Biofuel Ash Aging in Acidic Environment and Its Influence on Cd Immobilization
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials Selection
2.2. Aging Methods
2.2.1. Natural Aging
2.2.2. Artificial Acid Aging
2.3. Physical–Chemical Tests
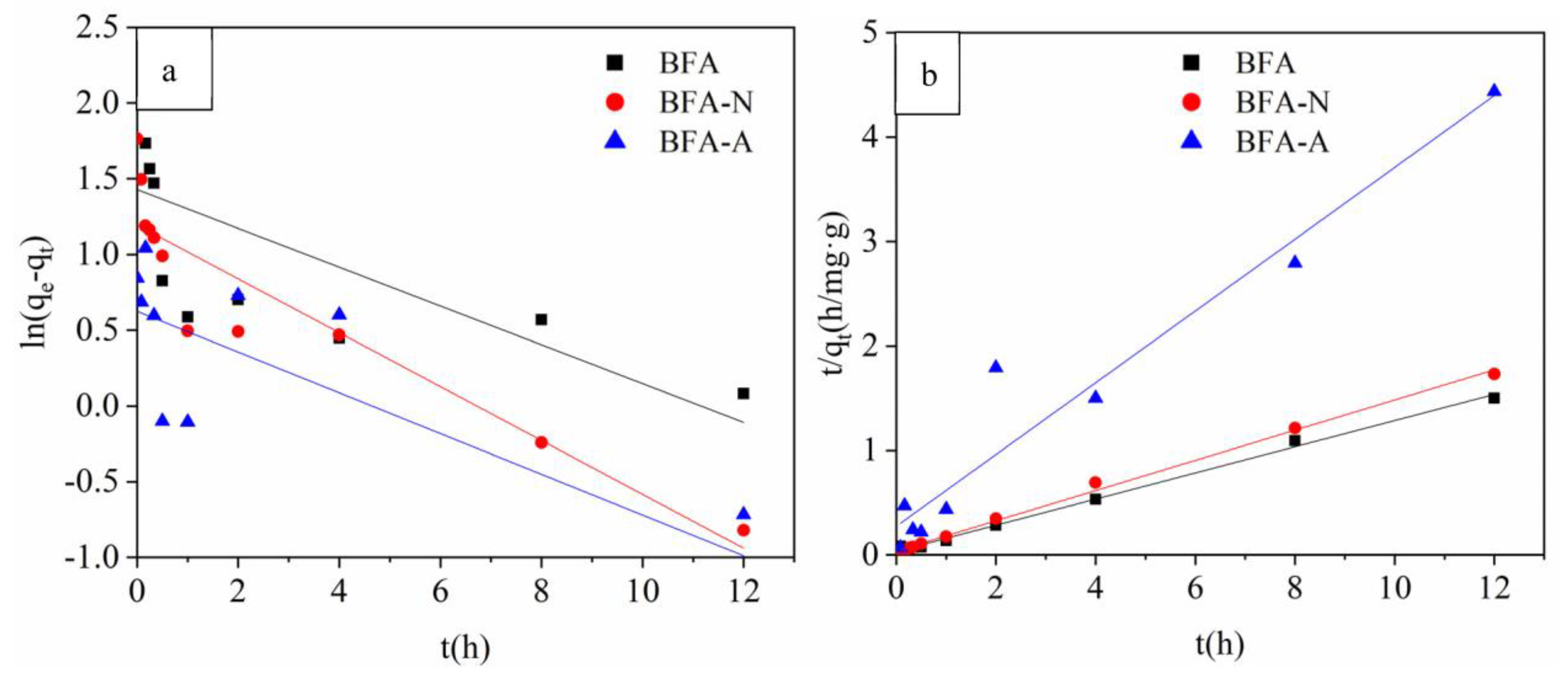
2.4. Adsorption Kinetic Experiment
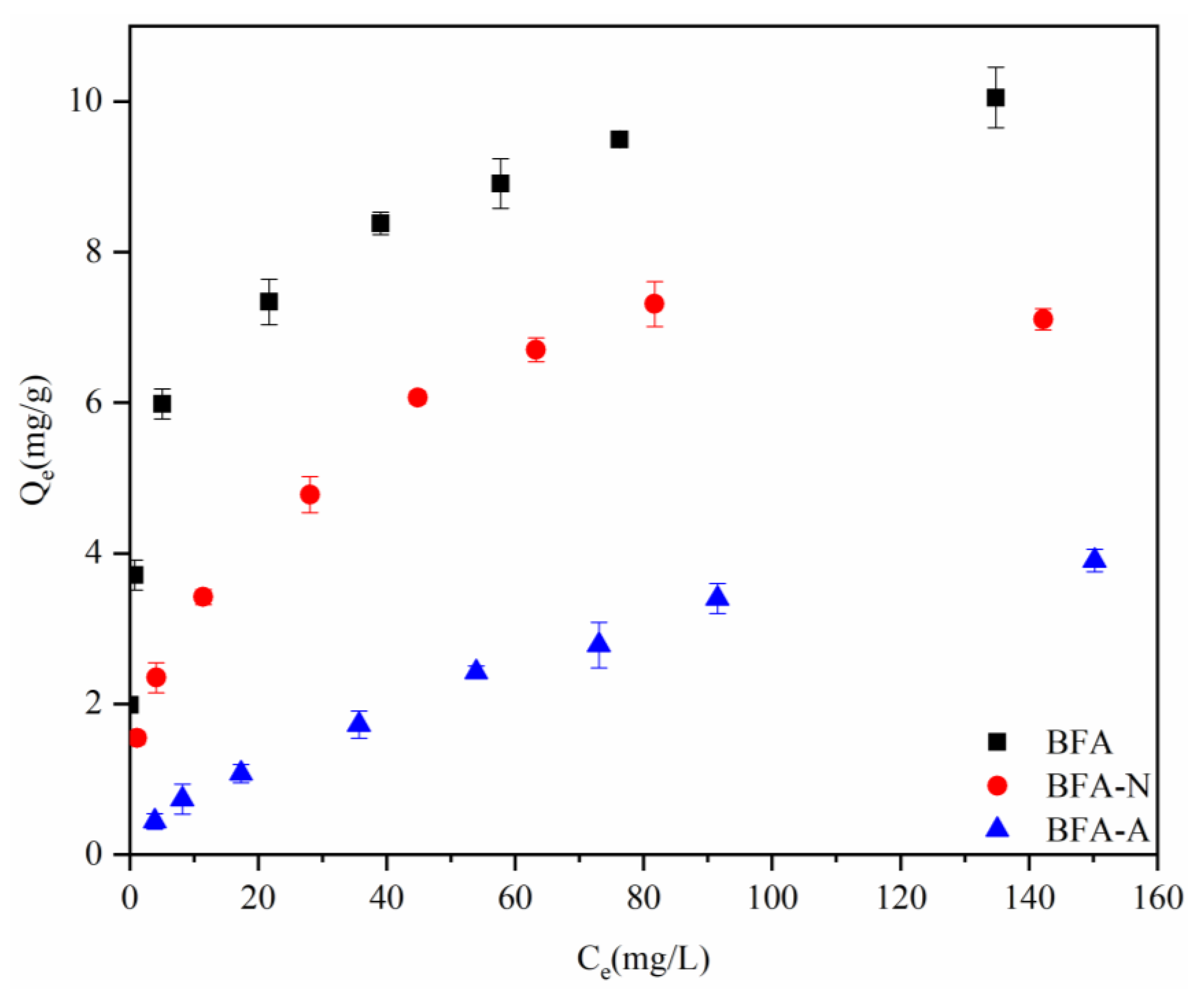
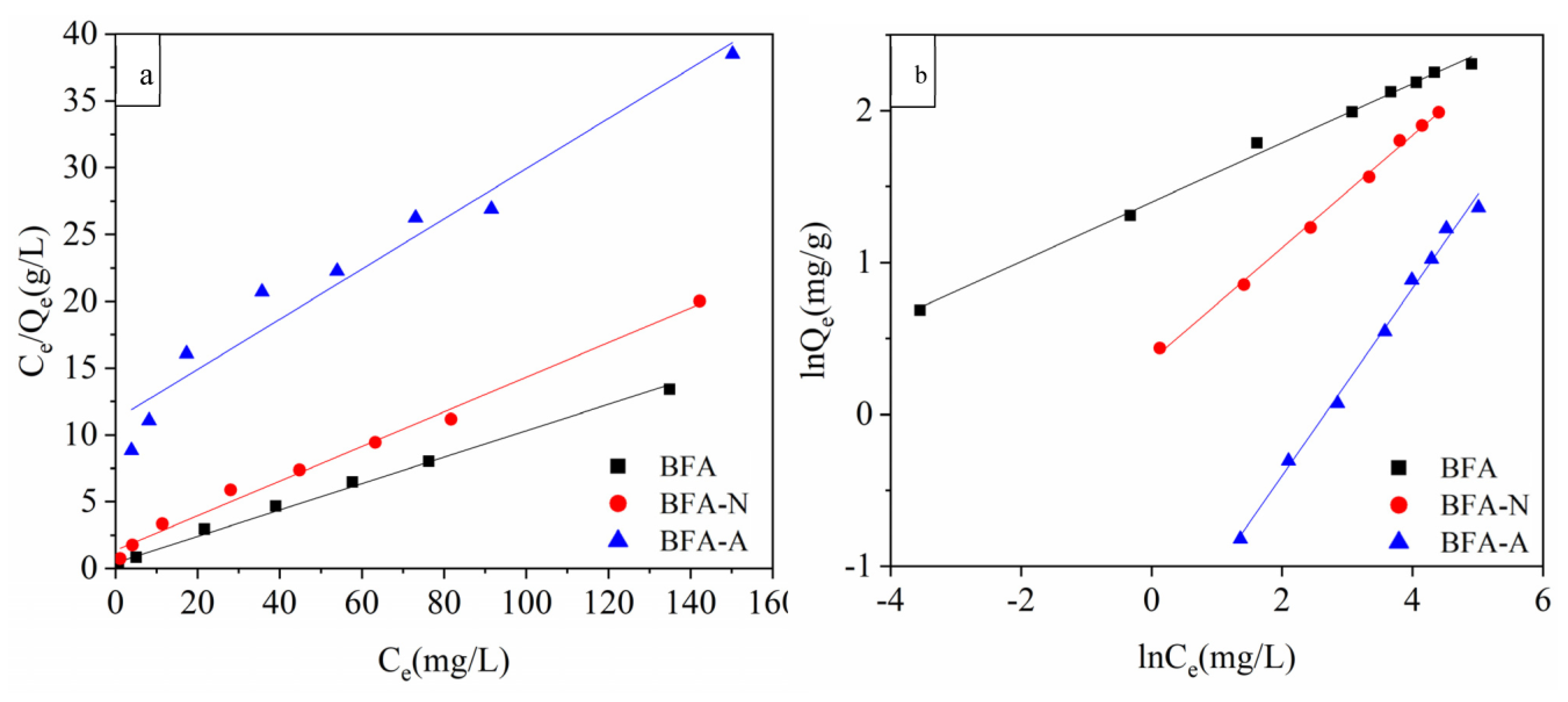
2.5. Isothermal Adsorption Experiment
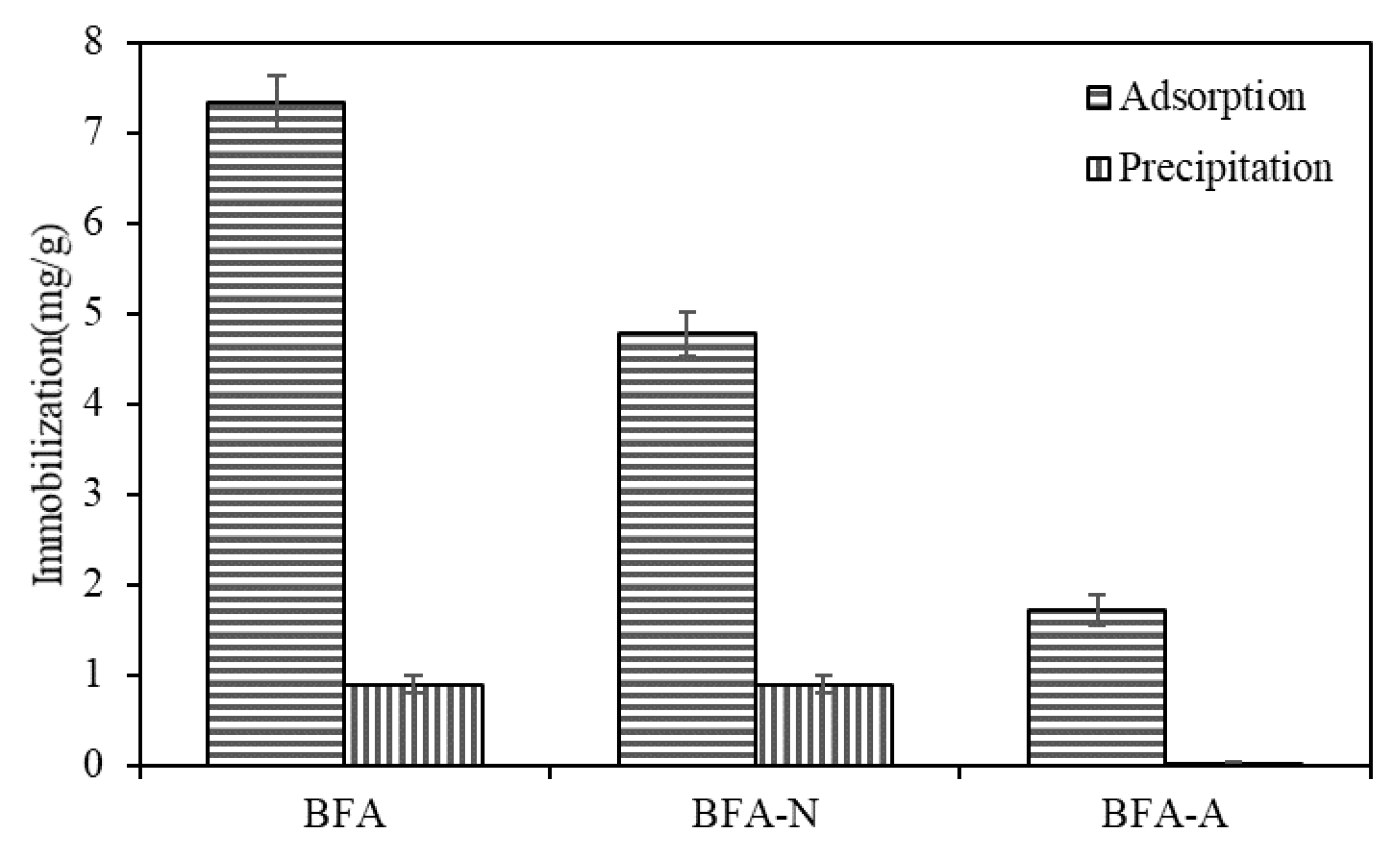
2.6. Precipitation Experiment
3. Results and Discussion
3.1. Changes in Physical and Chemical Properties of BFA induced by Aging
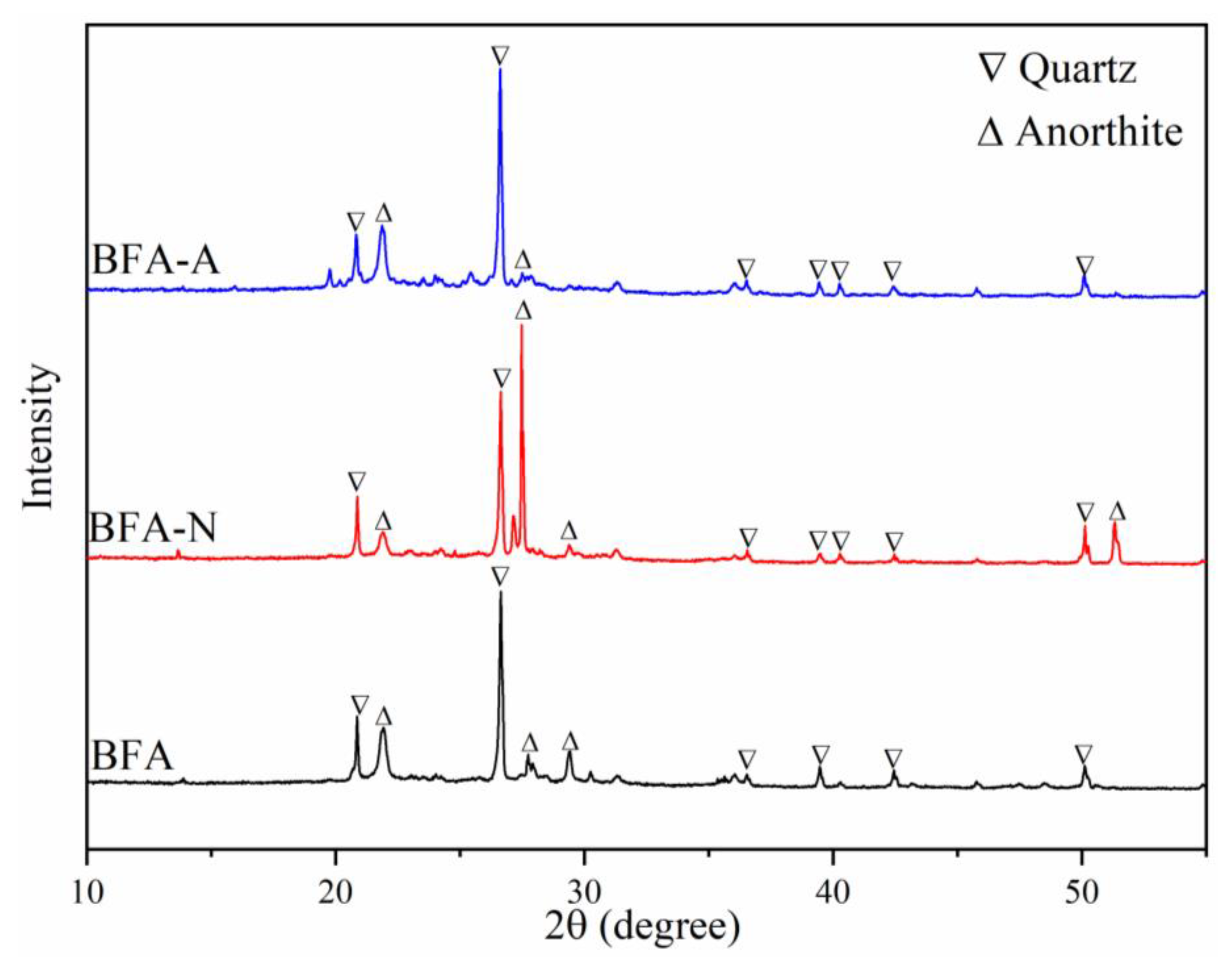
3.1.1. Mineral Composition Change by XRD
3.1.2. Functional Group Change by FTIR
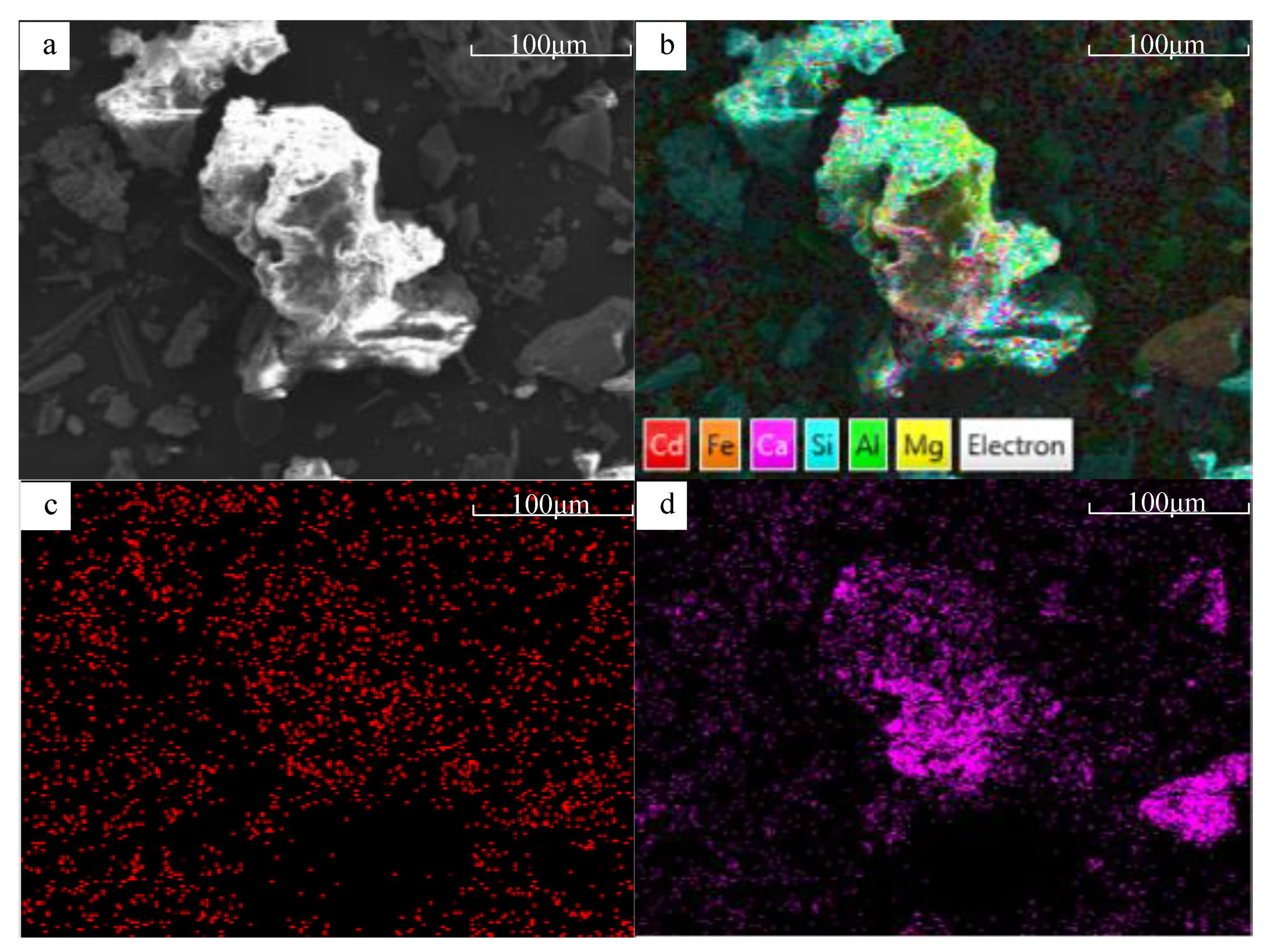
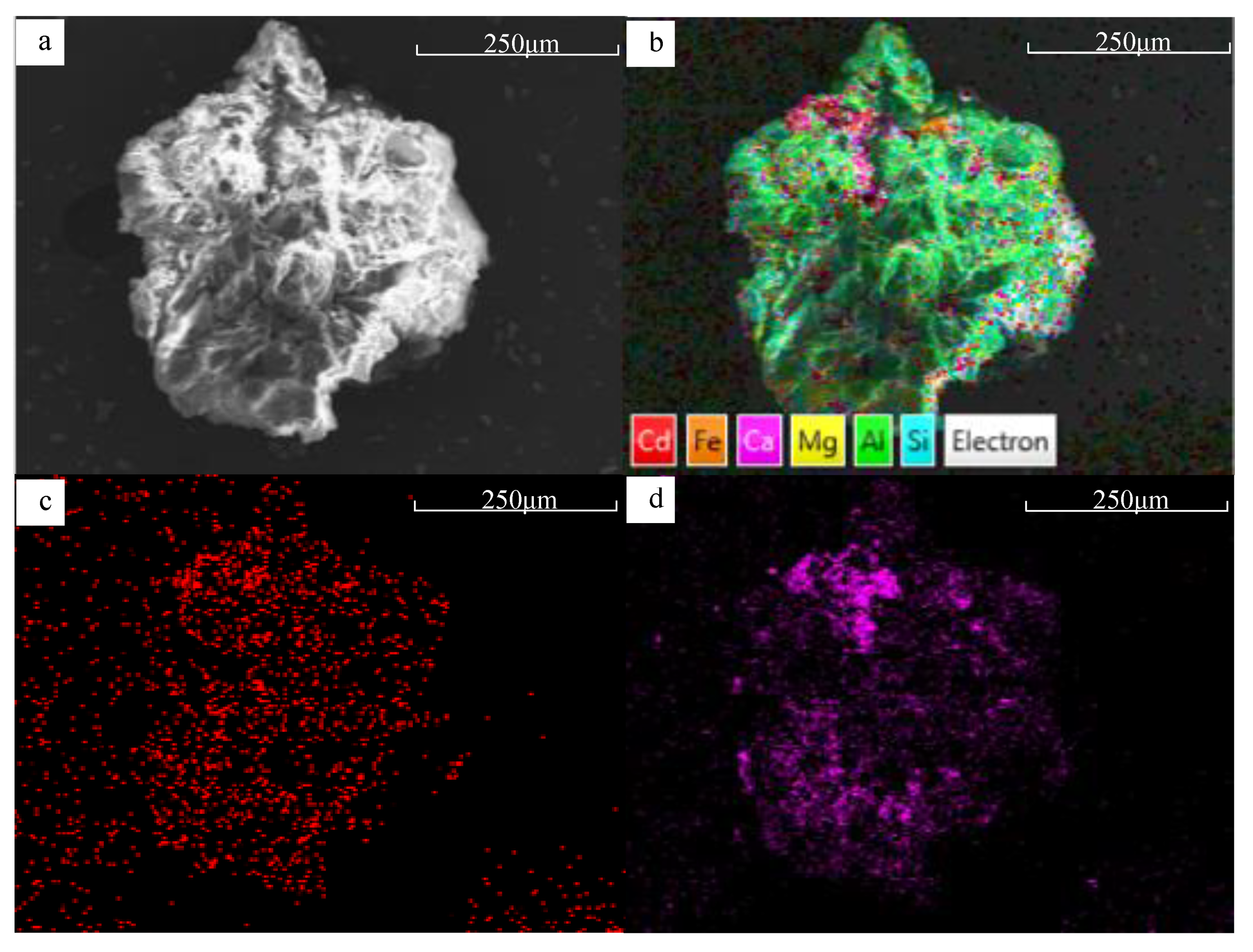
3.1.3. Surface Morphology and Specific Surface Area Changes
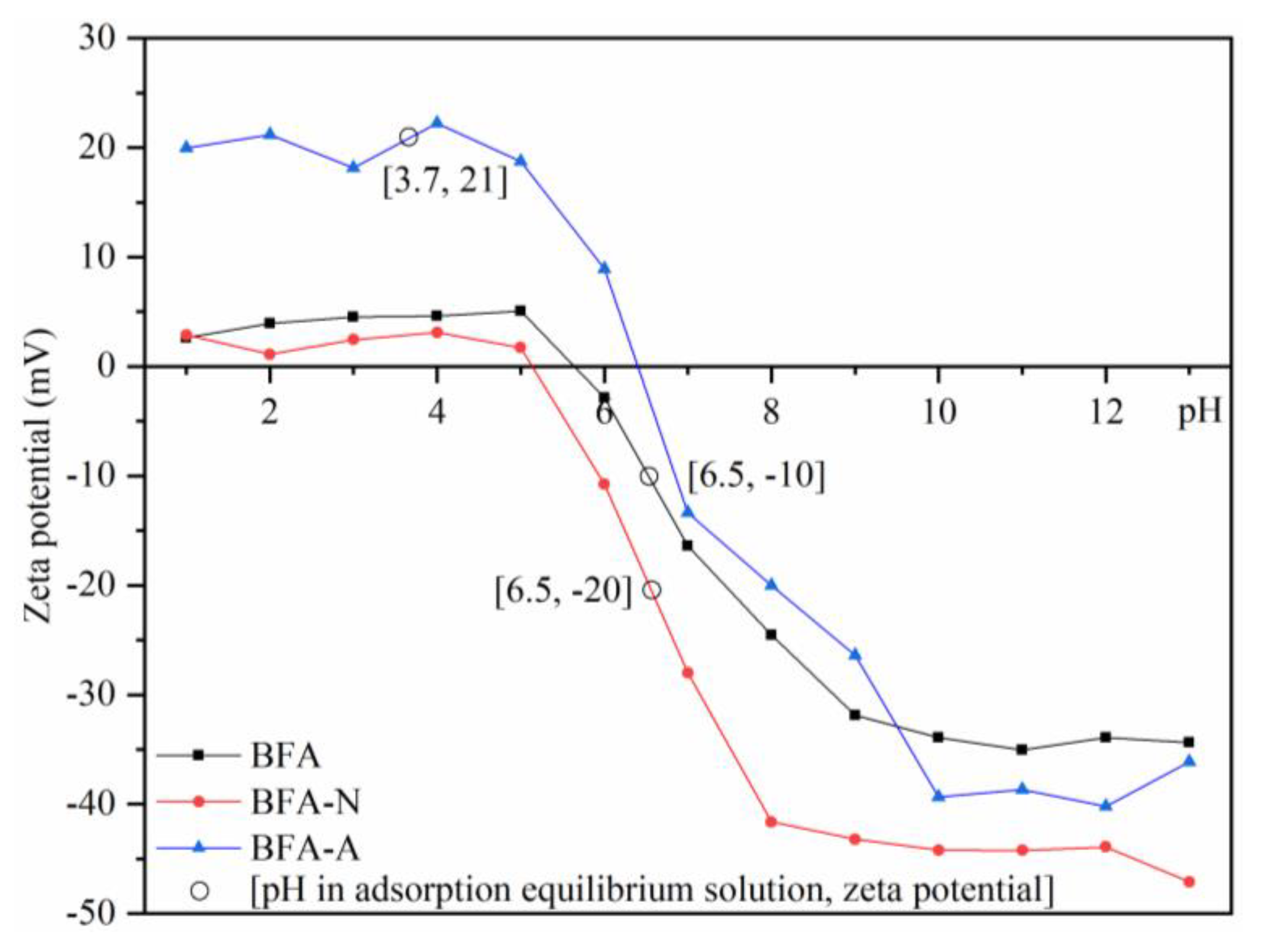
3.1.4. Zeta Potential Change
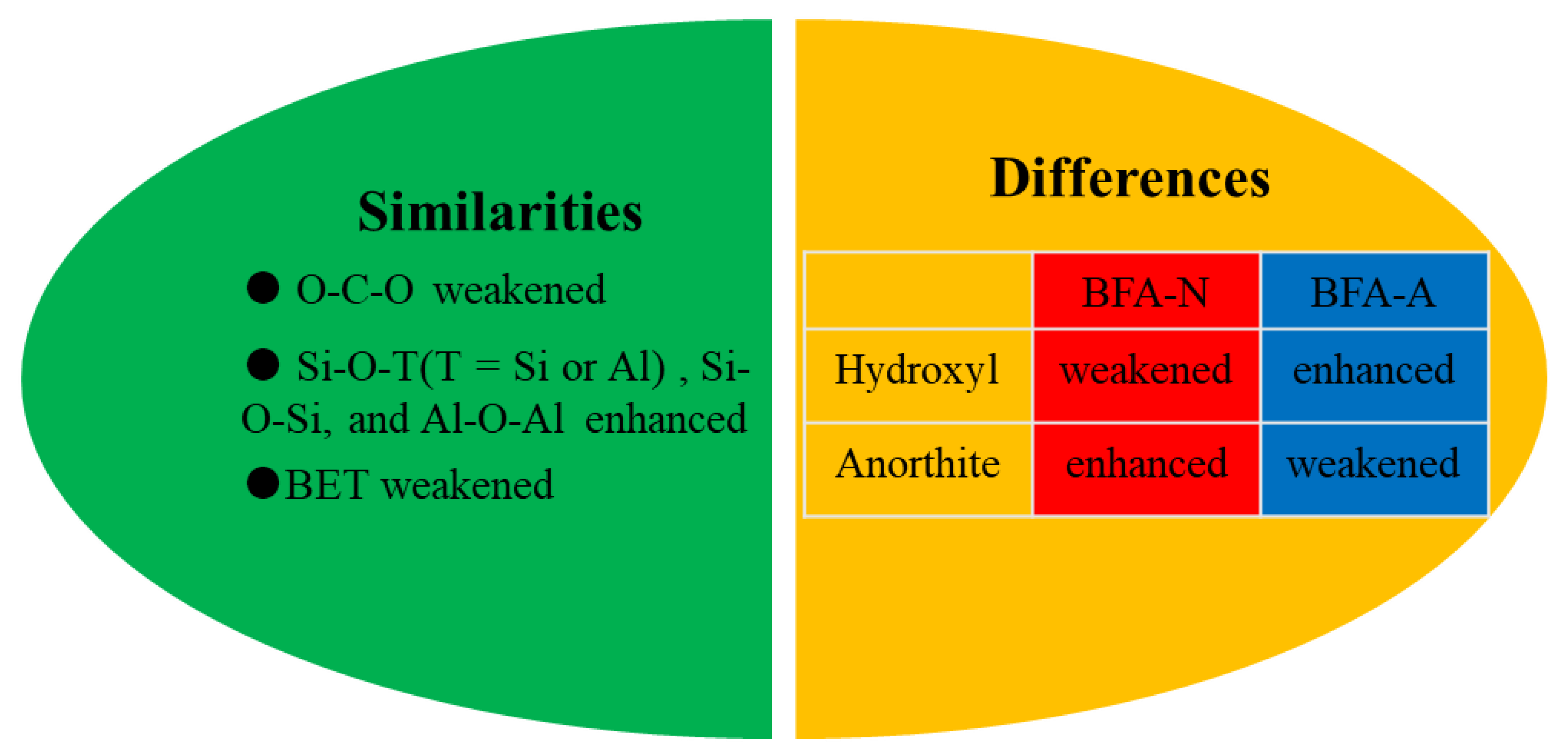
3.1.5. Comparison of Physicochemical Properties between BFA-N and BFA-A
3.2. Influence on Cd Immobilization Effect Induced by the Aging of BFA
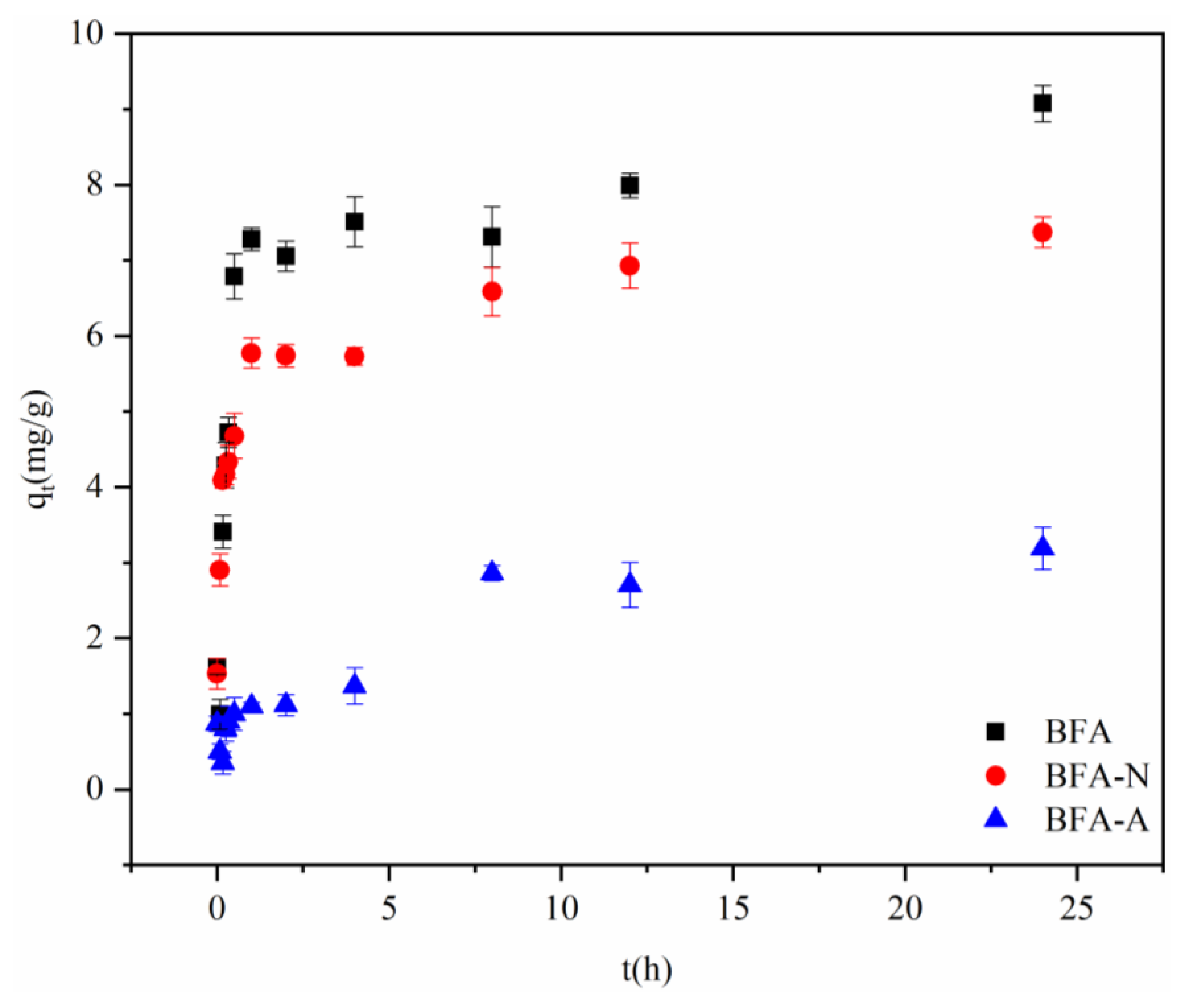
3.2.1. Adsorption Kinetic Experiment
3.2.2. Isothermal Adsorption Experiment
3.2.3. Precipitation Experiment
3.3. Influence on Cd Immobilization Mechanism Induced by the Aging of BFA
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhao, F.J.; Ma, Y.; Zhu, Y.G.; Tang, Z.; McGrath, S.P. Soil Contamination in China: Current Status and Mitigation Strategies. Environ. Sci. Technol. 2015, 49, 750–759. [Google Scholar] [CrossRef]
- GB 15618-2018; Soil Environmental Quality. Risk Control Standard for Soil Contamination of Agricultural Land. Ministry of Ecology and Environment (MEE): Beijing, China, 2018.
- Chen, H.P.; Zhang, W.W.; Yang, X.P.; Wang, P.; McGrath, S.P.; Zhao, F.J. Effective methods to reduce cadmium accumulation in rice grain. Chemosphere 2018, 207, 699–707. [Google Scholar] [CrossRef] [PubMed]
- He, S.R.; Lu, Q.; Li, W.Y.; Ren, Z.L.; Zhou, Z.; Feng, X.; Zhang, Y.L.; Li, Y.T. Factors controlling cadmium and lead activities in different parent material-derived soils from the Pearl River Basin. Chemosphere 2017, 182, 509–516. [Google Scholar] [CrossRef]
- Birke, M.; Reimann, C.; Rauch, U.; Ladenberger, A.; Zomeni, Z. Gemas: Cadmium distribution and its sources in agricultural and grazing land soil of Europe: Original data versus clr-transformed data. J. Geochem. Explor. 2017, 173, 13–30. [Google Scholar] [CrossRef]
- Holmgren, G.G.S.; Meyer, M.W.; Chaney, R.L.; Daniels, R.B. Cadmium, Lead, Zinc, Copper, and Nickel in Agricultural Soils of the United States of America. J. Environ. Qual. 1993, 22, 335–348. [Google Scholar] [CrossRef]
- Mcgrath, S.P.; Zhao, F.J. Concentrations of metals and metalloids in soils that have the potential to lead to exceedance of maximum limit concentrations of contaminants in food and feed. Soil Use Manag. 2013, 31, 34–45. [Google Scholar] [CrossRef]
- Liu, X.J.; Yang, K.; Guo, F.; Tang, S.Q.; Liu, Y.H.; Zhang, L.; Cheng, H.X.; Liu, F. Effects and mechanism of igneous rock on selenium in the tropical soil-rice system in Hainan Province, South China. China Geol. 2022, 5, 1–11. [Google Scholar] [CrossRef]
- Yu, H.Y.; Liu, C.P.; Zhu, J.S.; Li, F.B.; Deng, D.M.; Wang, Q.; Liu, C.S. Cadmium availability in rice paddy fields from a mining area: The effects of soil properties highlighting iron fractions and pH value. Environ. Pollut. 2016, 209, 38–45. [Google Scholar] [CrossRef]
- Wang, P.; Chen, H.; Kopittke, P.M.; Zhao, F.J. Cadmium contamination in agricultural soils of china and the impact on food safety. Environ. Pollut. 2019, 249, 1038–1048. [Google Scholar] [CrossRef]
- Chen, H.P.; Yang, X.P.; Wang, P.; Wang, Z.X.; Li, M.; Zhao, F.J. Dietary cadmium intake from rice and vegetables and potential health risk: A case study in xiangtan, southern china. Sci. Total Environ. 2018, 639, 271–277. [Google Scholar] [CrossRef]
- Tang, W.Z.; Xia, Q.; Shan, B.Q.; Ng, J.C. Relationship of bioaccessibility and fractionation of cadmium in long-term spiked soils for health risk assessment based on four in vitro gastrointestinal simulation models. Sci. Total Environ. 2018, 631–632, 1582–1589. [Google Scholar] [CrossRef] [PubMed]
- AlMulla, A.A.; Dahlawi, S.; Randhawa, M.A.; Zaman, Q.; Chen, Y.L.; Faraj, T.K. Toxic Metals and Metalloids in Hassawi Brown Rice: Fate during Cooking and Associated Health Risks. Int. J. Environ. Res. Public Health 2022, 19, 12125. [Google Scholar] [CrossRef] [PubMed]
- Gray, C.W.; Dunham, S.J.; Dennis, P.G.; Zhao, F.J.; McGrath, S.P. Field evaluation of in situ remediation of a heavy metal contaminated soil using lime and red-mud. Environ. Pollut. 2006, 142, 530–539. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Peng, Y.T.; Zhou, Y.Y.; Adeel, M.; Chen, Q. Effects of magnesium ferrite biochar on the cadmium passivation in acidic soil and bioavailability for packoi (Brassica chinensis L.). J. Environ. Manag. 2019, 251, 109–111. [Google Scholar] [CrossRef]
- Song, L.; Han, Z.T.; Li, Z.J.; Zhao, G.Z.; Yang, R.R. Effects of Atmospheric Precipitation on Heavy Metal Accumulation and Deactivation Amendment in Wheat Around a Lead Smelter. Water Air Soil Pollut. 2020, 231, 327. [Google Scholar] [CrossRef]
- Ljung, A.; Nordin, A. Theoretical feasibility for ecological biomass ash recirculation: Chemical equilibrium behavior of nutrient elements and heavy metals during combustion. Environ. Sci. Technol. 1997, 31, 2499–2503. [Google Scholar] [CrossRef]
- Song, L.; Qian, J.Z.; Zhang, F.W.; Kong, X.K.; Li, H.; Luan, S.; Zhang, Q.J.; Kang, Z.Q.; Han, Z.T.; Zhang, Z.J. An ecological remediation model combining optimal substrate amelioration and native hyperaccumulator colonization in non-ferrous metal tailings pond. J. Environ. Manag. 2022, 322, 116141. [Google Scholar] [CrossRef]
- Song, L.; Han, Z.T.; Lv, X.L.; Zhang, W.; Li, X.G.; Wang, L. Remediation of Cd-contaminated cropland soil in northen China via the amendment of modified biofuel ash. J. Agro-Environ. Sci. 2018, 37, 1484–1494. (In Chinese) [Google Scholar]
- Song, L.; Han, Z.T.; Zhang, W.; Ma, L.S.; Wang, L.; Li, X.G.; Wang, X.L. Remediation of Cd-contaminated cropland soil in South China with the amendment of modified biofuel ash and its long-term effects. China Environ. Sci. 2019, 39, 226–234. (In Chinese) [Google Scholar]
- Quan, G.X.; Fan, Q.Y.; Cui, L.Q.; Zimmerman, A.R.; Wang, H.L.; Zhu, Z.Y.; Gao, B.; Wu, L.M.; Yan, J.L. Simulated photocatalytic aging of biochar in soil ecosystem: Insight into organic carbon release, surface physicochemical properties and cadmium sorption. Environ. Res. 2020, 183, 109–241. [Google Scholar] [CrossRef]
- Zhou, H.; Wu, Y.; Huang, F.; Tang, X.F. Experimental simulation and dynamic model analysis of Cadmium (Cd) release in soil affected by rainfall leaching in a coal-mining area. J. Groundw. Sci. Eng. 2021, 9, 65–72. [Google Scholar]
- Chen, S.M.; Liu, F.T.; Zhang, Z.; Zhang, Q.; Wang, W. Changes of groundwater flow field of Luanhe River Delta under the human activities and its impact on the ecological environment in the past 30 years. China Geol. 2021, 4, 455–462. [Google Scholar] [CrossRef]
- Li, Q.; Gu, F.L.; Zhou, Y.; Xu, T.; Wang, L.; Zuo, Q.; Xiao, L.; Liu, J.Y.; Tian, Y. Changes in the Impacts of Topographic Factors, Soil Texture, and Cropping Systems on Topsoil Chemical Properties in the Mountainous Areas of the Subtropical Monsoon Region from 2007 to 2017: A Case Study in Hefeng, China. Int. J. Environ. Res. Public Health 2021, 18, 832. [Google Scholar] [CrossRef]
- Cong, J.Y.; Long, H.Y.; Zhang, Y.; Wang, N. Ecological environment response of benthic foraminifera to heavy metals and human engineering: A case study from Jiaozhou Bay, China. China Geol. 2022, 5, 12–25. [Google Scholar] [CrossRef]
- Ma, L.S.; Han, Z.T.; Wang, Y.Y. Dispersion performance of nanoparticles in water. J. Groundw. Sci. Eng. 2021, 9, 37–44. [Google Scholar]
- Kong, X.K.; Zhang, Z.X.; Wang, P.; Wang, Y.Y.; Zhang, Z.J.; Han, Z.T.; Ma, L.S. Transformation of ammonium nitrogen and response characteristics of nitrifying functional genes in tannery sludge contaminated soil. J. Groundw. Sci. Eng. 2022, 10, 223–232. [Google Scholar]
- Zhu, H.H.; Chen, C.; Xu, C.; Zhu, Q.H.; Huang, D.Y. Effects of soil acidification and liming on the phytoavailability of cadmium in paddy soils of central subtropical China. Environ. Pollut. 2016, 219, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Gong, S.T.; Wang, H.; Lou, F.; Qin, R.; Fu, T.L. Calcareous Materials Effectively Reduce the Accumulation of Cd in Potatoes in Acidic Cadmium-Contaminated Farmland Soils in Mining Areas. Int. J. Environ. Res. Public Health 2022, 19, 11736. [Google Scholar] [CrossRef]
- Wang, L.; O’Connor, D.; Rinklebe, J.; Ok, Y.S.; Tsang, D.C.W.; Shen, Z.T.; Hou, D.Y. Biochar Aging: Mechanisms, Physicochemical Changes, Assessment, And Implications for Field Applications. Environ. Sci. Technol. 2020, 54, 14797–14814. [Google Scholar] [CrossRef]
- Mukherjee, A.; Zimmerman, A.R.; Hamdan, R.; Cooper, W.T. Physicochemical changes in pyrogenic organic matter (biochar) after 15 months of field aging. Solid Earth 2014, 5, 693–704. [Google Scholar] [CrossRef]
- Li, H.X.; Lu, X.Q.; Xu, Y.; Liu, H.T. How close is artificial biochar aging to natural biochar aging in fields? A meta-analysis. Geoderma 2019, 352, 96–103. [Google Scholar] [CrossRef]
- Tan, L.S.; Ma, Z.H.; Yang, K.Q.; Cui, Q.L.; Wang, K.; Wang, T.T.; Wu, G.L.; Zheng, J.Y. Effect of three artificial aging techniques on physicochemical properties and Pb adsorption capacities of different biochars. Sci. Total Environ. 2020, 699, 134223. [Google Scholar] [CrossRef] [PubMed]
- Fan, Q.Y.; Sun, J.X.; Chu, L.; Cui, L.Q.; Quan, G.X.; Yan, J.L.; Hussain, Q.; Iqbal, M. Effects of chemical oxidation on surface oxygen-containing functional groups and adsorption behavior of biochar. Chemosphere 2018, 207, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Fan, Q.Y.; Cui, L.Q.; Quan, G.X.; Wang, S.F.; Sun, J.X.; Han, X.Y.; Wang, J.; Yan, J.L. Effects of Wet Oxidation Process on Biochar Surface in Acid and Alkaline Soil Environments. Materials 2018, 11, 2362. [Google Scholar] [CrossRef]
- Qin, J.; Chong, C.; Cui, X.; Hussain, A.; Yang, S. Recycling of lime mud and fly ash for fabrication of anorthite ceramic at low sintering temperature. Ceram. Int. 2015, 41, 5648–5655. [Google Scholar] [CrossRef]
- Singhal, A.; Gangwar, B.P.; Gayathry, J.M. CTAB modified large surface area nanoporous geopolymer with high adsorption capacity for copper ion removal. Appl. Clay Sci. 2017, 150, 106–114. [Google Scholar] [CrossRef]
- Siyal, A.A.; Shamsuddin, M.R.; Rabat, N.E.; Zulfiqar, M.; Man, Z.; Low, A. Fly ash based geopolymer for the adsorption of anionic surfactant from aqueous solution. J. Clean. Prod. 2019, 229, 232–243. [Google Scholar] [CrossRef]
- He, P.Y.; Zhang, Y.J.; Chen, H.; Han, Z.C.; Liu, L.C. Low-cost and facile synthesis of geopolymer-zeolite composite membrane for chromium(VI) separation from aqueous solution. J. Hazard Mater. 2020, 392, 122359. [Google Scholar] [CrossRef] [PubMed]
- Yan, C.; Guo, L.; Ren, D.; Duan, P. Novel composites based on geopolymer for removal of Pb(II). Mater. Lett. 2018, 239, 192–195. [Google Scholar] [CrossRef]
- Ghaffar, A.; Ghosh, S.; Li, F.; Dong, X.; Zhang, D.; Wu, M.; Li, H.; Pan, B. Effect of biochar aging on surface characteristics and adsorption behavior of dialkyl phthalates. Environ. Pollut. 2015, 206, 502–509. [Google Scholar] [CrossRef]
- Zafar, S.; Khalid, N.; Daud, M.; Mirza, M. Kinetic Studies of the Adsorption of Thorium Ions onto Rice Husk from Aqueous Media: Linear and Nonlinear Approach. Nucleus 2015, 52, 14–19. [Google Scholar]
- Zhu, M.; Zhu, L.; Wang, J.; Yue, T.; Li, R.; Li, Z. Adsorption of Cd(II) and Pb(II) by in situ oxidized Fe3O4 membrane grafted on 316L porous stainless steel filter tube and its potential application for drinking water treatment. J. Environ. Manag. 2017, 196, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Wan, J.M.; Zhang, F.W.; Han, Z.T.; Song, L.; Zhang, C.Y.; Zhang, J.S. Adsorption of Cd2+ and Pb2+ by biofuel ash-based geopolymer synthesized by one-step hydrothermal method. Arab. J. Chem. 2021, 14, 103234. [Google Scholar] [CrossRef]
- Qiu, R.; Cheng, F.; Huang, H. Removal of Cd2+ from aqueous solution using hydrothermally modified circulating fluidized bed fly ash resulting from coal gangue power plant. J. Clean. Prod. 2018, 172, 1918–1927. [Google Scholar] [CrossRef]
- Fei, Y.H.; Cao, S.T.; Zhang, G.H.; Nie, Z.L. Characteristics and Boundary Line between Adsorption and Precipition of Cadmium in Soil. Acta Geosci. Sin. 1998, 4, 409–414. (In Chinese) [Google Scholar]
- Rechberger, M.V.; Kloss, S.; Wang, S.L.; Lehmann, J.; Rennhofer, H.; Ottner, F.; Wriessnig, K.; Daudin, G.; Lichtenegger, H.; Soja, G.; et al. Enhanced Cu and Cd sorption after soil aging of woodchip-derived biochar: What were the driving factors? Chemosphere 2019, 216, 463–471. [Google Scholar] [CrossRef]
- Ratie, G.; Chrastny, V.; Guinoiseau, D.; Marsac, R.; Vankova, Z.; Komarek, M. Cadmium Isotope Fractionation during Complexation with Humic Acid. Environ. Sci. Technol. 2021, 55, 7430–7444. [Google Scholar] [CrossRef]
- Wang, J.; Wang, P.M.; Gu, Y.; Kopittke, P.M.; Zhao, F.J.; Wang, P. Iron-Manganese (Oxyhydro)oxides, Rather than Oxidation of Sulfides, Determine Mobilization of Cd during Soil Drainage in Paddy Soil Systems. Environ. Sci. Technol. 2019, 53, 2500–2508. [Google Scholar] [CrossRef]
- Liu, D.G.; Ke, Y.; Min, X.B.; Liang, Y.J.; Wang, Z.B.; Li, Y.C.; Fei, J.C.; Yao, L.W.; Xu, H.; Jiang, G.H. Cotreatment of MSWI Fly Ash and Granulated Lead Smelting Slag Using a Geopolymer System. Int. J. Environ. Res. Public Health 2019, 16, 156. [Google Scholar] [CrossRef]
- Tan, T.H.; Mo, K.H.; Ling, T.C.; Lai, S.H. Current development of geopolymer as alternative adsorbent for heavy metal removal. Environ. Technol. Innov. 2020, 18, 100684. [Google Scholar] [CrossRef]


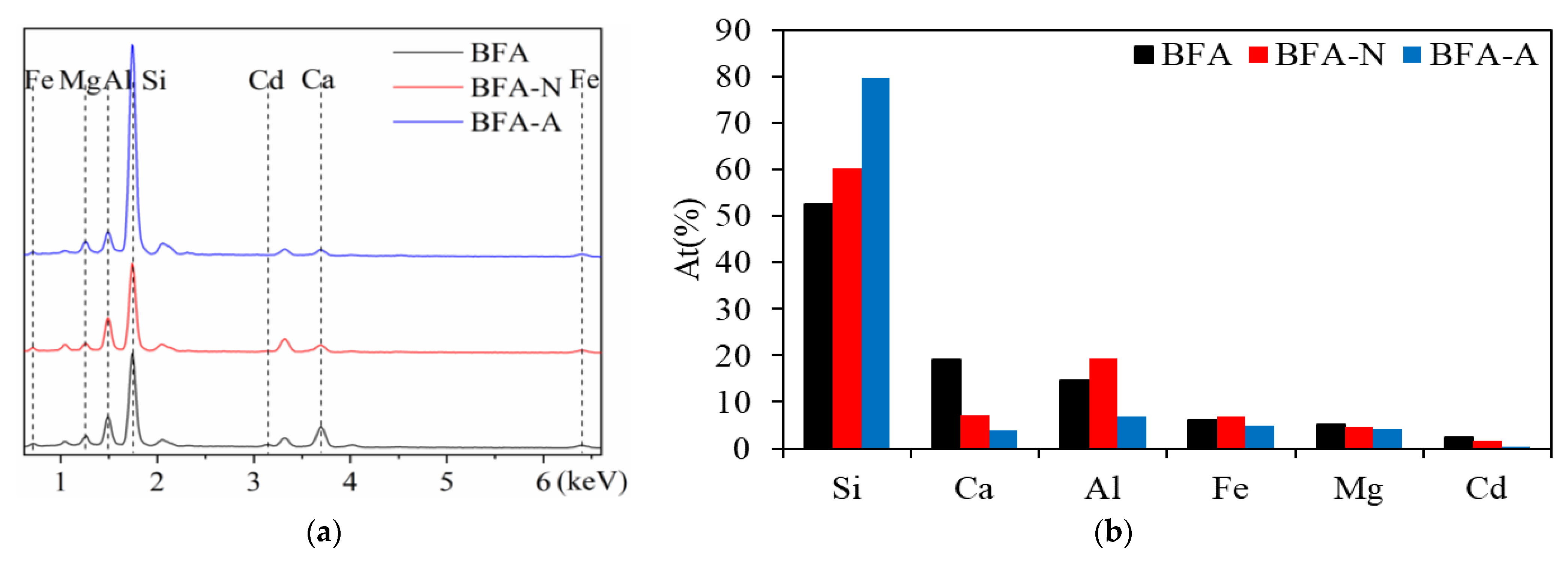
| Element | SiO2 | CaO | Al2O3 | Fe2O3 | K2O | MgO | SO3 | Na2O | P2O5 |
|---|---|---|---|---|---|---|---|---|---|
| Percentage (%) | 47.26 | 19.19 | 12.67 | 7.49 | 4.20 | 3.86 | 1.41 | 1.19 | 1.07 |
| Pseudo-First-Order Kinetic Equation | Pseudo-Second-Order Kinetic Equation | |||||
|---|---|---|---|---|---|---|
| (mg/g) | (1/h) | R2 | (mg/g) | (g/mg·h) | R2 | |
| BFA | 4.17 | 0.13 | 0.53 | 7.98 | 0.47 | 0.99 |
| BFA-N | 3.31 | 0.18 | 0.85 | 6.92 | 0.55 | 0.99 |
| BFA-A | 1.87 | 0.14 | 0.53 | 2.91 | 0.43 | 0.95 |
| Langmuir | Freundlich | |||||
|---|---|---|---|---|---|---|
| (mg/g) | (L/mg) | R2 | R2 | |||
| BFA | 10.11 | 0.23 | 0.98 | 4.05 | 5.12 | 0.99 |
| BFA-N | 7.71 | 0.09 | 0.97 | 1.43 | 2.70 | 0.99 |
| BFA-A | 5.32 | 0.02 | 0.95 | 0.19 | 1.62 | 0.99 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, L.; Zhao, F.; Cui, H.; Wan, J.; Li, H. Biofuel Ash Aging in Acidic Environment and Its Influence on Cd Immobilization. Int. J. Environ. Res. Public Health 2023, 20, 4635. https://doi.org/10.3390/ijerph20054635
Song L, Zhao F, Cui H, Wan J, Li H. Biofuel Ash Aging in Acidic Environment and Its Influence on Cd Immobilization. International Journal of Environmental Research and Public Health. 2023; 20(5):4635. https://doi.org/10.3390/ijerph20054635
Chicago/Turabian StyleSong, Le, Feng Zhao, Haiyang Cui, Jingmin Wan, and Hui Li. 2023. "Biofuel Ash Aging in Acidic Environment and Its Influence on Cd Immobilization" International Journal of Environmental Research and Public Health 20, no. 5: 4635. https://doi.org/10.3390/ijerph20054635
APA StyleSong, L., Zhao, F., Cui, H., Wan, J., & Li, H. (2023). Biofuel Ash Aging in Acidic Environment and Its Influence on Cd Immobilization. International Journal of Environmental Research and Public Health, 20(5), 4635. https://doi.org/10.3390/ijerph20054635





