Peruvian National Emergency COVID-19 Pandemic: A Multicentric Descriptive Study. Cancer Manag. Res. 2022, 14, 1075–1085. [Google Scholar] [CrossRef]
- Lai, A.G.; Pasea, L.; Banerjee, A.; Hall, G.; Denaxas, S.; Chang, W.H.; Katsoulis, M.; Williams, B.; Pillay, D.; Noursadeghi, M.; et al. Estimated impact of the COVID-19 pandemic on cancer services and excess 1-year mortality in people with cancer and multimorbidity: Near real-time data on cancer care, cancer deaths and a population-based cohort study. BMJ Open 2020, 10, e043828. [Google Scholar] [CrossRef]
- Han, X.; Hu, X.; Zhao, J.; Jemal, A.; Yabroff, K.R. Identification of Deaths Caused by Cancer and COVID-19 in the US during March to December 2020. JAMA Oncol. 2022, 8, 1696–1698. [Google Scholar] [CrossRef] [PubMed]
- Sari Motlagh, R.; Abufaraj, M.; Karakiewicz, P.I.; Rajwa, P.; Mori, K.; Mun, D.H.; Shariat, S.F. Association between SARS-CoV-2 infection and disease severity among prostate cancer patients on androgen deprivation therapy: A systematic review and meta-analysis. World J. Urol. 2022, 40, 907–914. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Global Health Estimates 2020: Deaths by Cause, Age, Sex, by Country and by Region. 2020. Available online: who.int/data/gho/data/themes/mortality-and-global-health-estimates/ghe-leading-causes-of-death (accessed on 30 December 2022).
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Mayo, M.; Potugari, B.; Bzeih, R.; Scheidel, C.; Carrera, C.; Shellenberger, R.A. Cancer Screening During the COVID-19 Pandemic: A Systematic Review and Meta-analysis. Mayo Clin. Proc. Innov. Qual. Outcomes 2021, 5, 1109–1117. [Google Scholar] [CrossRef]
- Torres-Roman, J.S.; Martinez-Herrera, J.F.; Carioli, G.; Ybaseta-Medina, J.; Valcarcel, B.; Pinto, J.A.; Aguilar, A.; McGlynn, K.A.; La Vecchia, C. Breast cancer mortality trends in Peruvian women. BMC Cancer 2020, 20, 1173. [Google Scholar] [CrossRef] [PubMed]
- Yong, J.H.; Mainprize, J.G.; Yaffe, M.J.; Ruan, Y.; Poirier, A.E.; Coldman, A.; Nadeau, C.; Iragorri, N.; Hilsden, R.J.; Brenner, D.R. The impact of episodic screening interruption: COVID-19 and population-based cancer screening in Canada. J. Med. Screen 2021, 28, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Gupta, N.; Chauhan, A.S.; Prinja, S.; Pandey, A.K. Impact of COVID-19 on Outcomes for Patients with Cervical Cancer in India. JCO Glob. Oncol. 2021, 7, 716–725. [Google Scholar] [CrossRef]
- Luo, Q.; O’Connell, D.L.; Yu, X.Q.; Kahn, C.; Caruana, M.; Pesola, F.; Sasieni, P.; Grogan, P.B.; Aranda, S.; Cabasag, C.J.; et al. Cancer incidence and mortality in Australia from 2020 to 2044 and an exploratory analysis of the potential effect of treatment delays during the COVID-19 pandemic: A statistical modelling study. Lancet Public Health 2022, 7, e537–e548. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Soto, M.C.; Ortega-Cáceres, G.; Arroyo-Hernández, H. Excess all-cause deaths stratified by sex and age in Peru: A time series analysis during the COVID-19 pandemic. BMJ Open 2022, 12, e057056. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramírez-Soto, M.C.; Salazar-Peña, M.; Vargas-Herrera, J. Estimating Excess Mortality Due to Prostate, Breast, and Uterus Cancer during the COVID-19 Pandemic in Peru: A Time Series Analysis. Int. J. Environ. Res. Public Health 2023, 20, 5156. https://doi.org/10.3390/ijerph20065156
Ramírez-Soto MC, Salazar-Peña M, Vargas-Herrera J. Estimating Excess Mortality Due to Prostate, Breast, and Uterus Cancer during the COVID-19 Pandemic in Peru: A Time Series Analysis. International Journal of Environmental Research and Public Health. 2023; 20(6):5156. https://doi.org/10.3390/ijerph20065156
Chicago/Turabian StyleRamírez-Soto, Max Carlos, Mariangel Salazar-Peña, and Javier Vargas-Herrera. 2023. "Estimating Excess Mortality Due to Prostate, Breast, and Uterus Cancer during the COVID-19 Pandemic in Peru: A Time Series Analysis" International Journal of Environmental Research and Public Health 20, no. 6: 5156. https://doi.org/10.3390/ijerph20065156
APA StyleRamírez-Soto, M. C., Salazar-Peña, M., & Vargas-Herrera, J. (2023). Estimating Excess Mortality Due to Prostate, Breast, and Uterus Cancer during the COVID-19 Pandemic in Peru: A Time Series Analysis. International Journal of Environmental Research and Public Health, 20(6), 5156. https://doi.org/10.3390/ijerph20065156





