Functional and Cognitive Occupational Therapy (FaCoT) Improves Self-Efficacy and Behavioral–Emotional Status of Individuals with Mild Stroke; Analysis of Secondary Outcomes
Abstract
1. Introduction
2. Materials and Methods
2.1. Design
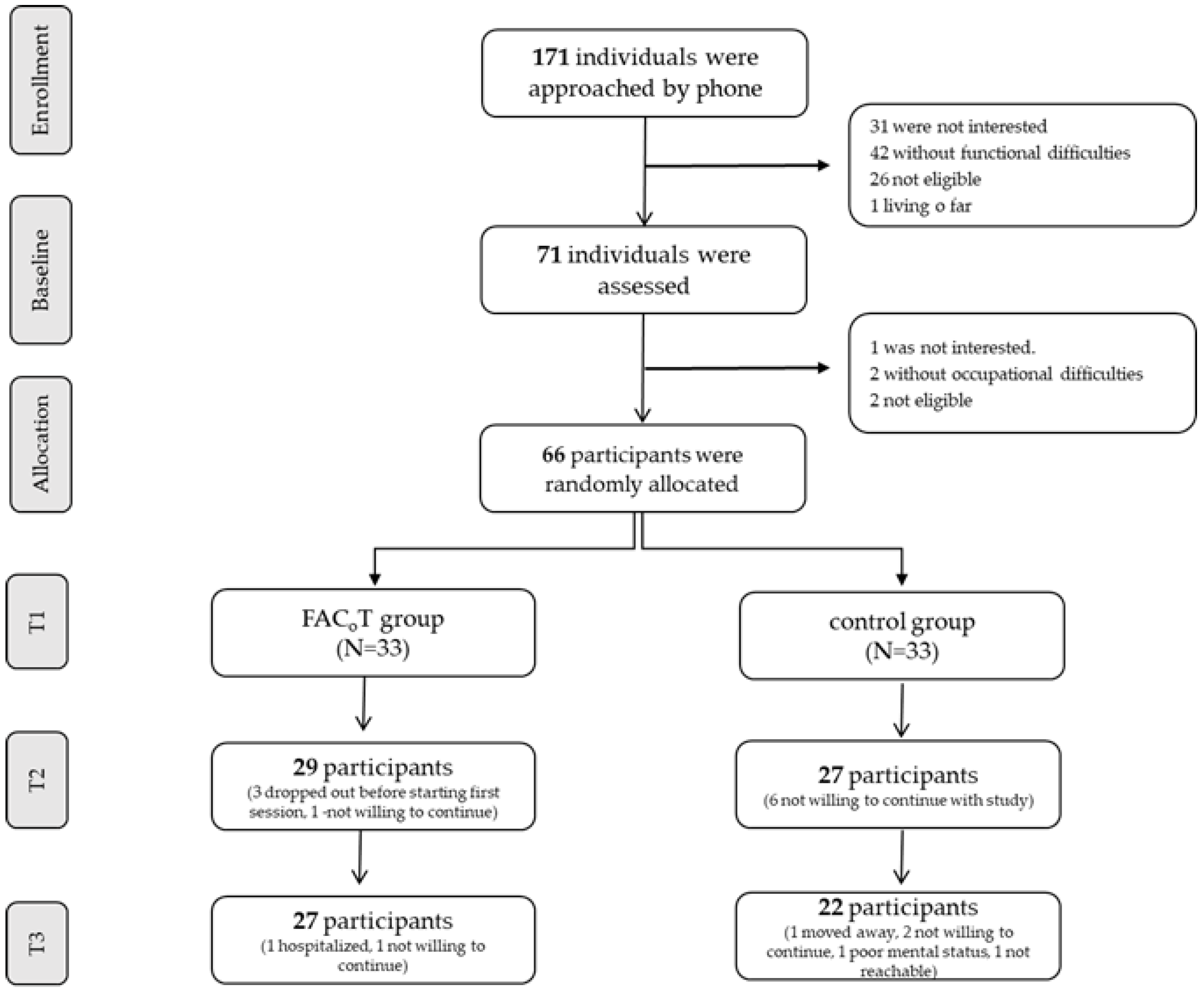
2.2. Participants
2.3. Randomization
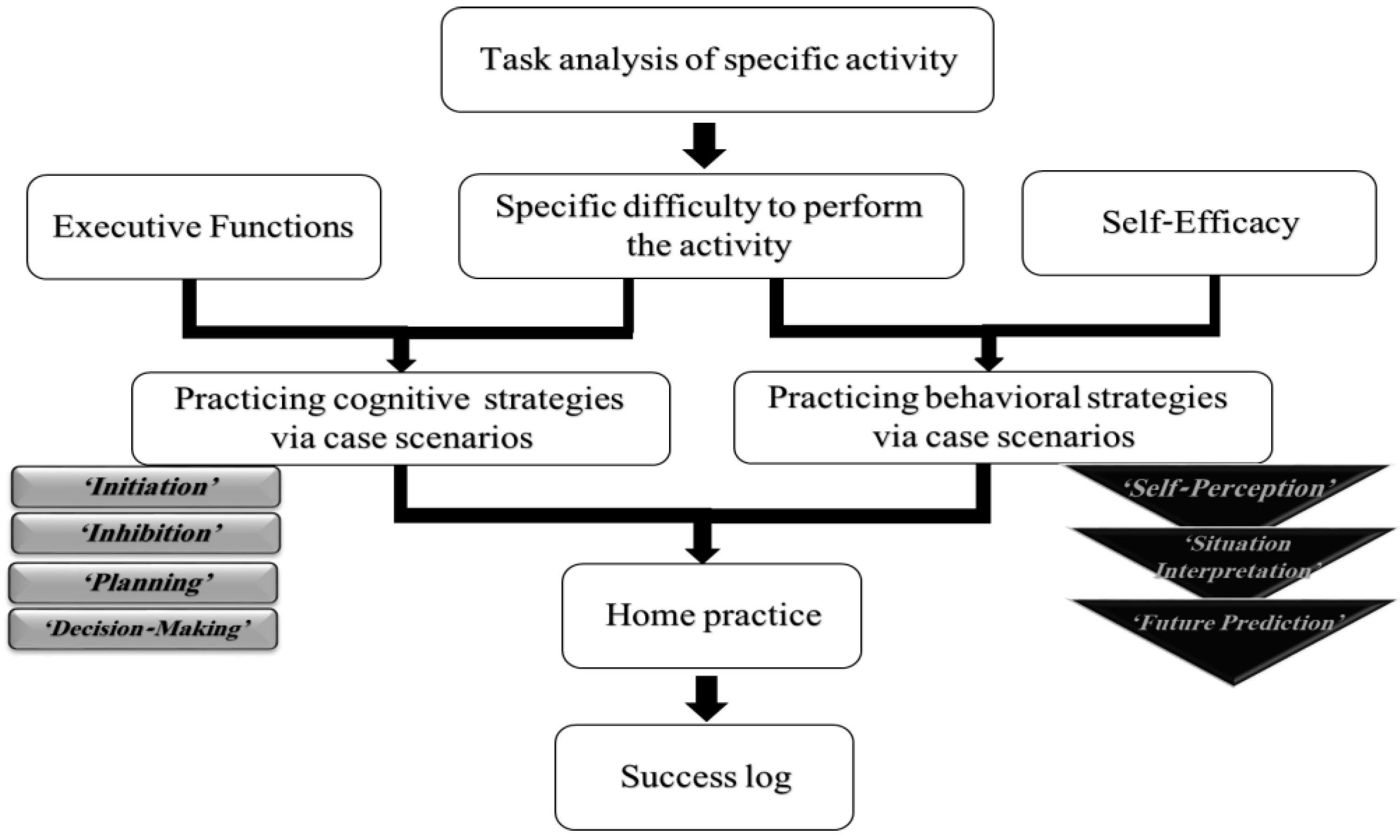
2.4. Intervention
2.5. Instruments
2.6. Data Analysis
3. Results
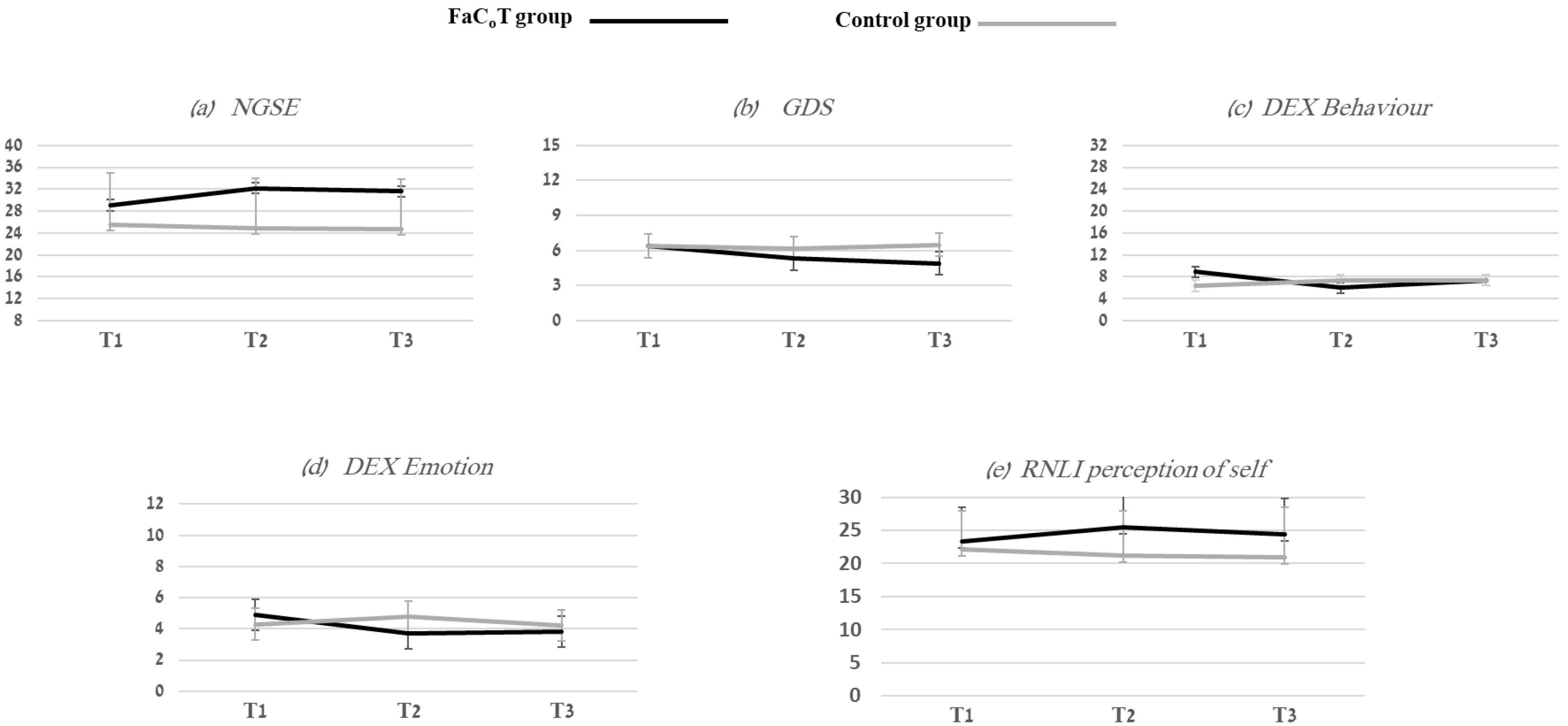
3.1. Self-Efficacy
3.2. Behavior and Emotional Status
3.3. RNLI Self-Perception
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Adamit, T.; Maeir, A.; Ben Assayag, E.; Bornstein, N.M.; Korczyn, A.D.; Katz, N. Impact of first-ever mild stroke on participation at 3 and 6 month post-event: The TABASCO study. Disabil. Rehabil. 2014, 37, 667–673. [Google Scholar] [CrossRef] [PubMed]
- Scott, S.L.; Burns, S.P.; Schwartz, J.; Kovic, M. Returning to work. Arch. Phys. Med. Rehabil. 2020, 101, 1243–1259. [Google Scholar] [CrossRef]
- Hu, X.; Heyn, P.C.; Schwartz, J.; Roberts, P. What Is Mild Stroke? Arch. Phys. Med. Rehabil. 2017, 98, 2347–2349. [Google Scholar] [CrossRef] [PubMed]
- Carlsson, G.E.; Möller, A.; Blomstrand, C. Consequences of Mild Stroke in Persons <75 Years—A 1-Year Follow-Up. Cerebrovasc. Dis. 2003, 16, 383–388. [Google Scholar] [CrossRef] [PubMed]
- Tellier, M.; Rochette, A. Falling Through the Cracks: A Literature Review to Understand the Reality of Mild Stroke Survivors. Top. Stroke Rehabil. 2009, 16, 454–462. [Google Scholar] [CrossRef]
- Carlsson, G.E.; Möller, A.; Blomstrand, C.H. A qualitative study of the consequences of ‘hidden dysfunctions’ one year after a mild stroke in persons Disabil. Rehabilitation 2004, 26, 1373–1380. [Google Scholar] [CrossRef]
- Edwards, D.F.; Hahn, M.; Baum, C.; Dromerick, A.W. The Impact of Mild Stroke on Meaningful Activity and Life Satisfaction. J. Stroke Cerebrovasc. Dis. 2006, 15, 151–157. [Google Scholar] [CrossRef]
- Jones, F.; Riazi, A. Self-efficacy and self-management after stroke: A systematic review. Disabil. Rehabil. 2011, 33, 797–810. [Google Scholar] [CrossRef]
- Korpershoek, C.; van der Bijl, J.; Hafsteinsdóttir, T.B. Self-efficacy and its influence on recovery of patients with stroke: A systematic review. J. Adv. Nurs. 2011, 67, 1876–1894. [Google Scholar] [CrossRef]
- Bandura, A. Self-Efficacy. In Social Foundations of thought and Action: A Social Cognitive Theory; Bandura, A., Ed.; Englewood Cliffs: Bergen County, NJ, USA, 1986; pp. 390–453. [Google Scholar]
- Benight, C.C.; Bandura, A. Social cognitive theory of posttraumatic recovery: The role of perceived self-efficacy. Behav. Res. Ther. 2004, 42, 1129–1148. [Google Scholar] [CrossRef]
- Dixon, G.; Thornton, E.W.; Young, C. Perceptions of self-efficacy and rehabilitation among neurologically disabled adults. Clin. Rehabil. 2007, 21, 230–240. [Google Scholar] [CrossRef]
- Bandura, A. The assessment and predictive generality of self-percepts of efficacy. J. Behav. Ther. Exp. Psychiatry 1982, 13, 195–199. [Google Scholar] [CrossRef]
- Bandura, A. Encyclopedia of human behavior. In Self-Efficacy; Ramachaudran, V.S., Ed.; Academic Press: New York, NY, USA, 1994; pp. 71–81. [Google Scholar]
- Frost, Y.; Weingarden, H.; Zeilig, G.; Nota, A.; Rand, D. Self-Care Self-Efficacy Correlates with Independence in Basic Activities of Daily Living in Individuals with Chronic Stroke. J. Stroke Cerebrovasc. Dis. 2015, 24, 1649–1655. [Google Scholar] [CrossRef]
- LeBrasseur, N.K.; Sayers, S.P.; Ouelette, M.M.; Fielding, R.A. Muscle impairments and behavioral factors mediate functional limitations and disability following stroke. PTJ 2006, 86, 1342–1350. [Google Scholar] [CrossRef]
- Pang, M.Y.C.; Eng, J.J. Fall-related self-efficacy, not balance and mobility performance, is related to accidental falls in chronic stroke survivors with low bone mineral density. Osteoporos. Int. 2008, 19, 919–927. [Google Scholar] [CrossRef]
- Salbach, N.M.; Mayo, N.E.; Robichaud-Ekstrand, S.; Hanley, J.A.; Richards, C.L.; Wood-Dauphinee, S. Balance Self-Efficacy and Its Relevance to Physical Function and Perceived Health Status After Stroke. Arch. Phys. Med. Rehabil. 2006, 87, 364–370. [Google Scholar] [CrossRef]
- Glass, T.A.; Berkman, L.F.; Hiltunen, E.F.; Furie, K.; Glymour, M.M.; Fay, M.E.; Ware, J. The families in recovery from stroke trial (FIRST): Primary study results. Psychosom Med. 2004, 66, 889–897. [Google Scholar] [CrossRef]
- Salbach, N.M.; Mayo, N.E.; Robichaud-Ekstrand, S.; Hanley, J.A.; Richards, C.L.; Wood-Dauphinee, S. The Effect of a Task-Oriented Walking Intervention on Improving Balance Self-Efficacy Poststroke: A Randomized, Controlled Trial. J. Am. Geriatr. Soc. 2005, 53, 576–582. [Google Scholar] [CrossRef]
- Hoffmann, T.; McKenna, K.; Worrall, L.; Read, S.J. Randomised trial of a computer-generated tailored written education package for patients following stroke. Age Ageing 2007, 36, 280–286. [Google Scholar] [CrossRef]
- Kendall, E.; Catalano, T.; Kuipers, P.; Posner, N.; Buys, N.; Charker, J. Recovery following stroke: The role of self-management education. Soc. Sci. Med. 2007, 64, 735–746. [Google Scholar] [CrossRef]
- Jones, F.; Mandy, A.; Partridge, C. Changing self-efficacy in individuals following a first time stroke: Preliminary study of a novel self-management intervention. Clin. Rehabil. 2009, 23, 522–533. [Google Scholar] [CrossRef] [PubMed]
- Lennon, S.; McKenna, S.; Jones, F. Self-management programmes for people post stroke: A systematic review. Clin. Rehabil. 2013, 27, 867–878. [Google Scholar] [CrossRef] [PubMed]
- Hale, L.; Jones, F.; Mulligan, H.; Levack, W.; Smith, C.; Claydon, L.; Milosavljevic, S.; Taylor, D.; Allan, J.; MacKenzie, N.; et al. Developing the Bridges self-management programme for New Zealand stroke survivors: A case study. Int. J. Ther. Rehabil. 2014, 21, 381–388. [Google Scholar] [CrossRef]
- McKenna, S.; Jones, F.; Glenfield, P.; Lennon, S. Bridges Self-Management Program for People with Stroke in the Community: A Feasibility Randomized Controlled Trial. Int. J. Stroke 2015, 10, 697–704. [Google Scholar] [CrossRef]
- Adamit, T.; Shames, J.; Rand, D. Effectiveness of the Functional and Cognitive Occupational Therapy (FaCoT) Intervention for Improving Daily Functioning and Participation of Individuals with Mild Stroke: A Randomized Controlled Trial. Int. J. Environ. Res. Public Health 2021, 18, 7988. [Google Scholar] [CrossRef]
- Hoffmann, T.C.; Glasziou, P.P.; Boutron, I.; Milne, R.; Perera, R.; Moher, D.; Altman, D.G.; Barbour, V.; Macdonald, H.; Johnston, M.; et al. Better reporting of interventions: Template for intervention description and replication (TIDieR) checklist and guide. BMJ 2014, 348, g1687. [Google Scholar] [CrossRef]
- Burns, S.P.; Schwartz, J.K.; Scott, S.L.; Devos, H.; Kovic, M.; Hong, I.; Akinwuntan, A. Interdisciplinary Approaches to Facilitate Return to Driving and Return to Work in Mild Stroke: A Position Paper. Arch. Phys. Med. Rehabil. 2018, 99, 2378–2388. [Google Scholar] [CrossRef]
- Langhorne, P.; Bernhardt, J.; Kwakkel, G. Stroke rehabilitation. Lancet 2011, 377, 1693–1702. [Google Scholar] [CrossRef]
- Legg, L.; Drummond, A.; Leonardi-Bee, J.; Gladman, J.; Corr, S.; Donkervoort, M.; Edmans, J.; Gilbertson, L.; Jongbloed, L.; Logan, P.; et al. Occupational therapy for patients with problems in personal activities of daily living after stroke: Systematic review of randomised trials. BMJ 2007, 335, 922–925. [Google Scholar] [CrossRef]
- Moher, D.; Hopewell, S.; Schulz, K.F.; Montori, V.; Gøtzsche, P.C.; Devereaux, P.; Elbourne, D.; Egger, M.; Altman, D.G. CONSORT 2010 explanation and elaboration: Updated guidelines for reporting parallel group randomised trials. Br. Med. J. 2010, 340, c869. [Google Scholar] [CrossRef]
- Adams, H.P.; Davis, P.H.; Leira, E.C.; Chang, K.-C.; Bendixen, B.H.; Clarke, W.R.; Woolson, R.F.; Hansen, M.D. Baseline NIH Stroke Scale score strongly predicts outcome after stroke: A report of the Trial of Org 10172 in Acute Stroke Treatment (TOAST). Neurology 1999, 53, 126. [Google Scholar] [CrossRef]
- Law, M.; Baptiste, S.; McColl, M.; Opzoomer, A.; Polatajko, H.; Pollock, N. The Canadian Occupational Performance Measure: An Outcome Measure for Occupational Therapy. Can. J. Occup. Ther. 1990, 57, 82–87. [Google Scholar] [CrossRef]
- Nasreddine, Z.S.; Phillips, N.A.; Bédirian, V.; Charbonneau, S.; Whitehead, V.; Collin, I.; Cummings, J.L.; Chertkow, H. The Montreal Cognitive Assessment, MoCA: A Brief Screening Tool For Mild Cognitive Impairment. J. Am. Geriatr. Soc. 2005, 53, 695–699. [Google Scholar] [CrossRef]
- Bandura, A. Self-efficacy: Toward a unifying theory of behavioral change. Psychol. Rev. 1977, 84, 191–215. [Google Scholar] [CrossRef]
- Chen, G.; Gully, S.M.; Eden, D. Validation of a New General Self-Efficacy Scale. Organ. Res. Methods 2001, 4, 62–83. [Google Scholar] [CrossRef]
- Yesavage, J.A.; Brink, T.L.; Rose, T.L.; Lum, O.; Huang, V.; Adey, M.; Leirer, V.O. Development and validation of a geriatric depression screening scale: A preliminary report. J. Psychiatr. Res. 1983, 17, 37–49. [Google Scholar] [CrossRef]
- Tang, W.K.; Chan, S.S.; Chiu, H.F.; Wong, K.S.L.; Kwok, T.C.; Mok, V.; Ungvari, G.S. Can the Geriatric Depression Scale detect poststroke depression in Chinese elderly? J. Affect. Disord. 2004, 81, 153–156. [Google Scholar] [CrossRef]
- Wilson, A.B.; Evans, J.; Alderman, N.; Burges, P.W.; Emslie, H. Behavioural Assessment of Dysexecutive Syndrome. In Methodology of Frontal and Executive Function, 1st ed.; Rabbitt, P., Ed.; Psychology Press: London, UK, 1996. [Google Scholar]
- Wilson, B.A.; Evans, J.J.; Emslie, H.; Alderman, N.; Burgess, P. The Development of an Ecologically Valid Test for Assessing Patients with a Dysexecutive Syndrome. Neuropsychol. Rehabil. 1998, 8, 213–222. [Google Scholar] [CrossRef]
- Wood-Dauphinee, S.L.; Opzoomer, M.A.; I Williams, J.; Marchand, B.; Spitzer, W.O. Assessment of global function: The Reintegration to Normal Living Index. Arch. Phys. Med. Rehabil. 1988, 69, 583–590. [Google Scholar]
- Granger, C.V.; Hamilton, B.B.; Keith, R.A.; Zielezny, M.; Sherwin, F.S. Advances in functional assessment for medical rehabilitation. Top. Geriatr. Rehabil. 1986, 1, 59–74. [Google Scholar] [CrossRef]
- Miles, J.; Shevlin, M. Applying Regression and Correlation: A Guide for Students and Researchers; Sage: London, UK, 2001. [Google Scholar]
- Gupta, S.K. Intention-to-treat concept: A review. Perspect. Clin. Res. 2011, 2, 109–112. [Google Scholar] [CrossRef] [PubMed]
- Jones, F. Strategies to enhance chronic disease self-management: How can we apply this to stroke? Disabil. Rehabil. 2006, 28, 841–847. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.S.; Peng, W.; Adams, J.; Sibbritt, D. The use of self-management strategies for stroke rehabilitation: A scoping review. Top. Stroke Rehabil. 2022, 27, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Nott, M.; Wiseman, L.; Seymour, T.; Pike, S.; Cuming, T.; Wall, G. Stroke self-management and the role of self-efficacy. Disabil. Rehabil. 2021, 43, 1410–1419. [Google Scholar] [CrossRef] [PubMed]
- Warner, G.; Packer, T.; Villeneuve, M.; Audulv, A.; Versnel, J. A systematic review of the effectiveness of stroke self-management programs for improving function and participation outcomes: Self-management programs for stroke survivors. Disabil. Rehabil. 2015, 37, 2141–2163. [Google Scholar] [CrossRef]
- Muus, I.; Petzold, M.; Ringsberg, K.C. Health-related quality of life among Danish patients 3 and 12 months after TIA or mild stroke. Scand. J. Caring Sci. 2010, 24, 211–218. [Google Scholar] [CrossRef]
- Naess, H.; Waje-Andreassen, U.; Thomassen, L.; Nyland, H.; Myhr, K.-M. Health-Related Quality of Life Among Young Adults With Ischemic Stroke on Long-Term Follow-Up. Stroke 2006, 37, 1232–1236. [Google Scholar] [CrossRef]
- van de Port, I.; Kwakkel, G.; Schepers, V.; Heinemans, C.; Lindeman, E. Is Fatigue an Independent Factor Associated with Activities of Daily Living, Instrumental Activities of Daily Living and Health-Related Quality of Life in Chronic Stroke? Cerebrovasc. Dis. 2007, 23, 40–45. [Google Scholar] [CrossRef]
- Whyte, E.M.; Mulsant, B.H. Post stroke depression: Epidemiology, pathophysiology, and biological treatment. Biol. Psychiatry 2002, 52, 253–264. [Google Scholar] [CrossRef]
- Dong, L.; Williams, L.S.; Brown, D.L.; Case, E.; Morgenstern, L.B.; Lisabeth, L.D. Prevalence and Course of Depression during the First Year After Mild to Moderate Stroke. J. Am. Hear Assoc. 2021, 10, e020494. [Google Scholar] [CrossRef]
- Guo, J.; Wang, J.; Sun, W.; Liu, X. The advances of post-storke depression: 2021 updtae. J. Neurol. 2022, 269, 1236–1249. [Google Scholar] [CrossRef]
- Woranush, W.; Moskopp, M.L.; Sedghi, A.; Stuckart, I.; Noll, T.; Barlinn, K.; Siepmann, T. Preventive Approaches for Post-Stroke Depression: Where Do We Stand? A Systematic Review. Neuropsychiatr. Dis. Treat. 2022, 17, 3359–3377. [Google Scholar] [CrossRef]
- Hackett, M.L.; Pickles, K. Part I: Frequency of Depression after Stroke: An Updated Systematic Review and Meta-Analysis of Observational Studies. Int. J. Stroke 2014, 9, 1017–1025. [Google Scholar] [CrossRef]
- Kutlubaev, M.A.; Hackett, M.L. Part II: Predictors of Depression after Stroke and Impact of Depression on Stroke Outcome: An Updated Systematic Review of Observational Studies. Int. J. Stroke 2014, 9, 1026–1036. [Google Scholar] [CrossRef]
- Tang, W.; Chen, Y.; Lam, W.W.; Mok, V.; Wong, A.; Ungvari, G.S.; Xiang, Y.; Wong, K.S. Emotional incontinence and executive function in ischemic stroke: A case-controlled study. J. Int. Neuropsychol. Soc. 2009, 15, 62–68. [Google Scholar] [CrossRef]
- Starkstein, S.E.; Robinson, R.G.; Price, T.R. Comparison of Patients With and Without Poststroke Major Depression Matched for Size and Location of Lesion. Arch. Gen. Psychiatry 1988, 46, 247–252. [Google Scholar] [CrossRef]
- Díez-Ascaso, O.; Martinez-Sanchez, P.; Fuentes, B.; Díez-Tejedor, E. Sociocultural study on the self-perception of stroke and an analysis of doctor-patient communication. Neurologia 2011, 26, 81–91. [Google Scholar] [CrossRef]
- Juniper, A.R.; Connor, L.T. Self-Perceived ADL/IADL Function is Influenced by Residual Neurological Impairment, Aphasia, and Anxiety. Can. J. Occup. Ther. 2022, 89, 307–317. [Google Scholar] [CrossRef]
- Nicholas, M.L.; Burch, K.; Mitchell, J.R.; Fox, A.B.; Baum, C.M.; Connor, L.T. Self-perception of physical function contributes to partici-pation in cognitively- and physically- demanding activities after stroke. Front. Neurol. 2020, 11, 110. [Google Scholar] [CrossRef]
| FaCoT (n = 33) | Control Group (n = 33) | Differences between Groups | |
|---|---|---|---|
| Mean (SD), Min–Max | Mean (SD), Min–Max | ||
| Age (years) | 64.6 (8.2), 49–77 | 64.4 (10.8), 48–84 | 0.9 a |
| Education (years) | 12.1 (1.9), 8–16 | 12.9 (2.8), 6–20 | 0.2 a |
| NIHSS (0–46) mean (SD) | 1.2 (1.2), 0–4 | 1.7 (1.6), 0–6 | 0.2 a |
| FIM (18–126) mean (SD) | 118.8 (7.2), 98–126 | 117.2 (7.1), 96–126 | 0.4 a |
| MoCA (0–30) | 21.5 (3. 9), 11–29 | 21.8 (4.1), 14–28 | 0.8 a |
| N (%) | N (%) | ||
| Sex female n (%) | 11 (33.3) | 15 (45.5) | 0.3 b |
| First stroke n (%) | 20 (60.6) | 19 (57.6) | 0.8 b |
| Stroke side R n (%) | 13 (39.4) | 14 (42.4) | 0.7 b |
| Type of stroke—ischemic/hemorrhage n (%) | 32/0 (100/0) | 31/2 (93.9/6.1) | 0.2 b |
| Lesion—cortical/subcortical n (%) | 9/17 (27.3/51.5) | 8/19 (24.2/57.6) | 0.8 b |
| Chronic stage n (%) | 28 (84.8) | 26 (78.8) | 0.5 b |
| Worked before stroke n (%) | 20 (60.6) | 13 (39.4) | 0.1 b |
| Returned to work since stroke n (%) | 11 (33.3) | 4 (12.1) | 0.2 b |
| FaCoT Group (n = 33) | Control Group (n = 33) | |||||
| T1 Mean (SD) | T2 Mean (SD) | T3 Mean (SD) | T1 Mean (SD) | T2 Mean (SD) | T3 Mean (SD) | |
| NGSE (8–40) | 29.1 (7.7) | 32.2 (6.4) | 31.6 (7.1) | 25.5 (9.5) | 24.8 (9.2) | 24.7 (9.1) |
| GDS (0–15) | 6.4 (3.3) | 5.3 (3.8) | 4.9 (4.1) | 6.4 (4.0) | 6.2 (3.6) | 6.5 (3.9) |
| DEX Behavioral (0–32) | 8.9 (6.4) | 6.0 (5.3) | 7.4 (6.5) | 6.4 (5.9) | 7.4 (5.7) | 7.4 (6.5) |
| DEX Emotional (0–12) | 4.9 (2.8) | 3.7 (2.6) | 3.8 (2.6) | 4.3 (3.1) | 4.8 (2.8) | 4.2 (2.9) |
| RNLI self-perception (0–30) | 23.3 (5.2) | 25.4 (6.3) | 24.4 (5.4) | 22.2 (5.9) | 21.2 (6.8) | 20.9 (7.6) |
| Interaction Effect (Time X Group) | Main Effect (Time) | Main Effect (Group) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| F | P | ɳP2 | F | P | ɳP2 | F | P | ɳP2 | |
| NGSE | 4.1 | 0.03 | 0.06 | 1.4 | 0.24 | 0.02 | 10.3 | 0.002 | 0.14 |
| GDS | 4.4 | 0.01 | 0.07 | 4.6 | 0.01 | 0.07 | 0.9 | 0.33 | 0.01 |
| DEX Behavioral | 5.4 | 0.006 | 0.08 | 1.3 | 0.28 | 0.02 | 0.1 | 0.78 | 0.001 |
| DEX Emotional | 4.3 | 0.02 | 0.07 | 2.3 | 0.11 | 0.04 | 0.3 | 0.62 | 0.004 |
| RNLI self-perception | 2.2 | 0.12 | 0.03 | 0.4 | 0.67 | 0.01 | 5.4 | 0.02 | 0.08 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adamit, T.; Shames, J.; Rand, D. Functional and Cognitive Occupational Therapy (FaCoT) Improves Self-Efficacy and Behavioral–Emotional Status of Individuals with Mild Stroke; Analysis of Secondary Outcomes. Int. J. Environ. Res. Public Health 2023, 20, 5052. https://doi.org/10.3390/ijerph20065052
Adamit T, Shames J, Rand D. Functional and Cognitive Occupational Therapy (FaCoT) Improves Self-Efficacy and Behavioral–Emotional Status of Individuals with Mild Stroke; Analysis of Secondary Outcomes. International Journal of Environmental Research and Public Health. 2023; 20(6):5052. https://doi.org/10.3390/ijerph20065052
Chicago/Turabian StyleAdamit, Tal, Jeffrey Shames, and Debbie Rand. 2023. "Functional and Cognitive Occupational Therapy (FaCoT) Improves Self-Efficacy and Behavioral–Emotional Status of Individuals with Mild Stroke; Analysis of Secondary Outcomes" International Journal of Environmental Research and Public Health 20, no. 6: 5052. https://doi.org/10.3390/ijerph20065052
APA StyleAdamit, T., Shames, J., & Rand, D. (2023). Functional and Cognitive Occupational Therapy (FaCoT) Improves Self-Efficacy and Behavioral–Emotional Status of Individuals with Mild Stroke; Analysis of Secondary Outcomes. International Journal of Environmental Research and Public Health, 20(6), 5052. https://doi.org/10.3390/ijerph20065052






