Abstract
Musculoskeletal (MSK) pain disorders are some of the most prevalent and disabling chronic pain conditions worldwide. These chronic conditions have a considerable impact on the quality of life of individuals, families, communities, and healthcare systems. Unfortunately, the burden of MSK pain disorders does not fall equally across the sexes. Females consistently demonstrate more prevalent and severe clinical presentations of MSK disorders, and this disparity increases in magnitude with age. The aim of the present article is to review recent studies that have examined sex differences between males and females in four of the most common MSK pain disorders: neck pain, low back pain, osteoarthritis, and rheumatoid arthritis.
1. Introduction
Musculoskeletal diseases are the leading cause of both chronic pain and disability worldwide [1]. In fact, it is estimated that approximately 1.71 billion people are living with a musculoskeletal condition [2]. Notably, low back pain, neck pain, osteoarthritis, and rheumatoid arthritis are among the most disabling musculoskeletal diseases [3]. Musculoskeletal diseases affect bones, muscle, ligaments, joints, tendons, and nerves. They are often characterized by pain (acute or chronic) and they limit movement and dexterity, impacting people’s ability to function in society [1]. Musculoskeletal pain can affect anyone regardless of demographic characteristics; however, there are clear disparities between demographic groups whereby the burden of the pain prevalence and experience is extremely unequal. In sum, previous studies suggest that females are disproportionately burdened by these painful conditions and present with more persistent pain symptoms than their male counterparts [4]. The mechanisms underlying these sex differences are still being elucidated, though it is hypothesized that differences in hormones, immune system functioning and differences in the perception of pain are primary contributors [5]. Physiological and behavioral differences in pain responses between males and females have also been well documented [6,7].
It should be noted that in this article, sex is defined as the biological processes that differ between males and females based on genetics and hormones, and not gender—the psychosocial construction of oneself as a man or a woman—in all cases. Thus, the primary objective of this topical review is to highlight recent studies that have examined differences between the biological sexes in the four most common musculoskeletal conditions—neck pain, chronic low back pain, osteoarthritis and rheumatoid arthritis (Figure 1) [8,9,10,11].
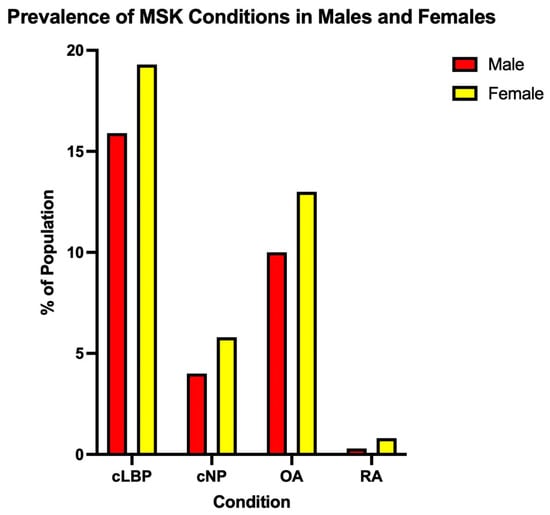
Figure 1.
Prevalence of the top four musculoskeletal conditions in the United States (Adapted from Kahere et al. (2021), Wang et al. (2016), Zhang et al. (2010) and Gabriel (2001)) [8,9,10,11].
1.1. Neck Pain
Neck pain (i.e., pain originating from/around cervical vertebrae 1–7) has become an increasingly common problem around the world. This is particularly exacerbated with the societal shift to working with technology and sitting at desks on computers, which is a primary risk factor for the development of persistent and disabling neck pain [12]. Neck pain has considerable impact on individuals, families, communities, and health care systems. Neck pain has an annual prevalence rate of over 30% in the United States, with nearly 50% of individuals experiencing some form of neck pain in their lifetime, and up to 67% of those individuals having pain that significantly detracts from their daily lives [13,14]. The etiology of neck pain is complex and believed to be a biopsychosocial problem, with the onset and course of neck pain being highly influenced by environmental and personal factors, some of which are modifiable. To better understand how multiple factors may contribute to increased risk of chronic neck pain, each domain of the biopsychosocial model must be investigated in order to predict and help prevent long-term neck pain. Having a history of acute neck or low back pain, poor self-assessed health and poor physiological status have been found to increase the risk of chronic neck pain onset [15]. In the physical domain, job-related exposure, routine physical activity and impairments in cervicoscapular strength, mobility and endurance may be associated with neck pain [16]. In the neurophysiological domain, evidence has shown alterations in pain processing that result in localized and widespread hypersensitivity to mechanical stimuli, as well as impairments in diffuse noxious inhibitory control (DNIC) are found in individuals with persistent pain [16]. Lastly, psychosocial factors including job satisfaction and general measures of psychological health have been shown to predict long-term symptoms and disability associated with neck pain.
In general, the prevalence of neck pain, regardless of source, is higher in females [15]. Data suggest that these differences in idiopathic neck pain may be due to muscle stiffness or alterations in neuromuscular control [17], though there is no consistent evidence suggesting one pathology over another. In 2016, a 5 year cross-sectional, observational study in Sweden was able to establish sex-related prognostic factors that show less favorable recovery in the female sex after neck trauma. Females were found to be twice as likely to have persistent pain after neck trauma likely due to higher vulnerability to tissue damage based on structural factors, higher risk for psychological distress, the pain sensitization process and possibly social factors [18]. Most studies indicate a higher incidence of neck pain among females and an increased risk of developing neck pain until the 35–49 year age group, after which the risk begins to decline [15,19]. However, it should be noted that these sex differences are also observed in younger individuals, with female children and adolescents reporting that they experienced pain more often in the past 7 days compared to males of their age [20].
Anatomically, females have smaller cervical vertebrae resulting in less segmental support area (disk and facet joints), less muscle strength, and ligament stiffness resulting in increased pain, reduced stability and increased range of motion, lower tolerance limit for lower neck shear force, and faster muscle reaction times resulting in greater tissue strain and injury potential [18]. Approximately 10% of neck pain cases are associated with conditions such as polymyalgia rheumatica, fibromyalgia, and rheumatoid arthritis, all of which are more prevalent in the female population [19,21,22,23]. In migraine, another female-predominant pain condition, cutaneous allodynia, cervical mobility, muscle function and overall migraine severity was greater in individuals who also demonstrated the presence of chronic neck pain [24]. Additionally, it has been reported that people who suffer from chronic neck pain are three times more likely to have chronic low back pain than individuals from the general population [25].
1.2. Low Back Pain
Low back Pain (LBP) is the most prevalent musculoskeletal condition in America and has been the single leading cause of disability worldwide since 1990 [26]. According to the Global Burden of Disease, the prevalence of LBP increased from 377.5 million in 1990 to 577 million in 2017 and continues to increase as the overall population ages [27]. Though the risk of developing LBP increases with age until peaking in the 7th and 8th decades of life [28], individuals between the ages of 50 and 54 constitute the largest group of people currently living with LBP [26]. It is estimated that 50–80% of the population will experience low back pain at some point in their lifetime [29]. While the long-term prognosis is favorable for most people who experience acute LBP, roughly one quarter of these individuals will go on to develop chronic low back pain [30,31]. Persistent or chronic low back pain (cLBP) is defined as pain that occurs in the lower back and persists for a period of 12 weeks or longer, and the overwhelming majority (~90%) of cLBP is “non-specific” and is not attributable to any distinguishable pathology associated with the anatomical structures positioned between the posterior margin of the ribcage and the gluteal fold (lower back) [32].
While cLBP can affect anyone regardless of biological sex, the prevalence is higher in females across all age groups. Similar to neck pain, a growing body of chronic pain studies endorses a biopsychosocial model of pain which posits that pain perception in cLBP is attributable to factors that are biological and psychosocial in nature [33]. It is well known that psychological distress contributes to the experience of cLBP and increases the risk of associated disability [34]. Depression is one of the strongest predictors of cLBP and females are nearly twice as likely to be diagnosed than males [35]. Further, depression is estimated to be 3–4 times greater in chronic pain patients than in the general population, of which females carry the burden [4]. Thus, early identification of depression and treatment of depressive symptoms might provide supplemental benefits to females who are suffering from cLBP. Anxiety has also been linked to chronic low back pain [36]. As in the case of depression, anxiety disorders and symptoms are twice as likely to occur in females than males [37], though more research is needed to determine whether anxiety moderates the relationship between biological sex and cLBP outcomes.
Aside from psychological correlates associated with low back pain, physiological responses to pregnancy, physical and emotional stress related to child-rearing, and perimenopausal weight gain in the abdominal area are also directly linked to this chronic condition [38]. In postmenopausal females, lumbar discs are susceptible to accelerated degeneration compared to age-matched males, further increasing the risk of developing cLBP for females over the age of 50 years [38]. In addition to these physiological factors, genetics might also contribute to the development of cLBP in females [28]. In a relatively large (n = 2256) cross-sectional study involving identical and fraternal twins, it was determined that genetic background and lumbar disc degeneration were the primary risk factors for severe and disabling LBC in this all-female sample [39,40]. More recently, a 2021 study revealed sex-specific epigenetic signatures in T cells that differentiated cLBP patients from pain-free controls. Female subjects with cLBP presented with hypomethylated expression in genes that have functions in the immune system [41]. A greater concentration of immune markers such as pro-inflammatory cytokines have been reported in cLBP [42] much like other MSK disorders such as osteoarthritic disease [43].
1.3. Osteoarthritis
Osteoarthritis (OA) is a widespread musculoskeletal condition, affecting approximately 300 million individuals worldwide. It is the leading cause of disability in older adults, with an estimated 10%–15% of individuals over the age of 60 having some degree of OA in one or more joints. OA is characterized by the degeneration of cartilage and bone as well as bony overgrowth (bone spurs), which eventually leads to pain, stiffness, and loss of function in the joint. The joints most commonly affected are those in the knees, hips, carpals and metacarpals, as well as the spine. OA is normally brought about by injury, overuse, and repetitive stress to the areas, but genetics and anatomy also play a distinct role.
Population-based studies have revealed that OA prevalence is higher in females compared to males, and that females generally have a higher risk of developing OA across the lifespan, especially after menopause [44]. Additionally, females over the age of 55 also tend to have more severe OA in the knee [45]. Interestingly, females are three times less likely to undergo hip or knee arthroplasty than males. In experiments aimed at understanding sex differences in pain severity of OA, results indicate that females report higher pain severity via visual analogue scale, greater prevalence of pain in both knees compared to just one knee, greater levels of inflammation, and more impaired function compared to males. In females but not males, low serum levels of endogenous estradiol, progesterone and testosterone were associated with increased pain, synovial inflammation, and decreased cartilage volume [46]. When analyzing synovial fluid from the knees of OA patients, the balance of pro- and anti-inflammatory factors appeared to differ as a function of sex; specifically, the anti-inflammatory chemokines MMP-10, IL-8, CCL-4, and monocyte chemoattractant protein (MCP)-2) were higher in males, whereas proinflammatory cytokines (IL-6, IL-10, IL-1β, TNF-α) were higher in females [47,48]. Such sex differences in inflammatory activity may contribute to the amplified pain sensitivity observed in females (compared to males) with osteoarthritis. Specifically, females with OA are more pain-sensitive on laboratory measures of pain sensitivity, and also demonstrate enhanced reactivity of IL-6 and blunted diurnal rhythms of anti-inflammatory neuroendocrine factors such as cortisol [49,50].
Psychosocial measures such as depression, anxiety, and social support, as well as physical activity appear to be similar between the sexes in the case of OA [48,51,52]. One study [53] used a single-blind placebo design to evaluate the placebo response to pain, depressive and anxiety symptoms, and performance-based tests in patients with knee OA. Results showed that males reported greater depressive symptoms due to their OA than females, while females showed greater walking resistance due to their OA than men.
1.4. Rheumatoid Arthritis
Rheumatoid arthritis, or RA, has many of the same symptoms as OA including pain and stiffness in the joints; however, their underlying pathologies differ. RA, as opposed to OA, is an autoimmune disorder in which the immune system attacks cells in the joints, causing inflammation, swelling, and pain at the affected areas [54]. RA commonly affects the tissue in the hands, wrists and knees. As this disease and subsequent tissue damage is long-lasting, it typically leads to chronic pain, lack of physical function, and deformity of the affected joints [55]. RA can also exert its effects extra-articularly on visceral organs, usually targeting the lungs, heart and eyes [56]. In addition to pain and stiffness, other symptoms such as fever, fatigue, weakness and weight loss can also accompany RA [57].
Many autoimmune disorders, including RA, display a disproportionate burden between the sexes, whereby females are afflicted at greater rates than males [58,59]. Research has documented sex differences in RA across several domains including epidemiology, disease-course, and management, leading the experience of the disease to differ between males and females [60]. Typically, females experience a more deleterious disease course, showing greater amounts of disease activity as well as prevalence of RA. These differences are what lead females to have poorer treatment outcomes compared to males with the same disorder. The reasons underlying this difference remains unclear, though it is thought that X-linked genetic factors [61], hormones, and immune system differences may be involved. In a study by Yu et al., the authors concluded that differences in expression of interleukin (IL)-4, a pro-inflammatory cytokine that participates in cytokine–cytokine receptor interactions, T-helper cell differentiation, and T cell receptor signaling pathway (among other things) between the sexes may contribute to the molecular mechanism of sex differences in RA [62]. Better disease activity scores in males may also be attributable to phenotypic factors such as muscle strength and bone density, which typically is greater in males compared to females [63]. These differences may allow for better compensatory strategies for males, making their daily activities less affected by RA. Because pharmaceutical treatment options do not differ by sex, this has likely led to decreased levels of adherence to RA-specific medications in females of child-bearing age. Because many of the drugs used to treat RA, such as methotrexate, can be teratogenic and passed along from mother to child, there is no current safe dosage recommended for females planning to conceive and breast feed [64,65]. Psychological factors associated with worse pain and RA outcomes, such as anxiety and depression, are also more common in females, and could also partially explain the sex differences seen in RA [66].
2. Conclusions
Musculoskeletal conditions, namely neck pain, back pain, osteoarthritis, and rheumatoid arthritis, are highly prevalent and impact females more frequently and more severely than males. We have highlighted biopsychosocial factors associated with MSK pain and more specifically, the mechanistic factors that may be contributing to sex differences in MSK pain including genetics, immunology, and hormones. By better understanding these mechanisms, we can develop targeted treatments to help ameliorate sex disparities in MSK pain. It is important to note that this review focused solely on sex as a biological variable and as such has not addressed the influence of gender, a social construct, which also has considerable effects on the pain experience. Future research should examine the intersectionality of sex and gender on pain.
Author Contributions
D.S.O., L.J.S., M.J.: Conceptualization, Visualization, Writing—Original Draft, Review and Editing; I.A.J., J.M.H.: Writing—Review & Editing; M.A.O., A.C.W.: Writing—Review & Editing; R.R.E.: Writing—Review & Editing, Supervision, Funding Acquisition. S.M.M.: Writing—Review & Editing, Supervision, Funding Acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
This investigation was supported by K23 AR077088 Meints (PI), K24NS126570 Edwards (PI) and R35 GM128691 (R35-Restricted Administrative Supplement Schreiber (PI), Overstreet (Co-I).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created/collected.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Murillo, C.; Vo, T.-T.; Vansteelandt, S.; Harrison, L.E.; Cagnie, B.; Coppieters, I.; Chys, M.; Timmers, I.; Meeus, M. How do psychologically based interventions for chronic musculoskeletal pain work? A systematic review and meta-analysis of specific moderators and mediators of treatment. Clin. Psychol. Rev. 2022, 94, 102160. [Google Scholar] [CrossRef] [PubMed]
- Cieza, A.; Causey, K.; Kamenov, K.; Hanson, S.W.; Chatterji, S.; Vos, T. Global estimates of the need for rehabilitation based on the Global Burden of Disease study 2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2021, 396, 2006–2017. [Google Scholar] [CrossRef]
- Vos, T.; Flaxman, A.D.; Naghavi, M.; Lozano, R.; Michaud, C.; Ezzati, M.; Shibuya, K.; Salomon, J.A.; Abdalla, S.; Aboyans, V.; et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990-2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012, 380, 2163–2196. [Google Scholar] [CrossRef]
- Sorge, R.E.; Strath, L.J. Sex differences in pain responses. Curr. Opin. Physiol. 2018, 6, 75–81. [Google Scholar] [CrossRef]
- Sorge, R.E.; Mapplebeck, J.C.; Rosen, S.; Beggs, S.; Taves, S.; Alexander, J.K.; Martin, L.J.; Austin, J.S.; Sotocinal, S.G.; Chen, D.; et al. Different immune cells mediate mechanical pain hypersensitivity in male and female mice. Nat. Neurosci. 2015, 18, 1081–1083. [Google Scholar] [CrossRef] [PubMed]
- Bartley, E.J.; Fillingim, R.B. Sex differences in pain: A brief review of clinical and experimental findings. Br. J. Anaesth. 2013, 111, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Templeton, K.J. Sex and Gender Issues in Pain Management. JBJS 2020, 102, 32–35. [Google Scholar] [CrossRef]
- Wang, H.; Naghavi, M.; Allen, C.; Barber, R.M.; Bhutta, Z.A.; Carter, A.; Casey, D.C.; Charlson, F.J.; Chen, A.Z.; Coates, M.M.; et al. Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980–2015: A systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016, 388, 1459–1544. [Google Scholar] [CrossRef]
- Kahere, M.; Ginindza, T. The prevalence and risk factors of chronic low back pain among adults in KwaZulu-Natal, South Africa: An observational cross-sectional hospital-based study. BMC Musculoskelet. Disord. 2021, 22, 955. [Google Scholar] [CrossRef]
- Zhang, Y.; Jordan, J.M. Epidemiology of osteoarthritis. Clin. Geriatr. Med. 2010, 26, 355–369. [Google Scholar] [CrossRef]
- Gabriel, S.E. The epidemiology of rheumatoid arthritis. Rheum. Dis. Clin. N. Am. 2001, 27, 269–281. [Google Scholar] [CrossRef] [PubMed]
- Dzakpasu, F.Q.S.; Carver, A.; Brakenridge, C.J.; Cicuttini, F.; Urquhart, D.M.; Owen, N.; Dunstan, D.W. Musculoskeletal pain and sedentary behaviour in occupational and non-occupational settings: A systematic review with meta-analysis. Int. J. Behav. Nutr. Phys. Act. 2021, 18, 159. [Google Scholar] [CrossRef] [PubMed]
- Sinnott, P.L.; Dally, S.K.; Trafton, J.; Goulet, J.L.; Wagner, T.H. Trends in diagnosis of painful neck and back conditions, 2002 to 2011. Medicine 2017, 96, e6691. [Google Scholar] [CrossRef]
- Cohen, S.P. Epidemiology, Diagnosis, and Treatment of Neck Pain. Mayo Clin. Proc. 2015, 90, 284–299. [Google Scholar] [CrossRef]
- Hoy, D.G.; Protani, M.; De, R.; Buchbinder, R. The epidemiology of neck pain. Best Pract. Res. Clin. Rheumatol. 2010, 24, 783–792. [Google Scholar] [CrossRef]
- Shahidi, B.; Curran-Everett, D.; Maluf, K.S. Psychosocial, Physical, and Neurophysiological Risk Factors for Chronic Neck Pain: A Prospective Inception Cohort Study. J. Pain 2015, 16, 1288–1299. [Google Scholar] [CrossRef]
- Wolff, W.L.; Heinemann, C.M.; Lipps, D.B. The influence of idiopathic chronic neck pain on upper trapezius and sternocleidomastoid muscle activity and elasticity during functional reaching: A cross-sectional study. J. Biomech. 2022, 141, 111223. [Google Scholar] [CrossRef]
- Westergren, H.; Larsson, J.; Freeman, M.; Carlsson, A.; Jöud, A.; Malmström, E.M. Sex-based differences in pain distribution in a cohort of patients with persistent post-traumatic neck pain. Disabil. Rehabil. 2018, 40, 1085–1091. [Google Scholar] [CrossRef] [PubMed]
- Kazeminasab, S.; Nejadghaderi, S.A.; Amiri, P.; Pourfathi, H.; Araj-Khodaei, M.; Sullman, M.J.M.; Kolahi, A.A.; Safiri, S. Neck pain: Global epidemiology, trends and risk factors. BMC Musculoskelet. Disord. 2022, 23, 26. [Google Scholar] [CrossRef]
- Keeratisiroj, O.; Siritaratiwat, W. Prevalence of self-reported musculoskeletal pain symptoms among school-age adolescents: Age and sex differences. Scand J. Pain 2018, 18, 273–280. [Google Scholar] [CrossRef]
- Narvaez, J.; Nolla-Solé, J.M.; Valverde-García, J.; Roig-Escofet, D. Sex differences in temporal arteritis and polymyalgia rheumatica. J. Rheumatol. 2002, 29, 321–325. [Google Scholar]
- Meester, I.; Rivera-Silva, G.F.; González-Salazar, F. Immune System Sex Differences May Bridge the Gap between Sex and Gender in Fibromyalgia. Front. Neurosci. 2020, 13, 1414. [Google Scholar] [CrossRef]
- Adawi, M.; Gurovich, B.; Firas, S.; Watad, A.; Bragazzi, N.l.; Amital, H.; Sirchan, R.; Blum, A. Gender differences in cardiovascular risk of patients with rheumatoid arthritis. QJM Int. J. Med. 2019, 112, 657–661. [Google Scholar] [CrossRef] [PubMed]
- Bragatto, M.M.; Bevilaqua-Grossi, D.; Benatto, M.T.; Lodovichi, S.S.; Pinheiro, C.F.; Carvalho, G.F.; Dach, F.; Fernández-de-Las-Peñas, C.; Florencio, L.L. Is the presence of neck pain associated with more severe clinical presentation in patients with migraine? A cross-sectional study. Cephalalgia 2019, 39, 1500–1508. [Google Scholar] [CrossRef]
- Guez, M.; Hildingsson, C.; Nasic, S.; Toolanen, G. Chronic low back pain in individuals with chronic neck pain of traumatic and non-traumatic origin: A population-based study. Acta Orthop. 2006, 77, 132–137. [Google Scholar] [CrossRef]
- IASP. The Global Burden of Low Back Pain. Updated 9 July 2021. Available online: https://www.iasp-pain.org/resources/fact-sheets/the-global-burden-of-low-back-pain/#:~:text=LBP%20has%20been%20the%20leading,a%20specific%20identifiable%20pathoanatomical%20cause (accessed on 28 September 2022).
- Wu, A.; March, L.; Zheng, X.; Huang, J.; Wang, X.; Zhao, J.; Blyth, F.M.; Smith, E.; Buchbinder, R.; Hoy, D. Global low back pain prevalence and years lived with disability from 1990 to 2017: Estimates from the Global Burden of Disease Study 2017. Ann. Transl. Med. 2020, 8, 299. [Google Scholar] [CrossRef]
- Wáng, Y.X.; Wáng, J.Q.; Káplár, Z. Increased low back pain prevalence in females than in males after menopause age: Evidences based on synthetic literature review. Quant. Imaging Med. Surg. 2016, 6, 199–206. [Google Scholar] [CrossRef]
- Fatoye, F.; Gebrye, T.; Odeyemi, I. Real-world incidence and prevalence of low back pain using routinely collected data. Rheumatol. Int. 2019, 39, 619–626. [Google Scholar] [CrossRef] [PubMed]
- Vlaeyen, J.W.S.; Maher, C.G.; Wiech, K.; Van Zundert, J.; Meloto, C.B.; Diatchenko, L.; Battié, M.C.; Goossens, M.; Koes, B.; Linton, S.J. Low back pain. Nat. Rev. Dis. Prim. 2018, 4, 52. [Google Scholar] [CrossRef] [PubMed]
- Nieminen, L.K.; Pyysalo, L.M.; Kankaanpää, M.J. Prognostic factors for pain chronicity in low back pain: A systematic review. Pain Rep. 2021, 6, e919. [Google Scholar] [CrossRef]
- NINDS. Low Back Pain fact Sheet. Available online: https://www.ninds.nih.gov/low-back-pain-fact-sheet#:~:text=Chronic%20back%20pain%20is%20defined,persistent%20symptoms%20at%20one%20year (accessed on 9 February 2023).
- Overstreet, D.S.; Michl, A.N.; Penn, T.M.; Rumble, D.D.; Aroke, E.N.; Sims, A.M.; King, A.L.; Hasan, F.N.; Quinn, T.L.; Long, D.L.; et al. Temporal summation of mechanical pain prospectively predicts movement-evoked pain severity in adults with chronic low back pain. BMC Musculoskelet. Disord. 2021, 22, 429. [Google Scholar] [CrossRef] [PubMed]
- Fisker, A.; Petersen, T.; Langberg, H.; Mortensen, O.S. The association between psychosocial distress, pain and disability in patients with persistent low back pain—A cross-sectional study. Cogent Med. 2018, 5, 1534536. [Google Scholar] [CrossRef]
- Shi, P.; Yang, A.; Zhao, Q.; Chen, Z.; Ren, X.; Dai, Q. A Hypothesis of Gender Differences in Self-Reporting Symptom of Depression: Implications to Solve Under-Diagnosis and Under-Treatment of Depression in Males. Front. Psychiatry 2021, 12, 589687. [Google Scholar] [CrossRef]
- Fernandez, M.; Colodro-Conde, L.; Hartvigsen, J.; Ferreira, M.L.; Refshauge, K.M.; Pinheiro, M.B.; Ordoñana, J.R.; Ferreira, P.H. Chronic low back pain and the risk of depression or anxiety symptoms: Insights from a longitudinal twin study. Spine J. 2017, 17, 905–912. [Google Scholar] [CrossRef] [PubMed]
- McLean, C.P.; Asnaani, A.; Litz, B.T.; Hofmann, S.G. Gender differences in anxiety disorders: Prevalence, course of illness, comorbidity and burden of illness. J. Psychiatr. Res. 2011, 45, 1027–1035. [Google Scholar] [CrossRef]
- Wang, Y.X.J. Postmenopausal Chinese women show accelerated lumbar disc degeneration compared with Chinese men. J. Orthop. Transl. 2015, 3, 205–211. [Google Scholar] [CrossRef]
- Pinheiro, M.B.; Ferreira, M.L.; Refshauge, K.; Colodro-Conde, L.; Carrillo, E.; Hopper, J.L.; Ordoñana, J.R.; Ferreira, P.H. Genetics and the environment affect the relationship between depression and low back pain: A co-twin control study of Spanish twins. Pain 2015, 156, 496–503. [Google Scholar] [CrossRef]
- Hestbaek, L.; Iachine, I.A.; Leboeuf-Yde, C.; Kyvik, K.O.; Manniche, C. Heredity of low back pain in a young population: A classical twin study. Twin Res. Hum. Genet. 2004, 7, 16–26. [Google Scholar] [CrossRef]
- Grégoire, S.; Cheishvili, D.; Salmon-Divon, M.; Dymov, S.; Topham, L.; Calderon, V.; Shir, Y.; Szyf, M.; Stone, L.S. Epigenetic signature of chronic low back pain in human T cells. Pain Rep. 2021, 6, e960. [Google Scholar] [CrossRef]
- Teodorczyk-Injeyan, J.A.; Triano, J.J.; Injeyan, H.S. Nonspecific Low Back Pain: Inflammatory Profiles of Patients with Acute and Chronic Pain. Clin. J. Pain 2019, 35, 818–825. [Google Scholar] [CrossRef]
- Kapoor, M.; Martel-Pelletier, J.; Lajeunesse, D.; Pelletier, J.-P.; Fahmi, H. Role of proinflammatory cytokines in the pathophysiology of osteoarthritis. Nat. Rev. Rheumatol. 2011, 7, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Tschon, M.; Contartese, D.; Pagani, S.; Borsari, V.; Fini, M. Gender and Sex Are Key Determinants in Osteoarthritis Not Only Confounding Variables. A Systematic Review of Clinical Data. J. Clin. Med. 2021, 10, 3178. [Google Scholar] [CrossRef] [PubMed]
- Laitner, M.H.; Erickson, L.C.; Ortman, E. Understanding the Impact of Sex and Gender in Osteoarthritis: Assessing Research Gaps and Unmet Needs. J. Women’s Health 2021, 30, 634–641. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Wang, B.H.; Wang, X.; Antony, B.; Zhu, Z.; Han, W.; Cicuttini, F.; Wluka, A.E.; Winzenberg, T.; Blizzard, L.; et al. Associations between endogenous sex hormones and MRI structural changes in patients with symptomatic knee osteoarthritis. Osteoarthr. Cartil. 2017, 25, 1100–1106. [Google Scholar] [CrossRef] [PubMed]
- Solheim, N.; Östlund, S.; Gordh, T.; Rosseland, L.A. Women report higher pain intensity at a lower level of inflammation after knee surgery compared with men. Pain Rep. 2017, 2, e595. [Google Scholar] [CrossRef]
- Perruccio, A.V.; Badley, E.M.; Power, J.D.; Canizares, M.; Kapoor, M.; Rockel, J.; Chandran, V.; Gandhi, R.; Mahomed, N.M.; Davey, J.R.; et al. Sex differences in the relationship between individual systemic markers of inflammation and pain in knee osteoarthritis. Osteoarthr. Cartil. Open 2019, 1, 100004. [Google Scholar] [CrossRef]
- Mun, C.J.; Letzen, J.E.; Nance, S.; Smith, M.T.; Khanuja, H.S.; Sterling, R.S.; Bicket, M.C.; Haythornthwaite, J.A.; Jamison, R.N.; Edwards, R.R.; et al. Sex Differences in Interleukin-6 Responses over Time Following Laboratory Pain Testing among Patients with Knee Osteoarthritis. J. Pain 2020, 21, 731–741. [Google Scholar] [CrossRef]
- Paschali, M.; Lazaridou, A.; Paschalis, T.; Moradian, J.R.; Sadora, J.; Vilsmark, E.S.; Edwards, R.R. Individual variation in diurnal cortisol in patients with knee osteoarthritis: Clinical correlates. Int. J. Psychophysiol. 2021, 167, 1–6. [Google Scholar] [CrossRef]
- Perruccio, A.V.; Chandran, V.; Power, J.D.; Kapoor, M.; Mahomed, N.N.; Gandhi, R. Systemic inflammation and painful joint burden in osteoarthritis: A matter of sex? Osteoarthr. Cartil. 2017, 25, 53–59. [Google Scholar] [CrossRef]
- Speed, T.J.; Richards, J.M.; Finan, P.H.; Smith, M.T. Sex moderates the effects of positive and negative affect on clinical pain in patients with knee osteoarthritis. Scand. J. Pain 2017, 16, 66–73. [Google Scholar] [CrossRef]
- Harden, R.N.; Saracoglu, M.; Connolly, S.; Kirsling, A.; Comstock, K.; Khazey, K.; Gerson, T.; Burns, J. “Managing” the Placebo Effect: The Single-Blind Placebo Lead-in Response in Two Pain Models. Pain Med. 2016, 17, 2305–2310. [Google Scholar] [CrossRef] [PubMed]
- Scherer, H.U.; Häupl, T.; Burmester, G.R. The etiology of rheumatoid arthritis. J. Autoimmun. 2020, 110, 102400. [Google Scholar] [CrossRef]
- Littlejohn, E.A.; Monrad, S.U. Early Diagnosis and Treatment of Rheumatoid Arthritis. Prim. Care Clin. Off. Pract. 2018, 45, 237–255. [Google Scholar] [CrossRef] [PubMed]
- Conforti, A.; Di Cola, I.; Pavlych, V.; Ruscitti, P.; Berardicurti, O.; Ursini, F.; Giacomelli, R.; Cipriani, P. Beyond the joints, the extra-articular manifestations in rheumatoid arthritis. Autoimmun. Rev. 2021, 20, 102735. [Google Scholar] [CrossRef]
- Grassi, W.; De Angelis, R.; Lamanna, G.; Cervini, C. The clinical features of rheumatoid arthritis. Eur. J. Radiol. 1998, 27, S18–S24. [Google Scholar] [CrossRef]
- Gold, S.M.; Willing, A.; Leypoldt, F.; Paul, F.; Friese, M.A. Sex differences in autoimmune disorders of the central nervous system. Semin. Immunopathol. 2019, 41, 177–188. [Google Scholar] [CrossRef]
- Lotter, H.; Altfeld, M. Sex differences in immunity. Semin. Immunopathol. 2019, 41, 133–135. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.; Park, E.H.; Kang, E.H.; Lee, Y.J.; Song, Y.W.; Ha, Y.-J. Sex differences in clinical characteristics and their influence on clinical outcomes in an observational cohort of patients with rheumatoid arthritis. Jt. Bone Spine 2021, 88, 105124. [Google Scholar] [CrossRef]
- Khalifa, O.; Pers, Y.-M.; Ferreira, R.; Sénéchal, A.; Jorgensen, C.; Apparailly, F.; Duroux-Richard, I. X-Linked miRNAs Associated with Gender Differences in Rheumatoid Arthritis. Int. J. Mol. Sci. 2016, 17, 1852. [Google Scholar] [CrossRef]
- Yu, C.; Liu, C.; Jiang, J.; Li, H.; Chen, J.; Chen, T.; Zhan, X. Gender Differences in Rheumatoid Arthritis: Interleukin-4 Plays an Important Role. J. Immunol. Res. 2020, 2020, 4121524. [Google Scholar] [CrossRef]
- Wysham, K.D.; Shoback, D.M.; Andrews, J.S.; Katz, P.P. Sex differences in frailty and its association with low bone mineral density in rheumatoid arthritis. Bone Rep. 2020, 12, 100284. [Google Scholar] [CrossRef] [PubMed]
- Ince-Askan, H.; Dolhain, R.J.E.M. Pregnancy and rheumatoid arthritis. Best Pract. Res. Clin. Rheumatol. 2015, 29, 580–596. [Google Scholar] [CrossRef] [PubMed]
- Balbi, G.G.M.; Domingues, V.; Balbi, G.G.M.; De Jesús, G.R.; Levy, R.A. Use of synthetic and biologic DMARDs during pregnancy. Expert Rev. Clin. Immunol. 2019, 15, 27–39. [Google Scholar] [CrossRef] [PubMed]
- Matcham, F.; Norton, S.; Scott, D.L.; Steer, S.; Hotopf, M. Symptoms of depression and anxiety predict treatment response and long-term physical health outcomes in rheumatoid arthritis: Secondary analysis of a randomized controlled trial. Rheumatology 2015, 55, 268–278. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).