Psychometric Properties of the Brazilian Version of the Quality of Dying and Death for Adult Family Members of ICU Patients
Abstract
1. Introduction
2. Methods
2.1. Study Design
2.2. Study Population
2.3. Data Collection Procedure
2.4. Statistical Analysis
3. Results
3.1. Construct Validity
3.1.1. Confirmatory Factor Analysis (CFA)
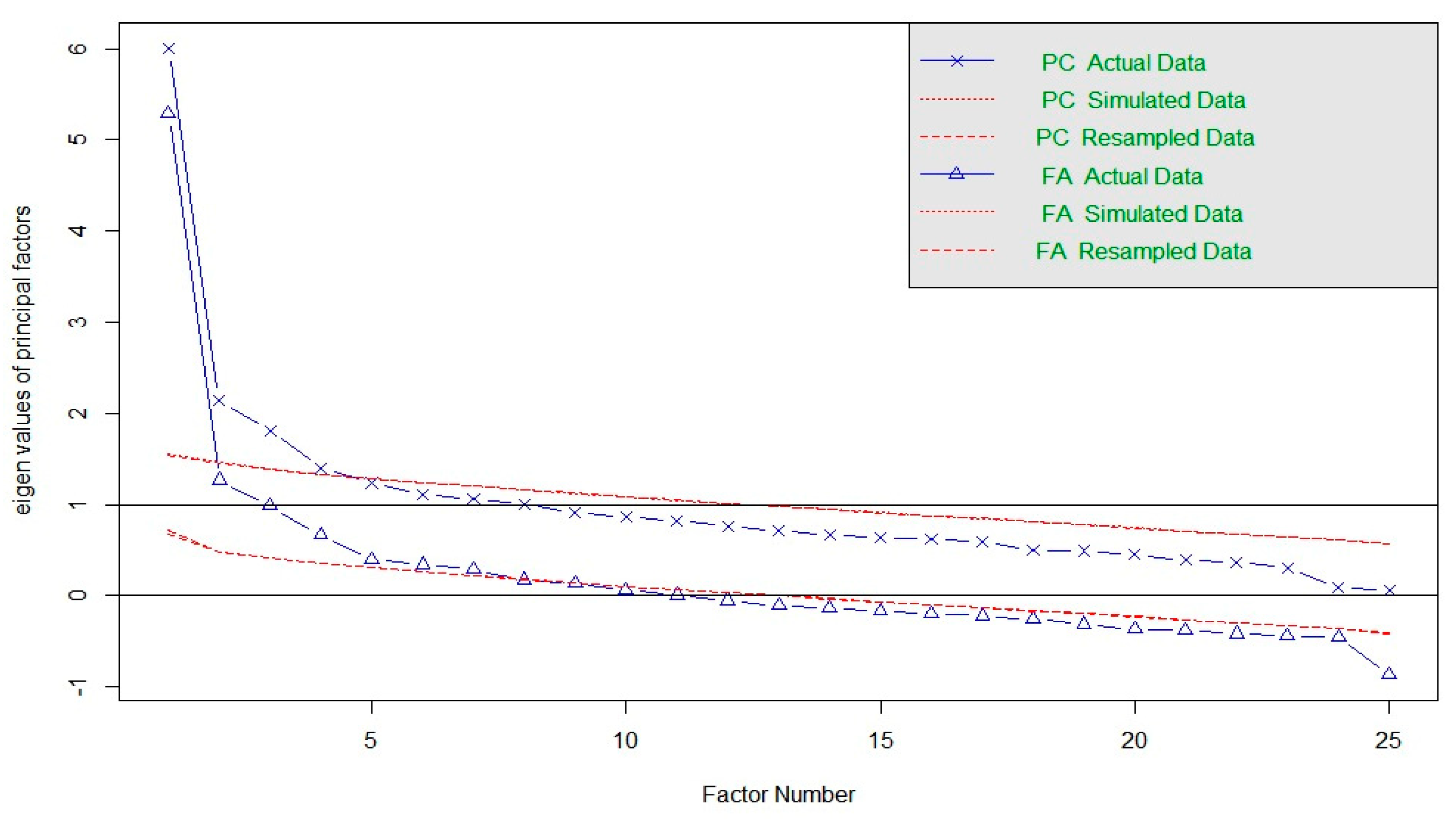
3.1.2. Exploratory Factor Analysis (EFA)
3.1.3. Confirmatory Factor Analysis (CFA)
3.2. Reliability
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gellie, A.; Mills, A.; Levinson, M.; Stephenson, G.; Flynn, E. Death: A foe to be conquered? Questioning the paradigm. Age Ageing 2015, 44, 7–10. [Google Scholar] [CrossRef] [PubMed]
- Gerritsen, R.T.; Hofhuis, J.G.; Koopmans, M.; van der Woude, M.; Bormans, L.; Hovingh, A.; Spronk, P.E. Perception by Family Members and ICU Staff of the Quality of Dying and Death in the ICU. Chest 2013, 143, 357–363. [Google Scholar] [CrossRef]
- Sellers, D.E.; Dawson, R.; Cohen-Bearak, A.; Solomond, M.Z.; Truog, R.D. Measuring the Quality of Dying and Death in the Pediatric Intensive Care Setting: The Clinician PICU-QODD. J. Pain Symptom Manag. 2015, 49, 66–78. [Google Scholar] [CrossRef] [PubMed]
- Moslemi, M.; Nikfarid, L.; Nourian, M.; Nasiri, M.; Rezayi, F. Translation, cultural, and age-related adaptation and psychometric properties of Persian version of “Quality of Dying and Death” in nurses working in neonatal intensive care units. Indian J. Palliat. Care 2020, 26, 34. [Google Scholar] [CrossRef] [PubMed]
- Han, X.P.; Mei, X.; Zhang, J.; Zhang, T.T.; Yin, A.N.; Qiu, F.; Liu, M.J. Validation of the Chinese Version of the Quality of Dying and Death Questionnaire for Family Members of ICU Patients. J. Pain Symptom Manag. 2021, 62, 599–608. [Google Scholar] [CrossRef]
- Brooks, L.A.; Manias, E.; Nicholson, P. Barriers, enablers and challenges to initiating end-of-life care in an Australian intensive care unit context. Aust. Crit. Care 2017, 30, 161–166. [Google Scholar] [CrossRef]
- Mularski, R.; Curtis, J.R.; Osborne, M.; Engelberg, R.A.; Ganzini, L. Agreement among family members in their assessment of the Quality of Dying and Death. J. Pain Symptom Manag. 2004, 28, 306–315. [Google Scholar] [CrossRef]
- Curtis, J.R.; Patrick, D.L.; Engelberg, R.A.; Norris, K.; Asp, C.; Byock, I. A Measure of the Quality of Dying and Death. J. Pain Symptom Manag. 2002, 24, 17–31. [Google Scholar] [CrossRef] [PubMed]
- Gerritsen, R.T.; Koopmans, M.; Hofhuis, J.G.; Curtis, J.R.; Jensen, H.I.; Zijlstra, J.G.; Spronk, P.E. Comparing Quality of Dying and Death Perceived by Family Members and Nurses for Patients Dying in US and Dutch ICUs. Chest 2017, 151, 298–307. [Google Scholar] [CrossRef]
- Curtis, J.R.; Downey, L.; Engelberg, R.A. The Quality of Dying and Death. Chest 2013, 143, 289–291. [Google Scholar] [CrossRef]
- Downey, L.; Curtis, J.R.; Lafferty, W.E.; Herting, J.R.; Engelberg, R.A. The Quality of Dying and Death Questionnaire (QODD): Empirical Domains and Theoretical Perspectives. J. Pain Symptom Manag. 2010, 39, 9–22. [Google Scholar] [CrossRef]
- Pérez-Cruz, P.E.; Pérez, O.P.; Bonati, P.; Parisi, O.T.; Satt, L.T.; Otaiza, M.G.; Morgado, A.M. Validation of the Spanish Version of the Quality of Dying and Death Questionnaire (QODD-ESP) in a Home-Based Cancer Palliative Care Program and Development of the QODD-ESP-12. J. Pain Symptom Manag. 2017, 53, 1042–1049.e3. [Google Scholar] [CrossRef]
- Gutiérrez Sánchez, D.; Cuesta-Vargas, A.I. Cross-cultural adaptation and psychometric testing of the Quality of Dying and Death Questionnaire for the Spanish population. Eur. J. Oncol. Nurs. 2018, 33, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Glavan, B.J.; Engelberg, R.A.; Downey, L.; Curtis, J.R. Using the medical record to evaluate the quality of end-of-life care in the intensive care unit. Crit. Care Med. 2008, 36, 1138–1146. [Google Scholar] [CrossRef] [PubMed]
- Meneguin, S.; Benichel, C.R.; Morais, J.F.; Oliveira, C.D. Translation and Cultural Adaptation into Portuguese of the Quality of Dying and Death Scale for Family Members of Patients in Intensive Care Units. Int. J. Environ. Res. Public Health 2022, 19, 3614. [Google Scholar] [CrossRef]
- Boateng, G.O.; Neilands, T.B.; Frongillo, E.A.; Melgar-Quiñonez, H.R.; Young, S.L. Best Practices for Developing and Validating Scales for Health, Social, and Behavioral Research: A Primer. Front. Public Health 2018, 6. [Google Scholar] [CrossRef] [PubMed]
- Little, R.; Rubin, D. Statistical Analysis with Missing Data, 3rd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2019. [Google Scholar]
- Bland, J.M.; Altman, D.G. Statistics notes: Cronbach’s alpha. BMJ 1997, 314, 572. [Google Scholar] [CrossRef]
- Hair, J.; Black, W.; Sant’Anna, A. Análise Multivariada de Dados, 6th ed.; Grupo A—Bookman: Taipei, Taiwan, 2000. [Google Scholar]
- Brown, T. Confirmatory Factor Analysis for Applied Research; Guilford Press: New York, NY, USA, 2015. [Google Scholar]
- Fabrigar, L.; Wegener, D. Exploratory Factor Analysis; Oxford University Press: Oxford, UK, 2011. [Google Scholar]
- Miot, H.A. Análise de correlação em estudos clínicos e experimentais. J. Vasc. Bras. 2018, 17, 275–279. [Google Scholar] [CrossRef]
- Ogrinc, G.; Davies, L.; Goodman, D.; Batalden, P.; Davidoff, F.; Stevens, D. SQUIRE 2.0 (Standards for QUality Improvement Reporting Excellence): Revised publication guidelines from a detailed consensus process: Table 1. BMJ Qual. Saf. 2016, 25, 986–992. [Google Scholar] [CrossRef]
- Mokkink, L.B.; Prinsen, C.A.C.; Bouter, L.M.; de Vet, H.C.W.; Terwee, C.B. The COnsensus-based Standards for the selection of health Measurement INstruments (COSMIN) and how to select an outcome measurement instrument. Braz. J. Phys. Ther. 2016, 20, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Heckel, M.; Bussmann, S.; Stiel, S.; Ostgathe, C.; Weber, M. Validation of the German Version of the Quality of Dying and Death Questionnaire for Health Professionals. Am. J. Hosp. Palliat. Med. 2016, 33, 760–769. [Google Scholar] [CrossRef] [PubMed]
- Vogt, A.; Stiel, S.; Heckel, M.; Goebel, S.; Mai, S.S.; Seifert, A.; Weber, M. Assessment of the quality of end-of-life care: Translation and validation of the German version of the “Care of the Dying Evaluation” (CODE-GER)—A questionnaire for bereaved relatives. Health Qual Life Outcomes 2020, 18, 311. [Google Scholar] [CrossRef] [PubMed]
- Byrne, B.M. Structural Equation Modeling with AMOS: Basic Concepts, Applications, and Programming, 2nd ed.; Taylor & Francis Group: Oxford, UK, 2010. [Google Scholar]
- Temel, J.S.; Greer, J.A.; Muzikansky, A. Early Palliative Care for Patients with Metastatic Non–Small-Cell Lung Cancer. New Engl. J. Med. 2010, 363, 733–742. [Google Scholar] [CrossRef] [PubMed]

| Questions 1–10a | Answers are scored on a scale from 0 to 6 |
| Questions 11–21a | Answers are scored on a scale ranging from 0 (yes) to 3 (I don’t know) |
| Question 22a | the participant indicates how their loved one was at the moment of death using a nominal scale ranging from 1 (awake) to 4 (I don’t know) |
| Questions 1b–22b | Answers are scored on a Likert scale ranging from 0 (terrible experience) to 10 (nearly perfect experience) but may not be answered depending on the answer given in part “a” of the item |
| Questions 23, 24 and 25 | Do not have subdivisions and the answers also range from 0 to 10 |
| Variables | Validation (n = 326) | Retest (n = 20) |
|---|---|---|
| Age * | 45 (±13.7) | 45.5 (±13.0) |
| Sex ** | ||
| Male | 116 (35.6%) | 5 (25%) |
| Female | 210 (64.4%) | 15 (75%) |
| Sex of loved one ** | ||
| Male | 190 (58.3%) | 8 (40%) |
| Female | 136 (41.7%) | 12 (60%) |
| Brazilian nationality ** | ||
| Yes | 324 (99.4%) | 19 (95%) |
| No | 2 (0.6%) | 1 (5%) |
| Ethnicity ** | ||
| White | 229 (70.2%) | 12 (60%) |
| Black | 23 (7.1%) | 1 (5%) |
| Asian | 2 (0.6%) | 0 (0.0) |
| Brown | 69 (21.2%) | 7 (35%) |
| Indigenous | 2 (0.6%) | 0 (0.0) |
| Other | 1 (0.3%) | 0 (0.0) |
| Schooling ** | ||
| Illiterate | 27 (8.3%) | 4 (20%) |
| Primary school | 34 (10.4%) | 2 (10%) |
| High school or trade school | 175 (53.7%) | 12 (60%) |
| Higher education | 72 (22.1%) | 1 (5%) |
| Postgraduate degree | 18 (5.5%) | 1 (5%) |
| Degree of relatedness ** | ||
| Spouse or companion | 48 (14.7%) | 2 (10%) |
| Son/daughter | 177 (54.3%) | 8 (40%) |
| Brother | 22 (6.7%) | 1 (5%) |
| Parent | 8 (2.5%) | 9 (45%) |
| Other relative | 67 (20.5%) | 0 (0.0) |
| Friend | 4 (1.2%) | 0 (0.0) |
| Resided with loved one ** | ||
| Yes | 48 (14.7%) | 15 (75%) |
| No | 64 (19.6%) | 5 (25%) |
| Knew loved one for (years) * | 37.7 (±16.2) | 34.4 (±15.0) |
| Item | Factor 1 | Factor 2 | Communality | Percentage of Variance |
|---|---|---|---|---|
| 1b | 0.22 | 0.048 | 0.95 | |
| 2b | 0.28 | 0.078 | 0.92 | |
| 3b | 0.39 | 0.150 | 0.85 | |
| 4b | 0.47 | 0.217 | 0.78 | |
| 5b | 0.38 | 0.141 | 0.86 | |
| 6b | 0.33 | 0.108 | 0.89 | |
| 7b | 0.28 | 0.076 | 0.92 | |
| 8b | 0.16 | 0.025 | 0.97 | |
| 9b | 0.62 | 0.380 | 0.62 | |
| 10b | 0.64 | 0.407 | 0.59 | |
| 11b | 0.52 | 0.272 | 0.73 | |
| 12b | 0.34 | 0.118 | 0.88 | |
| 13b | 0.57 | 0.325 | 0.68 | |
| 14b | 0.39 | 0.153 | 0.85 | |
| 15b | 0.69 | 0.477 | 0.52 | |
| 16b | 0.70 | 0.488 | 0.51 | |
| 17b | 0.47 | 0.224 | 0.78 | |
| 18b | 0.38 | 0.142 | 0.86 | |
| 19b | 0.30 | 0.090 | 0.91 | |
| 20b | 0.50 | 0.252 | 0.75 | |
| 21b | 0.55 | 0.303 | 0.70 | |
| 22b | 0.44 | 0.191 | 0.81 | |
| 23 | 0.60 | 0.364 | 0.64 | |
| 24 | 0.27 | 0.963 | 0.037 | |
| 25 | 0.29 | 0.919 | 0.081 |
| Items | 3b | 4b | 5b | 6b | 9b | 10b | 11b | 12b | 13b | 14b | 15b | 16b | 17b | 18b | 20b | 21b | 22b | 23b | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 3b | rho | 1.00 | 0.36 | 0.24 | 0.10 | 0.29 | 0.22 | 0.18 | 0.08 | 0.32 | 0.15 | 0.18 | 0.20 | 0.30 | 0.20 | 0.25 | 0.33 | 0.24 | 0.23 |
| 3b | p | 0.00 | 0.00 | 0.02 | 1.00 | 0.00 | 0.04 | 0.26 | 1.00 | 0.00 | 1.00 | 0.74 | 0.35 | 0.00 | 0.28 | 0.01 | 0.00 | 0.01 | 0.01 |
| 4b | rho | 1.00 | 0.37 | 0.11 | 0.38 | 0.38 | 0.29 | 0.15 | 0.30 | 0.21 | 0.35 | 0.32 | 0.46 | 0.14 | 0.31 | 0.34 | 0.29 | 0.30 | |
| 4b | p | 0.00 | 0.00 | 1.00 | 0.00 | 0.00 | 0.00 | 0.79 | 0.00 | 0.27 | 0.00 | 0.00 | 0.00 | 1.00 | 0.00 | 0.00 | 0.00 | 0.00 | |
| 5b | rho | 1.00 | 0.35 | 0.31 | 0.31 | 0.24 | 0.09 | 0.17 | 0.25 | 0.17 | 0.18 | 0.07 | 0.21 | 0.28 | 0.27 | 0.11 | 0.42 | ||
| 5b | p | 0.00 | 0.00 | 0.00 | 0.00 | 0.04 | 1.00 | 0.27 | 0.30 | 0.76 | 1.00 | 1.00 | 0.42 | 0.01 | 0.01 | 1.00 | 0.00 | ||
| 6b | rho | 1.00 | 0.23 | 0.37 | 0.25 | 0.12 | 0.21 | 0.20 | 0.23 | 0.22 | 0.18 | 0.30 | 0.19 | 0.28 | 0.12 | 0.22 | |||
| 6b | p | 0.00 | 0.02 | 0.00 | 0.02 | 1.00 | 0.03 | 0.47 | 0.10 | 0.15 | 0.67 | 0.00 | 0.91 | 0.01 | 1.00 | 0.30 | |||
| 9b | rho | 1.00 | 0.60 | 0.53 | 0.16 | 0.40 | 0.33 | 0.52 | 0.52 | 0.23 | 0.15 | 0.27 | 0.45 | 0.26 | 0.35 | ||||
| 9b | p | 0.00 | 0.00 | 0.00 | 0.41 | 0.00 | 0.00 | 0.00 | 0.00 | 0.01 | 1.00 | 0.00 | 0.00 | 0.01 | 0.00 | ||||
| 10b | rho | 1.00 | 0.35 | 0.37 | 0.39 | 0.34 | 0.50 | 0.53 | 0.36 | 0.33 | 0.39 | 0.36 | 0.25 | 0.41 | |||||
| 10b | p | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.02 | 0.00 | |||||
| 11b | rho | 1.00 | 0.11 | 0.34 | 0.14 | 0.37 | 0.38 | 0.20 | 0.11 | 0.18 | 0.33 | 0.28 | 0.28 | ||||||
| 11b | p | 0.00 | 1.00 | 0.00 | 1.00 | 0.00 | 0.00 | 0.05 | 1.00 | 0.27 | 0.00 | 0.00 | 0.00 | ||||||
| 12b | rho | 1.00 | 0.16 | 0.24 | 0.34 | 0.32 | 0.23 | 0.25 | 0.13 | 0.10 | 0.22 | 0.30 | |||||||
| 12b | p | 0.00 | 0.30 | 0.02 | 0.00 | 0.00 | 0.01 | 0.03 | 1.00 | 1.00 | 0.02 | 0.00 | |||||||
| 13b | rho | 1.00 | 0.50 | 0.45 | 0.48 | 0.35 | 0.23 | 0.41 | 0.41 | 0.36 | 0.38 | ||||||||
| 13b | p | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.01 | 0.00 | 0.00 | 0.00 | 0.00 | ||||||||
| 14b | rho | 1.00 | 0.34 | 0.34 | 0.22 | 0.24 | 0.42 | 0.29 | 0.23 | 0.26 | |||||||||
| 14b | p | 0.00 | 0.00 | 0.00 | 0.27 | 0.03 | 0.00 | 0.00 | 0.08 | 0.00 | |||||||||
| 15b | rho | 1.00 | 0.93 | 0.33 | 0.25 | 0.37 | 0.40 | 0.34 | 0.38 | ||||||||||
| 15b | p | 0.00 | 0.00 | 0.00 | 0.01 | 0.00 | 0.00 | 0.00 | 0.00 | ||||||||||
| 16b | rho | 1.00 | 0.36 | 0.27 | 0.41 | 0.39 | 0.33 | 0.39 | |||||||||||
| 16b | p | 0.00 | 0.00 | 0.01 | 0.00 | 0.00 | 0.00 | 0.00 | |||||||||||
| 17b | rho | 1.00 | 0.41 | 0.41 | 0.33 | 0.39 | 0.37 | ||||||||||||
| 17b | p | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | ||||||||||||
| 18b | rho | 1.00 | 0.32 | 0.33 | 0.25 | 0.31 | |||||||||||||
| 18b | p | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | |||||||||||||
| 20b | rho | 1.00 | 0.39 | 0.38 | 0.49 | ||||||||||||||
| 20b | p | 0.00 | 0.00 | 0.00 | 0.00 | ||||||||||||||
| 21b | rho | 1.00 | 0.37 | 0.40 | |||||||||||||||
| 21b | p | 0.00 | 0.00 | 0.00 | |||||||||||||||
| 22b | rho | 1.00 | 0.41 | ||||||||||||||||
| 22b | p | 0.00 | 0.00 | ||||||||||||||||
| 23 | rho | 1.00 | |||||||||||||||||
| 23b | p | 0.00 | |||||||||||||||||
| Score | rho |
| Item | Questions |
|---|---|
| 1 | How often was your loved one able to feed her/himself? |
| 2 | How often did your loved one appear to breathe comfortably? |
| 3 | How often did your loved one appear to feel at peace during the process of dying? |
| 4 | How often did your loved one appear to be unafraid of dying? |
| 5 | How often did your loved one spend time with his/her family or friends? |
| 6 | How often did your loved one spend time alone? |
| 7 | Was your loved one touched or hugged by his/her loved ones? |
| 8 | Were all of your loved one’s health care costs taken care of? |
| 9 | Did your loved one say goodbye to loved ones? |
| 10 | Did your loved one clear up any bad feelings with others? |
| 11 | Did your loved one have one or more visits from a religious or spiritual advisor? |
| 12 | Did your loved one have a spiritual service or ceremony before his/her death? |
| 13 | Did your loved one receive a mechanical ventilator (respirator) to breathe for him/her? |
| 14 | Did your loved one receive dialysis for his/her kidneys? |
| 15 | Did your loved one discuss his or her wishes for end-of-life care with his/her doctor -- for example, resuscitation or intensive care? |
| 16 | Was any family member present at the moment of your loved one’s death? |
| 17 | In the moment before your loved one’s death, was he/she: |
| 18 | Overall, how would you rate the quality of your loved one’s dying? |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Benichel, C.R.; Meneguin, S.; Pollo, C.F.; Oliveira, M.G.; de Oliveira, C. Psychometric Properties of the Brazilian Version of the Quality of Dying and Death for Adult Family Members of ICU Patients. Int. J. Environ. Res. Public Health 2023, 20, 5034. https://doi.org/10.3390/ijerph20065034
Benichel CR, Meneguin S, Pollo CF, Oliveira MG, de Oliveira C. Psychometric Properties of the Brazilian Version of the Quality of Dying and Death for Adult Family Members of ICU Patients. International Journal of Environmental Research and Public Health. 2023; 20(6):5034. https://doi.org/10.3390/ijerph20065034
Chicago/Turabian StyleBenichel, Cariston Rodrigo, Silmara Meneguin, Camila Fernandes Pollo, Mariele Gobo Oliveira, and Cesar de Oliveira. 2023. "Psychometric Properties of the Brazilian Version of the Quality of Dying and Death for Adult Family Members of ICU Patients" International Journal of Environmental Research and Public Health 20, no. 6: 5034. https://doi.org/10.3390/ijerph20065034
APA StyleBenichel, C. R., Meneguin, S., Pollo, C. F., Oliveira, M. G., & de Oliveira, C. (2023). Psychometric Properties of the Brazilian Version of the Quality of Dying and Death for Adult Family Members of ICU Patients. International Journal of Environmental Research and Public Health, 20(6), 5034. https://doi.org/10.3390/ijerph20065034






