The Effect of Quinolones on Common Duckweed Lemna minor L., a Hydrophyte Bioindicator of Environmental Pollution
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Biosorbent
2.2. Chemical Adsorbates
2.3. Lemna Test
- μi–j—average specific growth rate in time i to j;
- Ni—measurement variable in the test or control vessel at time i;
- Nj—measurement variable in the test or control vessel at time j;
- t—time from i to j;
- μc—mean value of µ in the control group;
- μT—mean value of µ in the treatment group;
- bc—final number of duckweed fronds and frond area minus the initial number of duckweed fronds and frond area in the control group;
- bT—final number of duckweed fronds and frond area minus the initial number of duckweed fronds and frond area in the treatment group.
- %IDW—percent reduction in dry weight;
- DWc—dry weight of duckweed fronds in the control group (mg);
- DWt—dry weight of duckweed fronds in the treatment group (mg).
2.4. Chlorophyll, Total Carotenoid Content and Phaeophytinization Quotient
2.5. Chlorophyll Fluorescence
2.6. Quinolone Biosorption
2.6.1. Spectrophotometric Measurements
2.6.2. Biosorption Measurements
2.7. Statistical Analysis
3. Results
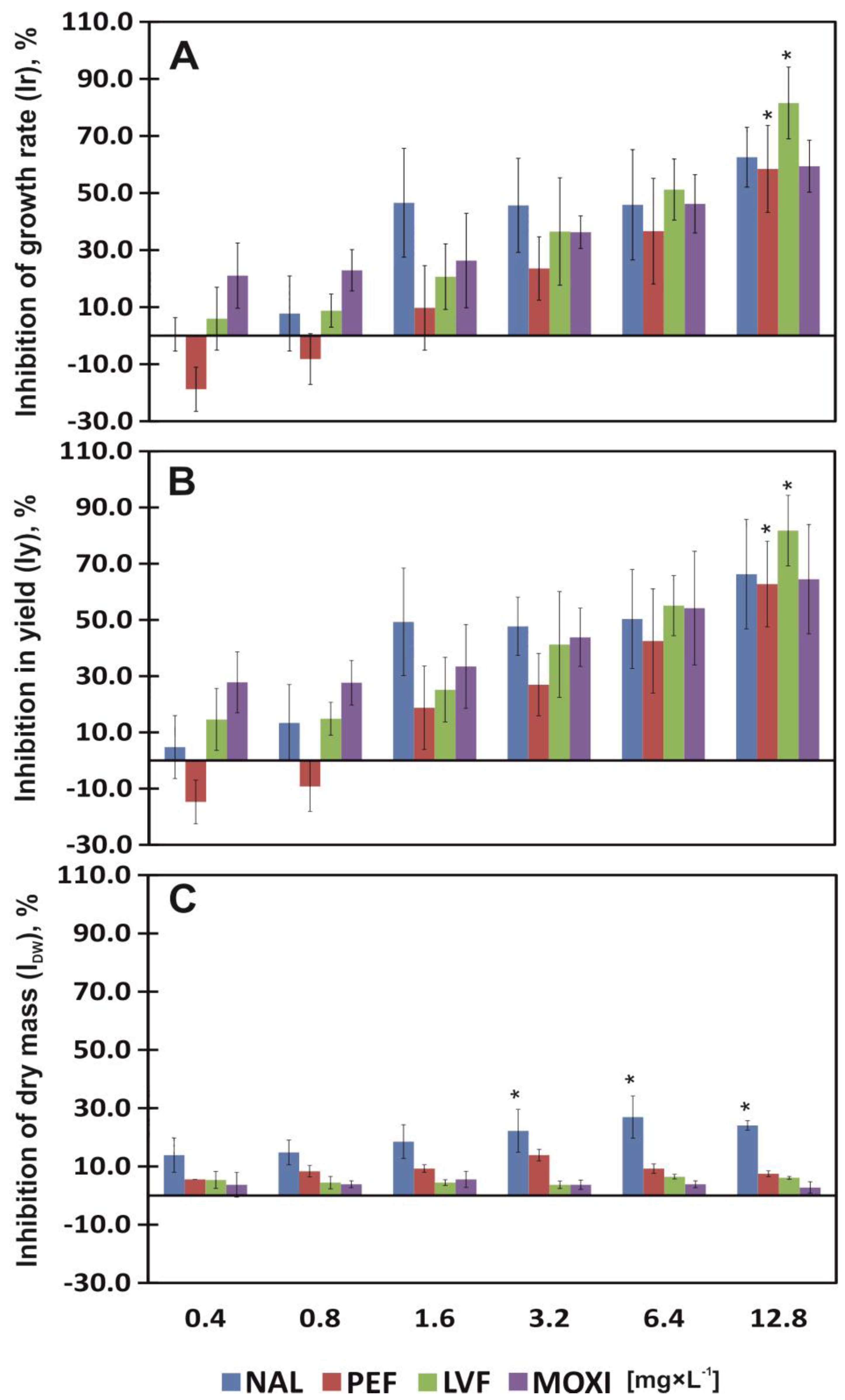
3.1. Effect of QNs on the Ir and Iy of Common Duckweed
3.2. Fresh Mass and Dry Matter Content
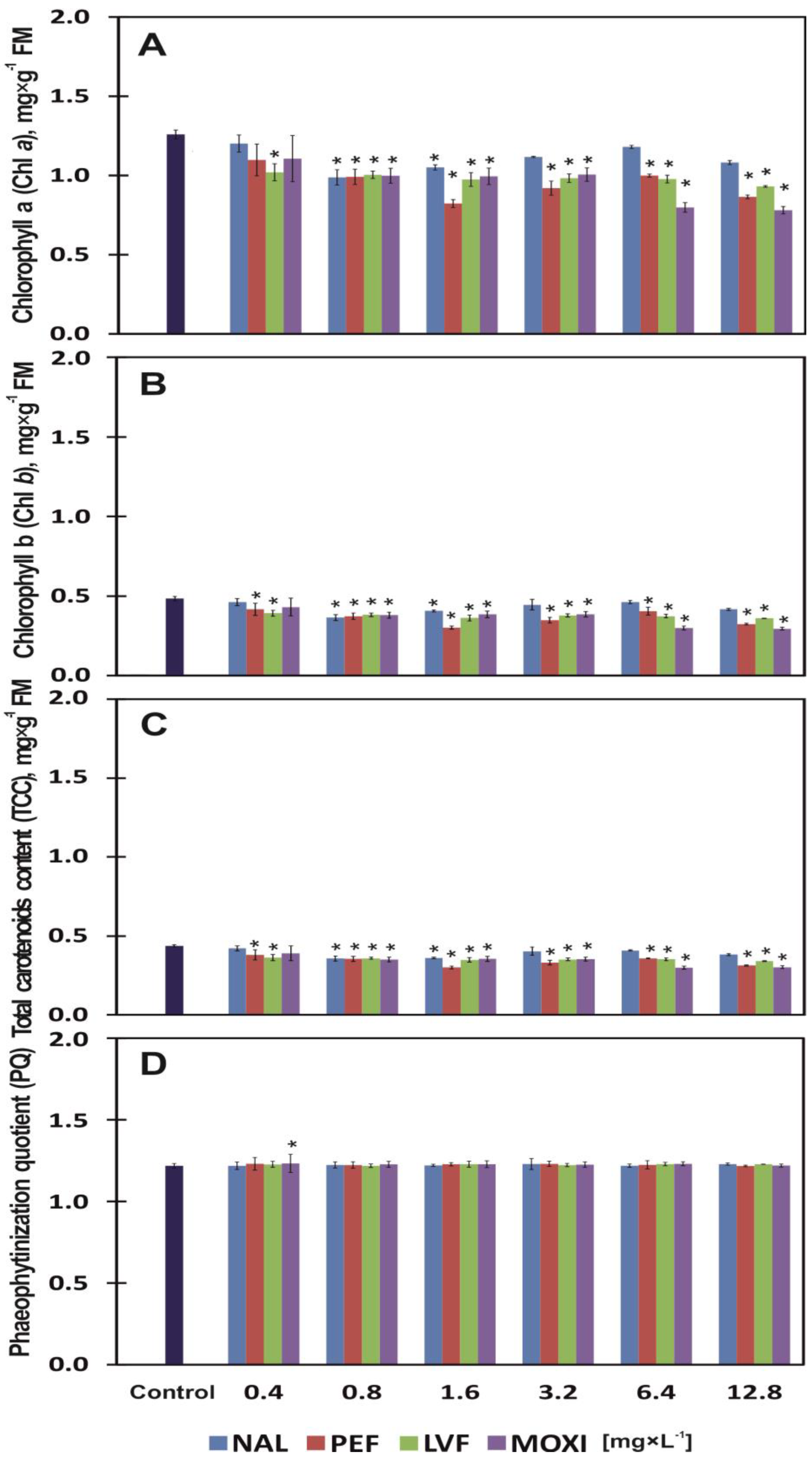
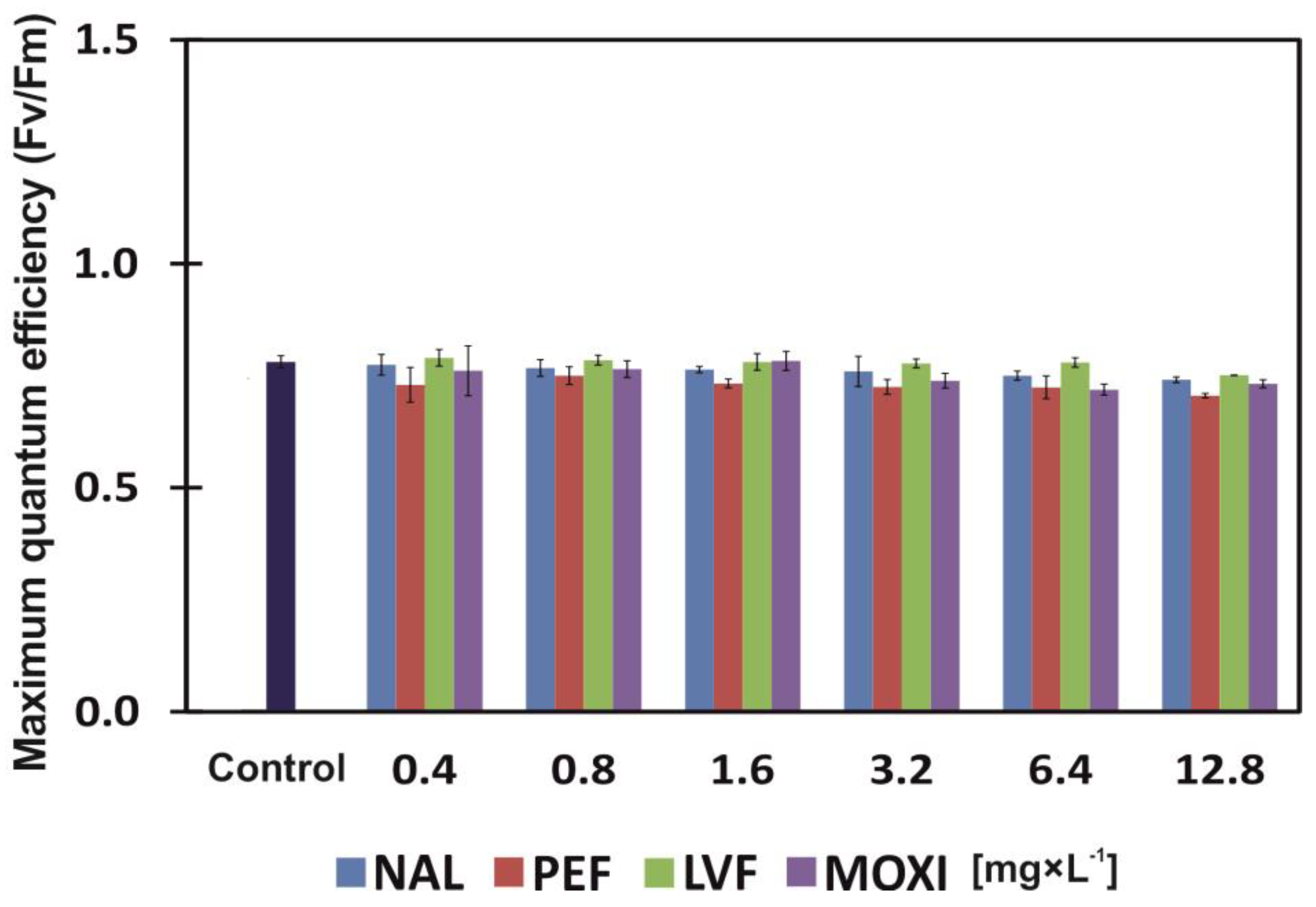
3.3. Chlorophylls, Carotenoids, Phaeophytinization and Fluorescence
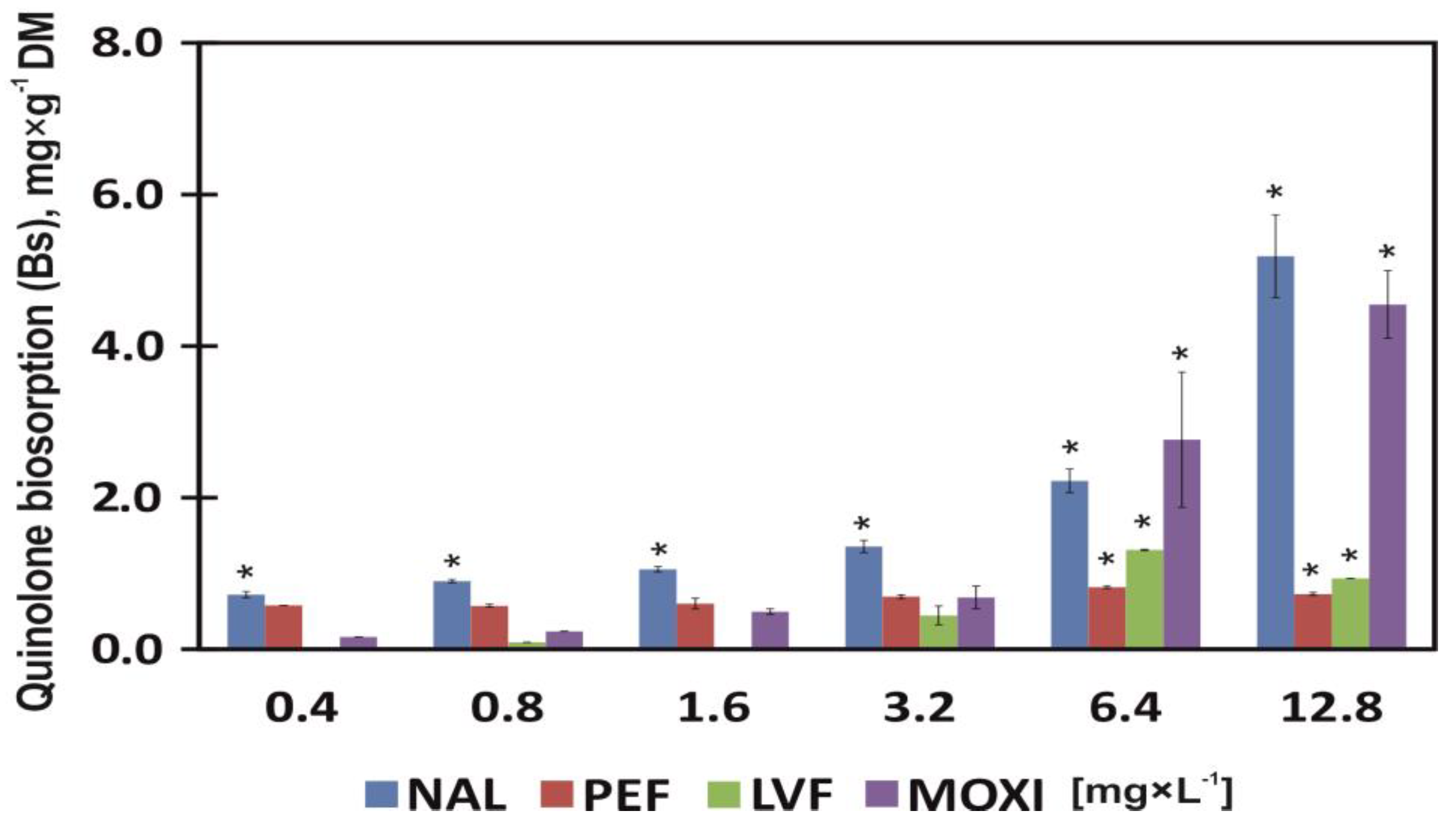
3.4. Quinolone Biosorption
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| QNs | quinolones |
| NAL | nalidixic acid |
| PEF | pefloxacin mesylate dihydrate |
| LVF | levofloxacin |
| MOXI | moxifloxacin hydrochloride |
| DM | dry matter content |
| FM | fresh mass |
| Ir | percent inhibition of growth rate |
| Iy | percent reduction in yield |
| IDW | percent reduction in dry weight |
| Chl a | chlorophyll a |
| Chl b | chlorophyll b |
| TCC | total carotenoid content |
| PQ | phaeophytinization quotient |
| Fv/Fm | maximum quantum efficiency |
| ECx | effective concentration associated with x% response (20% and 50%) |
| Bs | quinolone biosorption |
References
- Štefanac, T.; Grgas, D.; Landeka Dragičević, T. Xenobiotics-Division and Methods of Detection: A Review. J. Xenobiot. 2021, 11, 130–141. [Google Scholar] [CrossRef] [PubMed]
- Alduina, R. Antibiotics and Environment. Antibiotics 2020, 9, 202. [Google Scholar] [CrossRef] [PubMed]
- Huang, A.; Yan, M.; Lin, J.; Xu, L.; Gong, H.; Gong, H. A Review of Processes for Removing Antibiotics from Breeding Wastewater. Int. J. Environ. Res. Public. Health 2021, 18, 4909. [Google Scholar] [CrossRef] [PubMed]
- Danner, M.-C.; Robertson, A.; Behrends, V.; Reiss, J. Antibiotic pollution in surface fresh waters: Occurrence and effects. Sci. Total Environ. 2019, 664, 793–804. [Google Scholar] [CrossRef]
- Kumar, R.R.; Lee, J.T.; Cho, J.Y. Fate, occurrence, and toxicity of veterinary antibiotics in environment. J. Korean. Soc. Appl. Biol. Chem. 2012, 55, 701–709. [Google Scholar] [CrossRef]
- Nikolaou, A.; Meric, S.; Fatta, D. Occurrence patterns of pharmaceuticals in water and wastewater environments. Anal. Bioanal. Chem. 2007, 387, 1225–1234. [Google Scholar] [CrossRef]
- Sacher, F.; Lange, F.T.; Brauch, H.-J.; Blankenhorn, I. Pharmaceuticals in groundwaters. J. Chromatogr. A 2001, 938, 199–210. [Google Scholar] [CrossRef]
- Ternes, T.A.; Joss, A.; Siegrist, H. Scrutinizing pharmaceuticals and personal care products in wastewater treatment. Environ. Sci. Technol. 2004, 38, 392A–399A. [Google Scholar] [CrossRef]
- Roccaro, P.; Sgroi, M.; Vagliasindi, F.G. Removal of Xenobiotic Compounds from Wastewater for Environment Protection: Treatment Processes and Costs. Chem. Eng. Trans. 2013, 32, 505–510. [Google Scholar] [CrossRef]
- Narvaez, J.F.; Jiménez, C. Pharmaceutical products in the environment: Sources effects and risks. Vitae 2012, 19, 92–108. [Google Scholar] [CrossRef]
- Costanzo, S.D.; Murby, J.; Bates, J. Ecosystem response to antibiotics entering the aquatic environment. Mar. Pollut. Bull. 2005, 51, 218–223. [Google Scholar] [CrossRef]
- Göbel, A.a.; Thomsen, A.; McArdell, C.S.; Alder, A.C.; Giger, W.; Theiss, N.; Löffler, D.; Ternes, T.A. Extraction and determination of sulfonamides, macrolides, and trimethoprim in sewage sludge. J. Chromatogr. A 2005, 1085, 179–189. [Google Scholar] [CrossRef] [PubMed]
- Golet, E.M.; Alder, A.C.; Hartmann, A.; Ternes, T.A.; Giger, W. Trace determination of fluoroquinolone antibacterial agents in urban wastewater by solid-phase extraction and liquid chromatography with fluorescence detection. Anal. Chem. 2001, 73, 3632–3638. [Google Scholar] [CrossRef]
- Golet, E.M.; Strehler, A.; Alder, A.C.; Giger, W. Determination of fluoroquinolone antibacterial agents in sewage sludge and sludge-treated soil using accelerated solvent extraction followed by solid-phase extraction. Anal. Chem. 2002, 74, 5455–5462. [Google Scholar] [CrossRef]
- Gulkowska, A.; He, Y.; So, M.K.; Yeung, L.W.Y.; Leung, H.W.; Giesy, J.P.; Lam, P.K.S.; Martin, M.; Richardson, B.J. The occurrence of selected antibiotics in Hong Kong coastal waters. Mar. Pollut. Bull. 2007, 54, 1287–1293. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, R.; Ternes, T.A.; Haberer, K.; Mehlich, A.; Ballwanz, F.; Kratz, K.-L. Determination of antibiotics in different water compartments via liquid chromatography–electrospray tandem mass spectrometry. J. Chromatogr. A 1998, 815, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Kümmerer, K. Significance of antibiotics in the environment. J. Antimicrob. Chemother. 2003, 52, 5–7. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.-H.; Zhang, G.; Zou, S.-C.; Li, X.-D.; Liu, Y.-C. Determination of selected antibiotics in the Victoria Harbour and the Pearl River, South China using high-performance liquid chromatography-electrospray ionization tandem mass spectrometry. Environ. Pollut. 2007, 145, 672–679. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Zhang, G.; Li, X.; Zou, S.; Li, P.; Hu, Z.; Li, J. Occurrence and elimination of antibiotics at four sewage treatment plants in the Pearl River Delta (PRD), South China. Water Res. 2007, 41, 4526–4534. [Google Scholar] [CrossRef]
- Yiruhan; Wang, Q.-J.; Mo, C.-H.; Li, Y.-W.; Gao, P.; Tai, Y.-P.; Zhang, Y.; Ruan, Z.-L.; Xu, J.-W. Determination of four fluoroquinolone antibiotics in tap water in Guangzhou and Macao. Environ. Pollut. 2010, 158, 2350–2358. [Google Scholar] [CrossRef]
- Guo, X.; Kang, C.; Huang, H.; Chang, Y.; Zhong, C. Exploration of functional MOFs for efficient removal of fluoroquinolone antibiotics from water. Micropor. Mesopor. Mat. 2019, 286, 84–91. [Google Scholar] [CrossRef]
- Ye, Z.; Weinberg, H.S.; Meyer, M.T. Trace analysis of trimethoprim and sulfonamide, macrolide, quinolone, and tetracycline antibiotics in chlorinated drinking water using liquid chromatography electrospray tandem mass spectrometry. Anal. Chem. 2007, 79, 1135–1144. [Google Scholar] [CrossRef] [PubMed]
- Fick, J.; Söderström, H.; Lindberg, R.H.; Phan, C.; Tysklind, M.; Larsson, D.G.J. Contamination of surface, ground, and drinking water from pharmaceutical production. Environ. Toxicol. Chem. 2009, 28, 2522–2527. [Google Scholar] [CrossRef] [PubMed]
- Aldred, K.J.; Kerns, R.J.; Osheroff, N. Mechanism of quinolone action and resistance. Biochemistry 2014, 53, 1565–1574. [Google Scholar] [CrossRef]
- Hooper, D.C.; Jacoby, G.A. Mechanisms of drug resistance: Quinolone resistance. Ann. N. Y. Acad. Sci. 2015, 1354, 12–31. [Google Scholar] [CrossRef]
- Li, X.; Zhang, B.; Huang, W.; Cantwell, C.; Chen, B. Integration of Fuzzy Matter-Element Method and 3D-QSAR Model for Generation of Environmentally Friendly Quinolone Derivatives. Int. J. Environ. Res. Public. Health 2020, 17, 3239. [Google Scholar] [CrossRef]
- Pandey, A.; Aggarwal, N.; Adholeya, A.; Kochar, M. Resurrection of Nalidixic Acid: Evaluation of Water-Based Nanoformulations as Potential Nanomedicine. Nanoscale Res. Lett. 2018, 13, 298. [Google Scholar] [CrossRef]
- Göbel, A.; McArdell, C.S.; Joss, A.; Siegrist, H.; Giger, W. Fate of sulfonamides, macrolides, and trimethoprim in different wastewater treatment technologies. Sci. Total Environ. 2007, 372, 361–371. [Google Scholar] [CrossRef]
- Jia, A.; Wan, Y.; Xiao, Y.; Hu, J. Occurrence and fate of quinolone and fluoroquinolone antibiotics in a municipal sewage treatment plant. Water Res. 2012, 46, 387–394. [Google Scholar] [CrossRef]
- Kools, S.A.E.; Moltmann, J.F.; Knacker, T. Estimating the use of veterinary medicines in the European union. Regul. Toxicol. Pharmacol. 2008, 50, 59–65. [Google Scholar] [CrossRef]
- Ma, W.; Yang, M.; Wang, J.; Qi, R.; Ren, L. Treatment of antibiotics wastewater utilizing successive hydrolysis, denitrification and nitrification. Environ. Technol. 2002, 23, 685–694. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.-L.; Li, J.-J.; Li, S.-Y. Photocatalytic degradation of pefloxacin in water by modified nano-zinc oxide. Mater. Lett. 2017, 206, 146–149. [Google Scholar] [CrossRef]
- Ge, L.; Chen, J.; Wei, X.; Zhang, S.; Qiao, X.; Cai, X.; Xie, Q. Aquatic photochemistry of fluoroquinolone antibiotics: Kinetics, pathways, and multivariate effects of main water constituents. Environ. Sci. Technol. 2010, 44, 2400–2405. [Google Scholar] [CrossRef]
- Lübbert, C.; Baars, C.; Dayakar, A.; Lippmann, N.; Rodloff, A.C.; Kinzig, M.; Sörgel, F. Environmental pollution with antimicrobial agents from bulk drug manufacturing industries in Hyderabad, South India, is associated with dissemination of extended-spectrum beta-lactamase and carbapenemase-producing pathogens. Infection 2017, 45, 479–491. [Google Scholar] [CrossRef]
- Sellier, A.; Khaska, S.; Le Gal Salle, C. Assessment of the occurrence of 455 pharmaceutical compounds in sludge according to their physical and chemical properties: A review. J. Hazard. Mater. 2022, 426, 128104. [Google Scholar] [CrossRef] [PubMed]
- Knight, S.; Hauxwell, J.; Haber, E.A. Distribution and Abundance of Aquatic Plants—Human Impacts. Reference Module in Earth Systems and Environmental Sciences; Elsevier: Amsterdam, The Netherlands, 2014; ISBN 978-0-12-409548-9. [Google Scholar]
- Liu, L.; Liu, Y.-h.; Liu, C.-x.; Wang, Z.; Dong, J.; Zhu, G.-f.; Huang, X. Potential effect and accumulation of veterinary antibiotics in Phragmites australis under hydroponic conditions. Ecol. Eng. 2013, 53, 138–143. [Google Scholar] [CrossRef]
- Fomina, M.; Gadd, G. Biosorption: Current perspectives on concept, definition and application. Bioresour. Technol. 2014, 160, 3–14. [Google Scholar] [CrossRef]
- Nunes, B.; Veiga, V.; Frankenbach, S.; Serôdio, J.; Pinto, G. Evaluation of physiological changes induced by the fluoroquinolone antibiotic ciprofloxacin in the freshwater macrophyte species Lemna minor and Lemna gibba. Environ. Toxicol. Pharmacol. 2019, 72, 103242. [Google Scholar] [CrossRef]
- OECD. Guideline for Testing of Chemicals, Section 2 Test No.221: Lemma sp. Growth Inhibition Test; Organisation for Economic Co-Operation and Development: Paris, France, 2006. [Google Scholar]
- Lichtenthaler, H.K.; Wellburn, A.R. Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Biochem. Soc. Trans. 1983, 11, 591–592. [Google Scholar] [CrossRef]
- Barnes, J.D.; Balaguer, L.; Manrique, E.; Elvira, S.; Davison, A.W. A reappraisal of the use of DMSO for the extraction and determination of chlorophylls a and b in lichens and higher plants. Environ. Exp. Bot. 1992, 32, 85–100. [Google Scholar] [CrossRef]
- ISO 20079; ISO. Water Quality—Determination of Toxic Effect of Water Constituents and Wastewater on Duckweed (Lemma minor)—Duckweed Growth Inhibition Test. The International Organization for Standardization: Geneva, Switzerland, 2005.
- U.S. EPA 712-C-008; EPA. Ecological Effects Test Guidelines. OCSPP: 850.4400: Aquatic Plant Toxicity Test Using Lemma spp. United States Environmental Protection Agency: Washington, DC, USA, 2012.
- Baciak, M.; Sikorski, Ł.; Piotrowicz-Cieślak, A.; Adomas, B. Content of biogenic amines in Lemna minor (common duckweed) growing in medium contaminated with tetracycline. Aquat. Toxicol. 2016, 180, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Baciak, M.; Sikorski, Ł.; Piotrowicz-Cieślak, A.; Adomas, B. Role of decarboxylases in the biosynthesis of biogenic amines of pea growing in soil contaminated with lomefloxacin. Appl. Ecol. Env. Res. 2017, 15, 1131–1148. [Google Scholar] [CrossRef]
- Sikorski, Ł.; Baciak, M.; Piotrowicz-Cieślak, A.; Michalczyk, D.; Bęś, A.; Adomas, B. The bioaccumulation and metabolic effects of ciprofloxacin-HCl and ciprofloxacin free base in yellow lupin (Lupinus luteus L.) seedlings. Appl. Ecol. Environ. Res. 2017, 15, 1287–1300. [Google Scholar] [CrossRef]
- Sikorski, Ł.; Adomas, B.; Dobiesz, M.; Baciak, M.; Piotrowicz-Cieślak, A. Morphological and biochemical responses of Lemna minor L. (Common duckweed) to ciprofloxacin. Fresenius Environ. Bull. 2014, 23, 363–371. [Google Scholar]
- Migliore, L.; Cozzolino, S.; Fiori, M. Phytotoxicity to and uptake of enrofloxacin in crop plants. Chemosphere 2003, 52, 1233–1244. [Google Scholar] [CrossRef] [PubMed]
- Sikorski, Ł.; Piotrowicz-Cieślak, A.I.; Adomas, B. Phytotoxicity of Sodium Chloride towards Common Duckweed (Lemna Minor L.) and Yellow Lupin (Lupinus Luteus L.). Arch. Environ. Prot. 2013, 39, 117–128. [Google Scholar] [CrossRef]
- Sikorski, Ł.; Baciak, M.; Bęś, A.; Adomas, B. The effects of glyphosate-based herbicide formulations on Lemna minor, a non-target species. Aquat. Toxicol. 2019, 209, 70–80. [Google Scholar] [CrossRef]
- Kalčíková, G.; Žgajnar Gotvajn, A.; Kladnik, A.; Jemec, A. Impact of polyethylene microbeads on the floating freshwater plant duckweed Lemna minor. Environ. Pollut. 2017, 230, 1108–1115. [Google Scholar] [CrossRef]
- Moullan, N.; Mouchiroud, L.; Wang, X.; Ryu, D.; Williams, E.G.; Mottis, A.; Jovaisaite, V.; Frochaux, M.V.; Quiros, P.M.; Deplancke, B.; et al. Tetracyclines Disturb Mitochondrial Function across Eukaryotic Models: A Call for Caution in Biomedical Research. Cell. Rep. 2015, 10, 1681–1691. [Google Scholar] [CrossRef]
- Bęś, A.; Warmiński, K.; Adomas, B. Long-term responses of Scots pine (Pinus sylvestris L.) and European beech (Fagus sylvatica L.) to the contamination of light soils with diesel oil. Environ. Sci. Pollut. R. 2019, 26, 10587–10608. [Google Scholar] [CrossRef]
- Efroymson, R.; Will, M.; Suter II, G.; Wooten, A. Toxicological Benchmarks for Screening Contaminations of Potential Concern for Effects on Terrestrial Plants: 1997 Revision. U.S. Department of Energy. Available online: https://rais.ornl.gov/documents/tm85r3.pdf (accessed on 10 December 2022).
- Yang, T.; Jiang, Z.; Lin, D.; Guo, L.; Wu, T.; Li, Z.; He, Z.; Zhou, X. Effects of tetracycline and oxytetracycline on growth and chlorophyll fluorescence in lettuce seedlings. IOP Conf. Ser. Earth Environ. Sci. 2020, 474, 22013. [Google Scholar] [CrossRef]
- Adomas, B.; Sikorski, Ł.; Bęś, A.; Warmiński, K. Exposure of Lemna minor L. to gentian violet or Congo red is associated with changes in the biosynthesis pathway of biogenic amines. Chemosphere 2020, 254, 126752. [Google Scholar] [CrossRef] [PubMed]
- Iannelli, M.A.; Bellini, A.; Venditti, I.; Casentini, B.; Battocchio, C.; Scalici, M.; Ceschin, S. Differential phytotoxic effect of silver nitrate (AgNO3) and bifunctionalized silver nanoparticles (AgNPs-Cit-L-Cys) on Lemna plants (duckweeds). Aquat. Toxicol. 2022, 250, 106260. [Google Scholar] [CrossRef] [PubMed]
- Gadd, G.M. Biosorption: Critical review of scientific rationale, environmental importance and significance for pollution treatment. J. Chem. Technol. Biotechnol. 2009, 84, 13–28. [Google Scholar] [CrossRef]
- PubChem CID 4421. National Center for Biotechnology Information: PubChem Compound Summary for CID 4421, Nalidixic acid. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Nalidixic-acid (accessed on 7 December 2022).
- PubChem CID 101526. National Center for Biotechnology Information: PubChem Compound Summary for CID 101526, Moxifloxacin Hydrochloride. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Moxifloxacin-Hydrochloride (accessed on 7 December 2022).
- van Doorslaer, X.; Haylamicheal, I.D.; Dewulf, J.; van Langenhove, H.; Janssen, C.R.; Demeestere, K. Heterogeneous photocatalysis of moxifloxacin in water: Chemical transformation and ecotoxicity. Chemosphere 2015, 119, S75–S80. [Google Scholar] [CrossRef]
- Xiong, J.-Q.; Kurade, M.B.; Jeon, B.-H. Biodegradation of levofloxacin by an acclimated freshwater microalga, Chlorella vulgaris. Chem. Eng. J. 2017, 313, 1251–1257. [Google Scholar] [CrossRef]
- Park, D.; Yun, Y.-S.; Park, J.M. The past, present, and future trends of biosorption. Biotechnol. Bioprocess Eng. 2010, 15, 86–102. [Google Scholar] [CrossRef]

| Chemical Compound | Structural Formula | Empirical Formula | CAS Number | Molecular Weight (g × moL−1) | Form/Color |
|---|---|---|---|---|---|
| Nalidixic acid (NAL) | 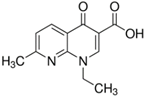 | C12H12N2O3 | 389-08-2 | 232.24 | powder/ beige |
| Pefloxacin mesylate dihydrate (PEF) | 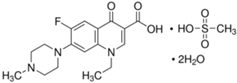 | C18H28FN3O8S | 149676-40-4 | 465.49 | powder/ white or almost white |
| Levofloxacin (LVF) | 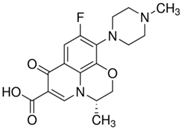 | C18H20FN3O4 | 100986-85-4 | 361.37 | powder/ light yellow |
| Moxifloxacin hydrochloride (MOXI) | 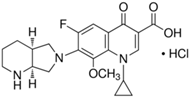 | C21H24FN3O4 · HCl | 186826-86-8 | 437.89 | crystalline/ colorless |
| SoV | Ir | Iy | IDW | FM | DM | Chl a | Chl b | TCC | PQ | Fv/Fm | Bs |
|---|---|---|---|---|---|---|---|---|---|---|---|
| F-value | |||||||||||
| Intercept | 87.48 * | 121.81 * | 137.12 * | 52,356.81 * | 10,574.14 * | 29,592.25 * | 27,794.50 * | 37,283.73 * | 12,050,801 * | 103,107.30 * | 1609.11 * |
| Antibiotic (A) | 1.76 | 2.22 | 20.54 * | 39.09 * | 9.19 * | 29.49 * | 29.52 * | 25.56 * | 4.00 * | 16.70 * | 144.85 * |
| Concentration (C) | 11.68 * | 12.41 * | 4.55 * | 27.09 * | 2.69 * | 52.11 * | 53.23 * | 49.61 * | 7.00 * | 7.50 * | 243.71 * |
| AxC | 0.50 | 0.38 | 1.08 | 2.24 * | 0.61 | 5.83 * | 7.45 * | 4.66 * | 6.00 * | 0.90 | 45.70 * |
| Antibiotic | Parameter | Effective Concentration, mg × L−1 | |
|---|---|---|---|
| EC20 | EC50 | ||
| NAL | Ir | 0.53 | 3.69 |
| Iy | 0.42 | 2.85 | |
| Mean Ir and Iy | 0.48 | 3.27 | |
| IDW | 0.94 | - | |
| FM | 1.09 | - | |
| DM | - | - | |
| Chl a | 0.63 | - | |
| Chl b | 0.56 | - | |
| TCC | - | - | |
| PQ | - | - | |
| Fv/Fm | - | - | |
| PEF | Ir | 1.59 | 6.67 |
| Iy | 1.31 | 5.30 | |
| Mean Ir and Iy | 1.45 | 5.99 | |
| IDW | - | - | |
| FM | - | - | |
| DM | - | - | |
| Chl a | 0.55 | - | |
| Chl b | 0.50 | - | |
| TCC | 0.55 | - | |
| PQ | - | - | |
| Fv/Fm | - | - | |
| LVF | Ir | 0.71 | 2.89 |
| Iy | 0.53 | 2.45 | |
| Mean Ir and Iy | 0.62 | 2.67 | |
| IDW | - | - | |
| FM | - | - | |
| DM | - | - | |
| Chl a | 0.61 | - | |
| Chl b | 0.49 | - | |
| TCC | 0.70 | - | |
| PQ | - | - | |
| Fv/Fm | - | - | |
| MOXI | Ir | 0.36 | 5.10 |
| Iy | 0.21 | 2.86 | |
| Mean Ir and Iy | 0.29 | 3.98 | |
| IDW | - | - | |
| FM | - | - | |
| DM | 0.85 | - | |
| Chl a | 0.58 | - | |
| Chl b | 0.57 | - | |
| TCC | 0.93 | - | |
| PQ | - | - | |
| Fv/Fm | - | - | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sikorski, Ł.; Bęś, A.; Warmiński, K. The Effect of Quinolones on Common Duckweed Lemna minor L., a Hydrophyte Bioindicator of Environmental Pollution. Int. J. Environ. Res. Public Health 2023, 20, 5089. https://doi.org/10.3390/ijerph20065089
Sikorski Ł, Bęś A, Warmiński K. The Effect of Quinolones on Common Duckweed Lemna minor L., a Hydrophyte Bioindicator of Environmental Pollution. International Journal of Environmental Research and Public Health. 2023; 20(6):5089. https://doi.org/10.3390/ijerph20065089
Chicago/Turabian StyleSikorski, Łukasz, Agnieszka Bęś, and Kazimierz Warmiński. 2023. "The Effect of Quinolones on Common Duckweed Lemna minor L., a Hydrophyte Bioindicator of Environmental Pollution" International Journal of Environmental Research and Public Health 20, no. 6: 5089. https://doi.org/10.3390/ijerph20065089
APA StyleSikorski, Ł., Bęś, A., & Warmiński, K. (2023). The Effect of Quinolones on Common Duckweed Lemna minor L., a Hydrophyte Bioindicator of Environmental Pollution. International Journal of Environmental Research and Public Health, 20(6), 5089. https://doi.org/10.3390/ijerph20065089






