Association of Maternal Smoking during Pregnancy with Neurophysiological and ADHD-Related Outcomes in School-Aged Children
Abstract
1. Introduction
2. Materials and Methods
2.1. Assessment of Maternal Prenatal Smoking
2.2. Assessment and Pre-Processing of Resting-State EEG Activity
2.3. Assessment of ADHD-Related Behavioral Difficulties
2.4. Statistical Analysis
3. Results
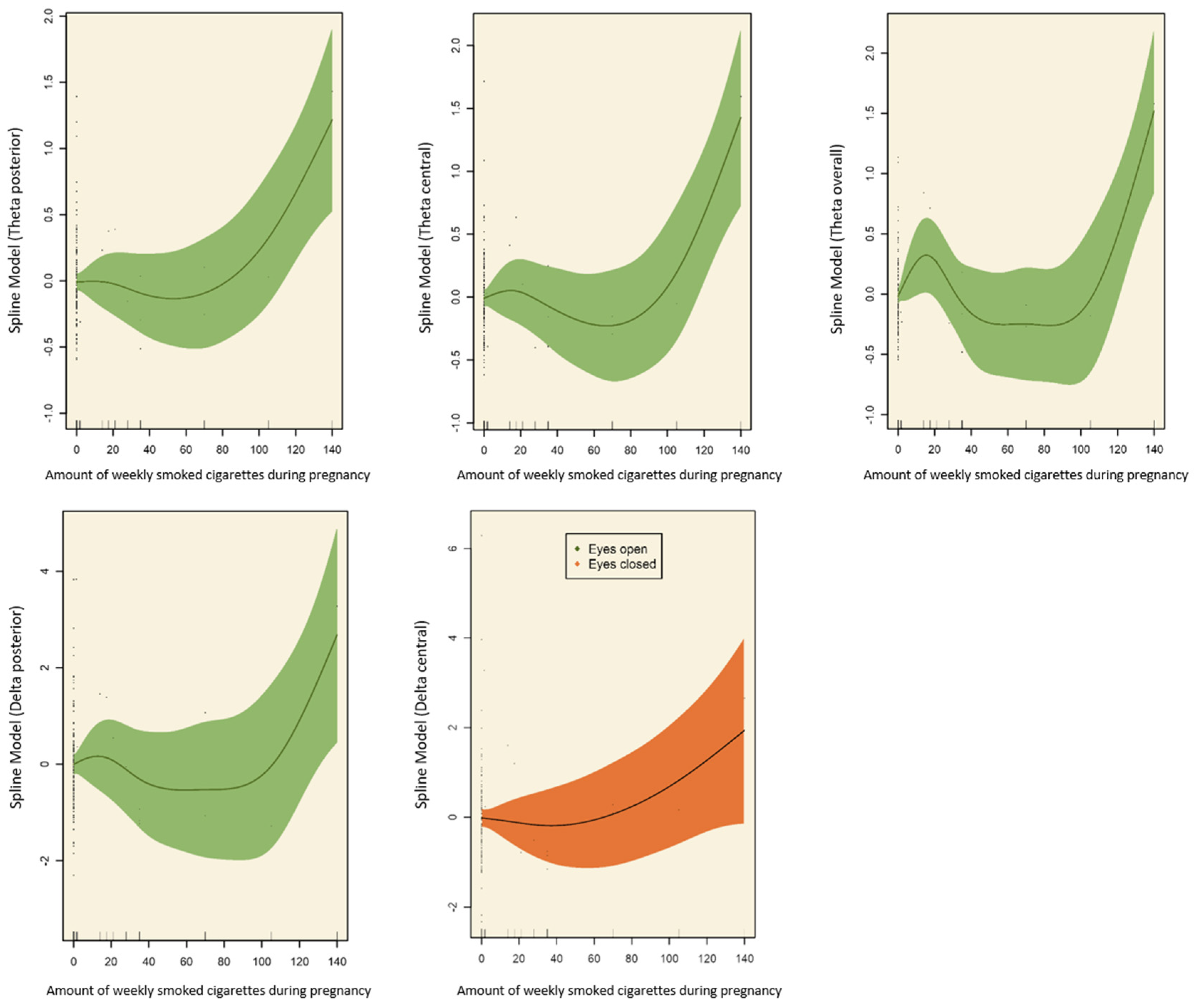
3.1. Effects of Prenatal Tobacco Exposure on Brain Activity
3.2. Effects of Prenatal Tobacco Exposure on ADHD
3.3. Interaction of Prenatal Tobacco Exposure, Brain Activity, and ADHD Symptoms
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rogers, J.M. Smoking and pregnancy: Epigenetics and developmental origins of the metabolic syndrome. Birth. Defects Res. 2019, 111, 1259–1269. [Google Scholar] [CrossRef] [PubMed]
- Banderali, G.; Martelli, A.; Landi, M.; Moretti, F.; Betti, F.; Radaelli, G.; Lassandro, C.; Verduci, E. Short and long term health effects of parental tobacco smoking during pregnancy and lactation: A descriptive review. J. Transl. Med. 2015, 13, 327. [Google Scholar] [CrossRef] [PubMed]
- Modabbernia, A.; Reichenberg, A.; Ing, A.; Moser, D.A.; Doucet, G.E.; Artiges, E.; Banaschewski, T.; Barker, G.J.; Becker, A.; Bokde, A.L.W.; et al. IMAGEN Consortium.: Linked patterns of biological and environmental covariation with brain structure in adolescence: A population-based longitudinal study. Mol. Psychiatry 2021, 26, 4905–4918. [Google Scholar] [CrossRef] [PubMed]
- Orleans, C.T.; Johnson, R.W.; Barker, D.C.; Kaufman, N.J.; Marx, J.F. Helping pregnant smokers quit: Meeting the challenge in the next decade. West. J. Med. 2001, 174, 276–281. [Google Scholar] [CrossRef]
- Braun, M.; Klingelhöfer, D.; Oremek, G.M.; Quarcoo, D.; Groneberg, D.A. Influence of Second-Hand Smoke and Prenatal Tobacco Smoke Exposure on Biomarkers, Genetics and Physiological Processes in Children-An Overview in Research Insights of the Last Few Years. Int. J. Environ. Res. Public Health 2020, 17, 3212. [Google Scholar] [CrossRef]
- Clifford, A.; Lang, L.; Chen, R. Effects of maternal cigarette smoking during pregnancy on cognitive parameters of children and young adults: A literature review. Neurotoxicology Teratol. 2012, 34, 560–570. [Google Scholar] [CrossRef]
- Laucht, M.; Schmidt, M.H. Mütterliches Rauchen in der Schwangerschaft: Risikofaktor für eine ADHS des Kindes? Maternal smoking during pregnancy: Risk factor for ADHD in the offspring? Z. Kinder Jugendpsychiatr. Psychother. 2004, 32, 177–185. [Google Scholar] [CrossRef]
- Melchior, M.; Hersi, R.; van der Waerden, J.; Larroque, B.; Saurel-Cubizolles, M.J.; Chollet, A.; Galéra, C.L. EDEN Mother-Child Cohort Study Group. Maternal tobacco smoking in pregnancy and children’s socio-emotional development at age 5: The EDEN mother-child birth cohort study. Eur. Psychiatry 2015, 30, 562–568. [Google Scholar] [CrossRef]
- Weitzman, M.; Byrd, R.S.; Aligne, C.A.; Moss, M. The effects of tobacco exposure on children’s behavioral and cognitive functioning: Implications for clinical and public health policy and future research. Neurotoxicol. Teratol. 2002, 24, 397–406. [Google Scholar] [CrossRef]
- Neuman, R.J.; Lobos, E.; Reich, W.; Henderson, C.A.; Sun, L.W.; Todd, R.D. Prenatal Smoking Exposure and Dopaminergic Genotypes Interact to Cause a Severe ADHD Subtype. Biol. Psychiatry 2007, 61, 1320–1328. [Google Scholar] [CrossRef]
- Dong, T.; Hu, W.; Zhou, X.; Lin, H.; Lan, L.; Hang, B.; Lv, W.; Geng, Q.; Xia, Y. Prenatal exposure to maternal smoking during pregnancy and attention-deficit/hyperactivity disorder in offspring: A meta-analysis. Reprod. Toxicol. 2018, 76, 63–70. [Google Scholar] [CrossRef]
- D’Onofrio, B.M.; Van Hulle, C.A.; Waldman, I.D.; Rodgers, J.L.; Harden, K.P.; Rathouz, P.J.; Lahey, B.B. Smoking during pregnancy and offspring externalizing problems: An exploration of genetic and environmental confounds. Dev. Psychopathol. 2008, 20, 139–164. [Google Scholar] [CrossRef]
- Sciberras, E.; Mulraney, M.; Silva, D.; Coghill, D. Prenatal risk factors and the etiology of ADHD—Review of existing evidence. Curr. Psychiatry Rep. 2017, 19, 1. [Google Scholar] [CrossRef]
- Tarver, J.; Daley, D.; Sayal, K. ADHD: An updated review of the essential facts. Child Care Health Dev. 2014, 40, 762–774. [Google Scholar] [CrossRef]
- Thapar, A.; Rice, F.; Hay, D.; Boivin, J.; Langley, K.; van den Bree, M.; Rutter, M.; Harold, G. Prenatal smoking might not cause attention-deficit/hyperactivity disorder: Evidence from a novel design. Biol. Psychiatry 2009, 66, 722–727. [Google Scholar] [CrossRef]
- Langley, K.; Heron, J.; Smith, G.D.; Thapar, A. Maternal and paternal smoking during pregnancy and risk of ADHD symptoms in offspring: Testing for intrauterine effects. Am. J. Epidemiol. 2012, 176, 261–268. [Google Scholar] [CrossRef]
- El Marroun, H.; Schmidt, M.N.; Franken, I.H.; Jaddoe, V.W.; Hofman, A.; van der Lugt, A.; Verhulst, F.C.; Tiemeier, H.; White, T. Prenatal tobacco exposure and brain morphology: A prospective study in young children. Neuropsychopharmacology 2014, 39, 792–800. [Google Scholar] [CrossRef]
- Derauf, C.; Lester, B.M.; Neyzi, N.; Kekatpure, M.; Gracia, L.; Davis, J.; Kallianpur, K.; Efird, J.T.; Kosofsky, B. Subcortical and cortical structural central nervous system changes and attention processing deficits in preschool-aged children with prenatal methamphetamine and tobacco exposure. Dev. Neurosci. 2012, 34, 327–341. [Google Scholar] [CrossRef]
- Paus, T.; Nawazkhan, I.; Leonard, G.; Perron, M.; Pike, G.B.; Pitiot, A.; Richer, L.; Veillette, S.; Pausova, Z. Corpus callosum in adolescent offspring exposed prenatally to maternal cigarette smoking. Neuroimage 2008, 40, 435–441. [Google Scholar] [CrossRef]
- Ekblad, M.; Korkeila, J.; Lehtonen, L. Smoking during pregnancy affects foetal brain development. Acta Paediatr. 2015, 104, 12–18. [Google Scholar] [CrossRef]
- Ekblad, M.; Korkeila, J.; Parkkola, R.; Lapinleimu, H.; Haataja, L.; Lehtonen, L.; PIPARI Study Group. Maternal Smoking during Pregnancy and Regional Brain Volumes in Preterm Infants. J. Pediatr. 2010, 156, 185–190.e1. [Google Scholar] [CrossRef] [PubMed]
- Holz, N.E.; Boecker, R.; Baumeister, S.; Hohm, E.; Zohsel, K.; Buchmann, A.F.; Blomeyer, D.; Jennen-Steinmetz, C.; Hohmann, S.; Wolf, I.; et al. Effect of prenatal exposure to tobacco smoke on inhibitory control: Neuroimaging results from a 25-year prospective study. JAMA Psychiatry 2014, 71, 786–796. [Google Scholar] [CrossRef] [PubMed]
- Baillet, S.; Mosher, J.C.; Leahy, R.M. “Electromagnetic Brain Mapping,” Signal Processing Magazine. IEEE 2001, 18, 14–30. [Google Scholar] [CrossRef]
- Brown, T.T.; Jernigan, T.L. Brain development during the preschool years. Neuropsychol. Rev. 2012, 22, 313–333. [Google Scholar] [CrossRef] [PubMed]
- Bridwell, D.A.; Cavanagh, J.F.; Collins, A.G.E.; Nunez, M.D.; Srinivasan, R.; Stober, S.; Calhoun, V.D. Moving Beyond ERP Components: A Selective Review of Approaches to Integrate EEG and Behavior. Front. Hum. Neurosci. 2018, 12, 106. [Google Scholar] [CrossRef]
- Shuffrey, L.C.; Shuffrey, L.C.; Myers, M.M.; Isler, J.R.; Lucchini, M.; Sania, A.; Pini, N.; Nugent, J.D.; Condon, C.; Ochoa, T.; et al. PASS Network. Association Between Prenatal Exposure to Alcohol and Tobacco and Neonatal Brain Activity: Results From the Safe Passage Study. JAMA Netw. Open 2020, 3, e204714. [Google Scholar] [CrossRef]
- Barry, R.J.; Clarke, A.R.; Johnstone, S.J.; Brown, C.R.; Bruggemann, J.M.; van Rijbroek, I. Caffeine effects on resting-state arousal in children. Int. J. Psychophysiol. 2009, 73, 355–361. [Google Scholar] [CrossRef]
- Machado, C.; Estévez, M.; Leisman, G.; Melillo, R.; Rodríguez, R.; DeFina, P.; Hernández, A.; Pérez-Nellar, J.; Naranjo, R.; Chinchilla, M.; et al. QEEG spectral and coherence assessment of autistic children in three different experimental conditions. J. Autism Dev. Disord. 2015, 45, 406–424. [Google Scholar] [CrossRef]
- Karakaş, S. A comparative review of the psychophysiology of attention in children with and without attention deficit hyperactivity disorder. Int. J. Psychophysiol. 2022, 177, 43–60. [Google Scholar] [CrossRef]
- Clarke, A.R.; Barry, R.J.; McCarthy, R. Correlation Between EEG Activity and Behavior in Children with Attention-Deficit/Hyperactivity Disorder. J. Neurother. 2011, 15, 193–199. [Google Scholar] [CrossRef]
- Rodríguez-Martínez, E.I.; Angulo-Ruiz, B.Y.; Arjona-Valladares, A.; Rufo, M.; Gómez-González, J.; Gómez, C.M. Frequency coupling of low and high frequencies in the EEG of ADHD children and adolescents in closed and open eyes conditions. Res. Dev. Disabil. 2020, 96, 103520. [Google Scholar] [CrossRef]
- Langley, K.; Turic, D.; Rice, F.; Holmans, P.; van den Bree, M.B.; Craddock, N.; Kent, L.; Owen, M.J.; O’Donovan, M.C.; Thapar, A. Testing for Gene x Environment Interaction Effects in Attention Deficit Hyperactivity Disorder and Associated Antisocial Behavior. Am. J. Med. Genet. Part B 2008, 147B, 49–53. [Google Scholar] [CrossRef]
- Reulbach, U.; Bleich, S.; Knorr, J.; Burger, P.; Fasching, P.A.; Kornhuber, J.; Beckmann, M.W.; Goecke, T.W. Pre-, peri- and postpartal depression. Fortschr. Neurol. Psychiat 2009, 77, 708–713. [Google Scholar] [CrossRef]
- Eichler, A.; Walz, L.; Grunitz, J.; Grimm, J.; van Doren, J.; Raabe, E.; Goecke, T.W.; Fasching, P.A.; Beckmann, M.W.; Kornhuber, J.; et al. Children of prenatally depressed mothers: Externalizing and internalizing symptoms are accompanied by reductions in specific social-emotional competencies. J. Child Fam. Stud. 2017, 26, 3135–3144. [Google Scholar] [CrossRef]
- Hein, A.; Rauh, C.; Engel, A.; Häberle, L.; Dammer, U.; Voigt, F.; Fasching, P.A.; Faschingbauer, F.; Burger, P.; Beckmann, M.W.; et al. Socioeconomic status and depression during and after pregnancy in the Franconian Maternal Health Evaluation Studies (FRAMES). Arch. Gynecol. Obstet. 2014, 289, 755–763. [Google Scholar] [CrossRef]
- Eichler, A.; Hudler, L.; Grunitz, J.; Grimm, J.; Raabe, E.; Goecke, T.W.; Fasching, P.A.; Beckmann, M.W.; Kratz, O.; Moll, G.H.; et al. Effects of prenatal alcohol consumption on cognitive development and ADHD-related behaviour in primary-school age: A multilevel study based on meconium ethyl glucuronide. J. Child Psychol. Psychiatry 2018, 59, 110–118. [Google Scholar] [CrossRef]
- Breuer, D.; Döpfner, M. Entwicklung eines Fragebogens zur Erfassung von Aufmerksamkeitsdefizit-/Hyperaktivitätsstörungen (ADHS) bei Vorschulkindern im Eltern- und im Erziehungsurteil. Z. Für Entwickl. Und Pädagogische Psychol. 2008, 40, 40–48. [Google Scholar] [CrossRef]
- Wood, S.N. Stable and efficient multiple smoothing parameter estimation for generalized additive models. J. Amer. Statist. Ass. 2004, 99, 673–686. [Google Scholar] [CrossRef]
- Wood, S.N. Fast stable restricted maximum likelihood and marginal likelihood estimation of semiparametric generalized linear models. J. R. Stat. Soc. B 2011, 73, 3–36. [Google Scholar] [CrossRef]
- Chen, C. Generalized additive mixed models. Commun. Stat. Theory Methods 2000, 29, 1257–1271. [Google Scholar] [CrossRef]
- Baayen, H.; Vasishth, S.; Kliegl, R.; Bates, D. The cave of shadows: Addressing the human factor with generalized additive mixed models. J. Mem. Lang. 2017, 94, 206–234. [Google Scholar] [CrossRef]
- Lin, X.; Zhang, D. Inference in generalized additive mixed models by using smoothing splines. J. R. Stat. Soc. Ser. B Stat. Methodol. 1999, 61, 381–400. [Google Scholar] [CrossRef]
- Lees, B.; Mewton, L.; Jacobus, J.; Valadez, E.A.; Stapinski, L.A.; Teesson, M.; Tapert, S.F.; Squeglia, L.M. Association of Prenatal Alcohol Exposure With Psychological, Behavioral, and Neurodevelopmental Outcomes in Children From the Adolescent Brain Cognitive Development Study. Am. J. Psychiatry 2020, 177, 1060–1072. [Google Scholar] [CrossRef] [PubMed]
- Rivkin, M.J.; Davis, P.E.; Lemaster, J.L.; Cabral, H.J.; Warfield, S.K.; Mulkern, R.V.; Robson, C.D.; Rose-Jacobs, R.; Frank, D.A. Volumetric MRI study of brain in children with intrauterine exposure to cocaine, alcohol, tobacco, and marijuana. Pediatrics 2008, 121, 741–750. [Google Scholar] [CrossRef]
- Albers, L.; Sobotzki, C.; Kuß, O.; Ajslev, T.; Batista, R.F.; Bettiol, H.; Brabin, B.; Buka, S.L.; Cardoso, V.C.; Clifton, V.L.; et al. Maternal smoking during pregnancy and offspring overweight: Is there a dose-response relationship? An individual patient data meta-analysis. Int. J. Obes. 2018, 42, 1249–1264. [Google Scholar] [CrossRef]
- Havlicek, V.; Childiaeva, R.; Chernick, V. EEG Frequency spectrum characteristics of sleep states in infants ofalcoholic mothers. Neuropadiatrie 1977, 8, 360–373. [Google Scholar] [CrossRef]
- Loffe, S.; Childiaeva, R.; Chernick, V. Prolonged effects of maternal alcohol ingestion on the neonatal electroencephalogram. Pediatrics 1984, 74, 330–335. [Google Scholar] [CrossRef]
- Jacobsen, L.K.; Slotkin, T.A.; Mencl, W.E.; Frost, S.J.; Pugh, K.R. Gender-specific effects of prenatal and adolescent exposure to tobacco smoke on auditory and visual attention. Neuropsychopharmacology 2007, 32, 2453–2464. [Google Scholar] [CrossRef]
- Cornelius, M.D.; Day, N.L. Developmental consequences of prenatal tobacco exposure. Curr. Opin. Neurol. 2009, 22, 121–125. [Google Scholar] [CrossRef]
- Rice, F.; Langley, K.; Woodford, C.; Davey Smith, G.; Thapar, A. Identifying the contribution of prenatal risk factors to offspring development and psychopathology: What designs to use and a critique of literature on maternal smoking and stress in pregnancy. Dev. Psychopathol. 2018, 30, 1107–1128. [Google Scholar] [CrossRef]
- Abraham, M.; Alramadhan, S.; Iniguez, C.; Duijts, L.; Jaddoe, V.W.; Den Dekker, H.T.; Crozier, S.; Godfrey, K.M.; Hindmarsh, P.; Vik, T.; et al. A systematic review of maternal smoking during pregnancy and fetal measurements with meta-analysis. PLoS ONE 2017, 12, e0170946. [Google Scholar] [CrossRef]
- Gustavson, K.; Ystrom, E.; Stoltenberg, C.; Susser, E.; Surén, P.; Magnus, P.; Knudsen, G.P.; Smith, G.D.; Langley, K.; Rutter, M.; et al. Smoking in Pregnancy and Child ADHD. Pediatrics 2017, 139, e20162509. [Google Scholar] [CrossRef]
- Moylan, S.; Gustavson, K.; Øverland, S.; Karevold, E.B.; Jacka, F.N.; Pasco, J.A.; Berk, M. The impact of maternal smoking during pregnancy on depressive and anxiety behaviors in children: The Norwegian Mother and Child Cohort Study. BMC Med. 2015, 3, 13–24. [Google Scholar] [CrossRef]
- Kitsune, G.L.; Cheung, C.H.; Brandeis, D.; Banaschewski, T.; Asherson, P.; McLoughlin, G.; Kuntsi, J. A matter of time: The influence of recording context on EEG spectral power in adolescents and young adults with ADHD. Brain Topogr. 2015, 28, 580–590. [Google Scholar] [CrossRef]
- Tye, C.; Rijsdijk, F.; McLoughlin, G. Genetic overlap between ADHD symptoms and EEG theta power. Brain Cogn. 2014, 87, 168–172. [Google Scholar] [CrossRef]

| Characteristic | Tobacco Unexposed Children (n = 116) | Children With Prenatal Tobacco Exposure (n = 26) | |||
|---|---|---|---|---|---|
| Youth Variables | |||||
| n | % | n | % | p | |
| Sex | 0.434 | ||||
| Male Female | 59 57 | 50.9 49.1 | 11 15 | 42.3 57.7 | |
| Prenatal alcohol exposure | 0.562 | ||||
| No Yes | 95 21 | 81.9 18.1 | 20 6 | 76.9 23.1 | |
| Mean | SD | Mean | SD | p | |
| Age (years) | 7.72 | 0.65 | 7.76 | 0.74 | 0.781 |
| Gestational age | 39.41 | 1.33 | 39.23 | 1.45 | 0.533 |
| Birth weight | 3453.91 | 494.28 | 3370.77 | 520.34 | 0.446 |
| Intelligence quotient * | 104.23 | 10.25 | 104.31 | 7.18 | 0.972 |
| Parent Variables | |||||
| Age at giving birth | 32.86 | 4.12 | 31.88 | 5.15 | 0.299 |
| n | % | n | % | p | |
| Prenatal marital status | 0.068 | ||||
| Married Single parent | 113 3 | 97.4 2.6 | 23 3 | 88.5 11.5 | |
| Maternal Psychopathology | 0.536 | ||||
| No Yes Unknown | 68 29 19 | 58.6 25.0 16.4 | 17 5 4 | 65.4 19.2 15.3 | |
| Paternal Psychopathology | 0.461 | ||||
| No Yes Unknown | 92 12 12 | 79.3 10.3 8.3 | 20 2 4 | 76.9 7.7 15.3 | |
| Smoked | Did Not Smoke | Passive Smoking | |
|---|---|---|---|
| n (%) [Min, Max] M (SD) | n (%) | n (%) [Min, Max] M (SD) | |
| Before pregnancy | 40 (28.2) [2, 300] 25.21 (50.72) | 102 (71.8) | 33 (32.4) [3, 210] 102.39 (57.44) |
| 1. Trimester | 26 (18.3) [2, 140] 5.25 (19.28) | 116 (81.7) | 40 (34.5) [4, 210] 98.16 (57.09) |
| 2. Trimester | 24 (16.9) [2, 140] 5.07 (19.25) | 118 (83.1) | 37 (31.4) [4, 210] 99.05 (56.39) |
| 3. Trimester | 24 (16.9) [2, 140] 4.83 (19.01) | 118 (83.1) | 35 (29.7) [4, 210] 99.00 (56.48) |
| During pregnancy | 26 (18.3) [2, 140] 5.09 (19.23) | 116 (81.7) | 35 (30.2) [4, 210] 100.51 (55.27) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jansone, K.; Eichler, A.; Fasching, P.A.; Kornhuber, J.; Kaiser, A.; Millenet, S.; Banaschewski, T.; Nees, F.; on behalf of the IMAC-Mind Consortium. Association of Maternal Smoking during Pregnancy with Neurophysiological and ADHD-Related Outcomes in School-Aged Children. Int. J. Environ. Res. Public Health 2023, 20, 4716. https://doi.org/10.3390/ijerph20064716
Jansone K, Eichler A, Fasching PA, Kornhuber J, Kaiser A, Millenet S, Banaschewski T, Nees F, on behalf of the IMAC-Mind Consortium. Association of Maternal Smoking during Pregnancy with Neurophysiological and ADHD-Related Outcomes in School-Aged Children. International Journal of Environmental Research and Public Health. 2023; 20(6):4716. https://doi.org/10.3390/ijerph20064716
Chicago/Turabian StyleJansone, Karina, Anna Eichler, Peter A. Fasching, Johannes Kornhuber, Anna Kaiser, Sabina Millenet, Tobias Banaschewski, Frauke Nees, and on behalf of the IMAC-Mind Consortium. 2023. "Association of Maternal Smoking during Pregnancy with Neurophysiological and ADHD-Related Outcomes in School-Aged Children" International Journal of Environmental Research and Public Health 20, no. 6: 4716. https://doi.org/10.3390/ijerph20064716
APA StyleJansone, K., Eichler, A., Fasching, P. A., Kornhuber, J., Kaiser, A., Millenet, S., Banaschewski, T., Nees, F., & on behalf of the IMAC-Mind Consortium. (2023). Association of Maternal Smoking during Pregnancy with Neurophysiological and ADHD-Related Outcomes in School-Aged Children. International Journal of Environmental Research and Public Health, 20(6), 4716. https://doi.org/10.3390/ijerph20064716









