Safety and Efficacy of Bojungikki-Tang in Advanced NSCLC Patients Receiving Treatment with Immune Checkpoint Inhibitors: Protocol for a Multicenter, Double-Blind, Randomized, Placebo-Controlled Pilot Trial
Abstract
1. Introduction
2. Materials and Methods
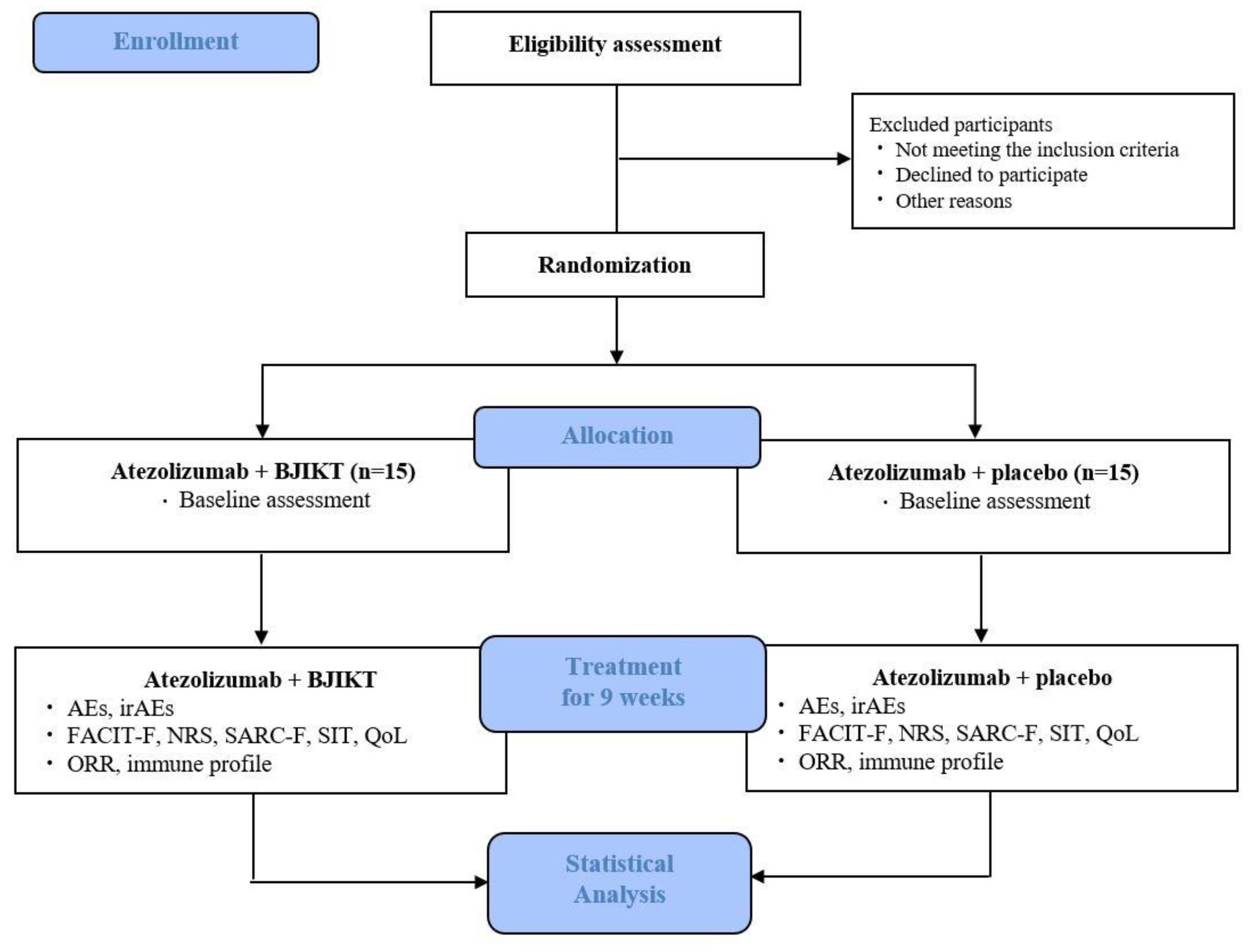
2.1. Study Design and Setting
2.2. Recruitment
2.3. Participants
2.3.1. Inclusion Criteria
2.3.2. Exclusion Criteria
2.4. Randomization and Allocation Concealment
2.5. Blinding
2.6. Interventions
2.7. Outcome Measures
2.7.1. Primary Outcomes
2.7.2. Secondary Outcomes
2.7.3. Exploratory Outcomes
2.7.4. Safety Outcomes
2.8. Sample Size
2.9. Statistical Analysis
2.10. Data Management and Monitoring
2.11. Ethics and Dissemination
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hong, S.; Won, Y.J.; Park, Y.R.; Jung, K.W.; Kong, H.J.; Lee, E.S.; Community of Population-Based Regional Cancer Registries. Cancer statistics in Korea: Incidence, mortality, survival, and prevalence in 2017. Cancer Res. Treat. 2020, 52, 335–350. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2021. CA Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.G.; Kim, H.C.; Choi, C.M. Recent trends of lung cancer in Korea. Tuberc. Respir. Dis. 2021, 84, 89–95. [Google Scholar] [CrossRef] [PubMed]
- National Comprehensive Cancer Network. National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) Non-Small Cell Lung Cancer. Version 4 2021. 2021. Available online: https://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf (accessed on 9 January 2023).
- Komiya, K.; Nakamura, T.; Abe, T.; Ogusu, S.; Nakashima, C.; Takahashi, K.; Kimura, S.; Sueoka-Aragane, N. Discontinuation due to immune-related adverse events is a possible predictive factor for immune checkpoint inhibitors in patients with non-small cell lung cancer. Thorac. Cancer 2019, 10, 1798–1804. [Google Scholar] [CrossRef] [PubMed]
- Park, R.; Lopes, L.; Cristancho, C.R.; Riano, I.M.; Saeed, A. Treatment-related adverse events of combination immune checkpoint inhibitors: Systematic review and meta-analysis. Front. Oncol. 2020, 10, 258. [Google Scholar] [CrossRef] [PubMed]
- Arnaud-Coffin, P.; Maillet, D.; Gan, H.K.; Stelmes, J.J.; You, B.; Dalle, S.; Péron, J. A systematic review of adverse events in randomized trials assessing immune checkpoint inhibitors. Int. J. Cancer 2019, 145, 639–648. [Google Scholar] [CrossRef]
- West, H.; McCleod, M.; Hussein, M.; Morabito, A.; Rittmeyer, A.; Conter, H.J.; Kopp, H.G.; Daniel, D.; McCune, S.; Mekhail, T.; et al. Atezolizumab in combination with carboplatin plus nab-paclitaxel chemotherapy compared with chemotherapy alone as first-line treatment for metastatic non-squamous non-small-cell lung cancer (IMpower130): A multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2019, 20, 924–937. [Google Scholar] [CrossRef]
- Peng, M.; Li, X.; Lei, G.; Weng, Y.M.; Hu, M.X.; Song, Q.B. The efficacy and safety of immune checkpoint inhibitor combination therapy in lung cancer: A systematic review and meta-analysis. Oncol. Targets Ther. 2018, 11, 7369–7383. [Google Scholar] [CrossRef]
- Spigel, D.R.; Chaft, J.E.; Gettinger, S.; Chao, B.H.; Dirix, L.; Schmid, P.; Chow, L.Q.M.; Hicks, R.J.; Leon, L.; Fredrickson, J.; et al. FIR: Efficacy, safety, and biomarker analysis of a phase II open-label study of atezolizumab in PD-L1-selected patients with NSCLC. J. Thorac. Oncol. 2018, 13, 1733–1742. [Google Scholar] [CrossRef]
- Choi, J.; Yang, Z.; Lee, J.; Lee, J.H.; Kim, H.K.; Yong, H.S.; Lee, S.Y. Usefulness of pulmonary rehabilitation in non-small cell lung cancer patients based on pulmonary function tests and muscle analysis using computed tomography images. Cancer Res. Treat. 2022, 54, 793–802. [Google Scholar] [CrossRef]
- Jeong, M.K.; Kim, Y.E.; Kim, A.; Jung, J.; Son, M.J. The herbal drug, Bu-Zhong-Yi-Qi-Tang, for the treatment of atopic dermatitis: Protocol for a systematic review. Medicine 2019, 98, e13938. [Google Scholar] [CrossRef]
- Jeong, J.S.; Ryu, B.H.; Kim, J.S.; Park, J.W.; Choi, W.C.; Yoon, S.W. Bojungikki-tang for cancer-related fatigue: A pilot randomized clinical trial. Integr. Cancer Ther. 2010, 9, 331–338. [Google Scholar] [CrossRef]
- Yae, S.; Takahashi, F.; Yae, T.; Yamaguchi, T.; Tsukada, R.; Koike, K.; Minakata, K.; Murakami, A.; Nurwidya, F.; Kato, M.; et al. Hochuekkito (TJ-41), a Kampo formula, ameliorates cachexia induced by colon 26 adenocarcinoma in mice. Evid. Based Complement. Alternat. Med. 2012, 2012, 976926. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Lee, J.K.; Shin, H.K. Analysis of studies on Bojungikgi-tang (Buzhongyiqi-tang) to establish the fundament for evidence based medicine (EBM). Korean J. Orient. Med. 2011, 17, 135–167. [Google Scholar]
- Kido, T.; Mori, K.; Daikuhara, H.; Tsuchiya, H.; Ishige, A.; Sasaki, H. The protective effect of hochu-ekki-to (TJ-41), a Japanese herbal medicine, against HSV-1 infection in Mitomycin C-treated mice. Anticancer Res. 2000, 20, 4109–4113. [Google Scholar] [PubMed]
- Li, T.; Tamada, K.; Abe, K.; Tada, H.; Onoe, Y.; Tatsugami, K.; Harada, M.; Kubo, C.; Nomoto, K. The restoration of the antitumor T cell response from stress-induced suppression using a traditional Chinese herbal medicine Hochu-ekki-to (TJ-41:Bu-Zhong-Yi-Qi-Tang). Immunopharmacology 1999, 43, 11–21. [Google Scholar] [CrossRef]
- Kimura, M.; Sasada, T.; Kanai, M.; Kawai, Y.; Yoshida, Y.; Hayashi, E.; Iwata, S.; Takabayashi, A. Preventive effect of a traditional herbal medicine, Hochu-ekki-to, on immunosuppression induced by surgical stress. Surg. Today 2008, 38, 316–322. [Google Scholar] [CrossRef]
- Qi, F.; Zhao, L.; Zhou, A.; Zhang, B.; Li, A.; Wang, Z.; Han, J. The advantages of using traditional Chinese medicine as an adjunctive therapy in the whole course of cancer treatment instead of only terminal stage of cancer. BioSci. Trends 2015, 9, 16–34. [Google Scholar] [CrossRef]
- Park, S.R.; Kim, K.H.; Kwun, M.J.; Lee, J.Y.; Won, R.; Han, C.W.; Choi, J.Y.; Joo, M. Differential regulation of NF-κB and Nrf2 by Bojungikki-tang is associated with suppressing lung inflammation. Evid. Based Complement. Altern. Med. 2018, 2018, 5059469. [Google Scholar] [CrossRef]
- Han, Y.J.; Lee, S.D.; Choi, J.H.; Park, J.G.; Jang, I.S.; Park, H.M. Effects of high frequency herbal medication administrations on the liver functions in rats—Focusing on Sipjeondaebo-tang, Yukmaijihwang-tang, Bojungikgi-tang, and Ojeoksan. J. Korean Med. 2006, 27, 78–90. Available online: https://koreascience.kr/article/JAKO200631036998460.jsp-k1ff8j=SSMHB4&py=2012&vnc=v27n6&sp=588 (accessed on 19 August 2022).
- Lee, J.H.; Hwang, Y.H.; Kwak, D.H.; Kim, T.S.; Ma, J.Y. Single oral dose toxicity test of Bojungikgi-tang (Buzhongyiqi-tang) and fermented Bojungikgi-tang (Buzhongyiqi-tang) extracts in mice. Korean J. Intern. Med. 2011, 32, 599–609. Available online: https://koreascience.kr/article/JAKO201113036235933.kr&sa=U (accessed on 9 January 2023).
- Kuboniwa, H.; Maemura, S.; Minematsu, S.; Onishi, M. Mutagenicity studies of Hochu-ekki-to (TJ-41). Jpn. Pharmacol. Ther. 1999, 27 (Suppl. S6), s1379–s1384. Available online: https://lifescience.co.jp/yk/yk99/yke99s6.html (accessed on 19 August 2022).
- Satoh, H.; Ishikawa, H.; Ohtsuka, M.; Sekizawa, K. Japanese herbal medicine in patients with advanced lung cancer: Prolongation of survival. J. Altern. Complement. Med. 2002, 8, 107–108. [Google Scholar] [CrossRef] [PubMed]
- El-Saber Batiha, G.; Magdy Beshbishy, A.; El-Mleeh, A.; Abdel-Daim, M.M.; Prasad Devkota, H. Traditional uses, bioactive chemical constituents, and pharmacological and toxicological activities of Glycyrrhiza glabra L. (Fabaceae). Biomolecules 2020, 10, E352. [Google Scholar] [CrossRef]
- Shimada, Y.; Fujimoto, M.; Nogami, T.; Watari, H. Adverse events associated with ethical Kampo formulations: Analysis of the domestic adverse-event data reports of the Ministry of Health, Labor, and Welfare in Japan. Evid. Based Complement. Alternat. Med. 2019, 2019, 1643804. [Google Scholar] [CrossRef]
- Iwase, S.; Yamaguchi, T.; Miyaji, T.; Terawaki, K.; Inui, A.; Uezono, Y. The clinical use of Kampo medicines (traditional Japanese herbal treatments) for controlling cancer patients’ symptoms in Japan: A national cross-sectional survey. BMC Complement. Altern. Med. 2012, 12, 222. [Google Scholar] [CrossRef] [PubMed]
- Li, T.M.; Yu, Y.H.; Tsai, F.J.; Cheng, C.F.; Wu, Y.C.; Ho, T.J.; Liu, X.; Tsang, H.; Lin, T.H.; Liao, C.C.; et al. Characteristics of Chinese herbal medicine usage and its effect on survival of lung cancer patients in Taiwan. J. Ethnopharmacol. 2018, 213, 92–100. [Google Scholar] [CrossRef]
- Wang, C.Y.; Huang, H.S.; Su, Y.C.; Tu, C.Y.; Hsia, T.C.; Huang, S.T. Conventional treatment integrated with Chinese herbal medicine improves the survival rate of patients with advanced non-small cell lung cancer. Complement. Ther. Med. 2018, 40, 29–36. [Google Scholar] [CrossRef]
- Herbst, R.S.; Giaccone, G.; de Marinis, F.; Reinmuth, N.; Vergnenegre, A.; Barrios, C.H.; Morise, M.; Felip, E.; Andric, Z.; Geater, S.; et al. Atezolizumab for first-line treatment of PD-L1-selected patients with NSCLC. N. Engl. J. Med. 2020, 383, 1328–1339. [Google Scholar] [CrossRef]
- US Department of Health and Human Services. Common Terminology Criteria for Adverse Events (CTCAE); Version 4.03; National Institutes of Health—National Cancer Institute. 2010. Available online: http://evs.nci.nih.gov/ftp1/CTCAE/About.html (accessed on 19 August 2022).
- Socinski, M.A.; Jotte, R.M.; Cappuzzo, F.; Orlandi, F.; Stroyakovskiy, D.; Nogami, N.; Rodríguez-Abreu, D.; Moro-Sibilot, D.; Thomas, C.A.; Barlesi, F.; et al. Atezolizumab for first-line treatment of metastatic nonsquamous NSCLC. N. Engl. J. Med. 2018, 378, 2288–2301. [Google Scholar] [CrossRef]
- Fehrenbacher, L.; Spira, A.; Ballinger, M.; Kowanetz, M.; Vansteenkiste, J.; Mazieres, J.; Park, K.; Smith, D.; Artal-Cortes, A.; Lewanski, C.; et al. Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (Poplar): A multicentre, open-label, phase 2 randomised controlled trial. Lancet 2016, 387, 1837–1846. [Google Scholar] [CrossRef]
- Webster, K.; Cella, D.; Yost, K. The Functional Assessment of Chronic Illness Therapy (FACIT) measurement system: Properties, applications, and interpretation. Health Qual. Life Outcomes 2003, 1, 79. [Google Scholar] [CrossRef]
- Minton, O.; Stone, P. A systematic review of the scales used for the measurement of cancer-related fatigue (CRF). Ann. Oncol. 2009, 20, 17–25. [Google Scholar] [CrossRef]
- Storey, D.J.; Waters, R.A.; Hibberd, C.J.; Rush, R.W.; Cargill, A.T.; Wall, L.R.; Fallon, M.T.; Strong, V.A.; Walker, J.; Sharpe, M. Clinically relevant fatigue in cancer outpatients: The Edinburgh Cancer Centre symptom study. Ann. Oncol. 2007, 18, 1861–1869. [Google Scholar] [CrossRef]
- Fayers, P.; Aaronson, N.K.; Bjordal, K.; Groenvold, M.; Curran, D.; Bottomley, A. EORTC QLQ-C30 Scoring Manual, 3rd ed.; European Organization for Research and Treatment of Cancer: Brussels, Belgium, 2001. [Google Scholar]
- Kim, S.; Kim, M.; Won, C.W. Validation of the Korean version of the SARC-F questionnaire to assess sarcopenia: Korean Frailty and Aging Cohort Study. J. Am. Med. Dir. Assoc. 2018, 19, 40–45.e1. [Google Scholar] [CrossRef]
- Yeo, M.; Park, K.; Bae, K.; Jang, E.S.; Lee, Y.S. Development on the Questionnaire of Cold-Heat Pattern Identification Based on Usual Symptoms for Health Promotion—Focused on Reliability Study’. J. Physiol. Pathol. Korean Med. 2016, 30, 116–123. [Google Scholar] [CrossRef]
- Bae, K.H.; Yoon, Y.; Yeo, M.; Kim, H.S.; Lee, Y.M.; Lee, S. Development on the questionnaire of cold-heat pattern identification based on usual symptoms for health promotion—Focused on agreement study. J. Soc. Prev. Korean Med. 2016, 20, 17–26. Available online: https://koreascience.kr/article/JAKO201625653102971.pdf (accessed on 9 January 2023).
- Xing, P.Y.; Zhu, Y.X.; Wang, L.; Hui, Z.G.; Liu, S.M.; Ren, J.S.; Zhang, Y.; Song, Y.; Liu, C.C.; Huang, Y.C.; et al. What are the clinical symptoms and physical signs for non-small cell lung cancer before diagnosis is made? A nation-wide multicenter 10-year retrospective study in China. Cancer Med. 2019, 8, 4055–4069. [Google Scholar] [CrossRef]
- Bower, J.E. Cancer-related fatigue—Mechanisms, risk factors, and treatments. Nat. Rev. Clin. Oncol. 2014, 11, 597–609. [Google Scholar] [CrossRef]
- Bower, J.E. The role of neuro-immune interactions in cancer-related fatigue: Biobehavioral risk factors and mechanisms. Cancer 2019, 125, 353–364. [Google Scholar] [CrossRef]
- Bower, J.E.; Lamkin, D.M. Inflammation and cancer-related fatigue: Mechanisms, contributing factors, and treatment implications. Brain Behav. Immun. 2013, 30, S48–S57. [Google Scholar] [CrossRef]
- Shiroyama, T.; Nagatomo, I.; Koyama, S.; Hirata, H.; Nishida, S.; Miyake, K.; Fukushima, K.; Shirai, Y.; Mitsui, Y.; Takata, S.; et al. Impact of sarcopenia in patients with advanced non-small cell lung cancer treated with PD-1 inhibitors: A preliminary retrospective study. Sci. Rep. 2019, 9, 2447. [Google Scholar] [CrossRef]
- Cheville, A.L.; Novotny, P.J.; Sloan, J.A.; Basford, J.R.; Wampfler, J.A.; Garces, Y.I.; Jatoi, A.; Yang, P. The value of a symptom cluster of fatigue, dyspnea, and cough in predicting clinical outcomes in lung cancer survivors. J. Pain Symptom Manag. 2011, 42, 213–221. [Google Scholar] [CrossRef]
- Tanaka, K.; Akechi, T.; Okuyama, T.; Nishiwaki, Y.; Uchitomi, Y. Impact of dyspnea, pain, and fatigue on daily life activities in ambulatory patients with advanced lung cancer. J. Pain Symptom Manag. 2002, 23, 417–423. [Google Scholar] [CrossRef]
- Zhu, Y.J.; Zhang, H.B.; Liu, L.R.; Liu, Y.H.; Zhang, F.L.; Bai, J.P.; Li, Y.; Qu, Y.C.; Qu, X.; Chen, X.; et al. Yin-Cold or Yang-Heat Syndrome Type of Traditional Chinese Medicine Was Associated with the Epidermal Growth Factor Receptor Gene Status in Non-Small Cell Lung Cancer Patients: Confirmation of a TCM Concept. Evid. Based Complement. Alternat. Med. 2017, 2017, 7063859. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, P. Clinical distribution and molecular basis of traditional Chinese medicine ZHENG in cancer. Evid. Based Complement. Altern. Med. 2012, 2012, 783923. [Google Scholar] [CrossRef]
- Su, S.B.; Lu, A.; Li, S.; Jia, W. Evidence-based ZHENG: A traditional Chinese medicine syndrome. Evid. Based Complement. Altern. Med. 2012, 2012, 246538. [Google Scholar] [CrossRef]
- Zhu, Y.J.; Zhang, H.B.; Liu, Y.H.; Zhang, F.L.; Zhu, Y.Z.; Li, Y.; Bai, J.P.; Liu, L.R.; Qu, Y.C.; Qu, X.; et al. Quantitative cell-free circulating EGFR mutation concentration is correlated with tumor burden in advanced NSCLC patients. Lung Cancer 2017, 109, 124–127. [Google Scholar] [CrossRef]
- Chen, Z.; Chen, L.Y.; Wang, P.; Dai, H.Y.; Gao, S.; Wang, K. Tumor microenvironment varies under different TCM ZHENG models and correlates with treatment response to herbal medicine. Evid. Based Complement. Altern. Med. 2012, 2012, 635702. [Google Scholar] [CrossRef]
| Study Period | |||||
|---|---|---|---|---|---|
| Screening | Treatment & F/U | ||||
| Baseline | V1 | V2 | V3 c | ||
| Time Point | ~4 Weeks | C1D1 (±7 Days) | C2D1 (±7 Days) | C3D1 (±7 Days) | C3D21 (±7 Days) |
| Enrollment | |||||
| Informed consent | X | ||||
| Eligibility screen | X | X | |||
| Demographic characteristics | X | ||||
| Medical history | X | X | |||
| PD-L1 testing (immunohistochemistry), EGFR/ALK mutation test | X | ||||
| Physical examination and vital signs | X | X | X | X | |
| Electrocardiography | X | ||||
| ECOG PS | X | X | X | X | |
| Random allocation | X | ||||
| Interventions | |||||
| Arm 1: atezolizumab + BJIKT |  | ||||
| Arm 2: atezolizumab + placebo |  | ||||
| Assessments | |||||
| EORTC-QLQ-C30 | X | X | X | ||
| Fatigue subscale/NRS | X | X | X | ||
| FACIT-F(TOI-F) | X | X | X | ||
| SARC-F | X | X | X | ||
| Muscle test (InBodyTM, STS test) | X | X | X | ||
| Cold/heat pattern | X | X | X | ||
| Laboratory testing a | X | b | X | X | |
| Immunological testing (blood collection) | X | b | X | ||
| Chest CT | X | X | |||
| X-ray examination | X | X | X | ||
| Body composition test d | X | X | X | X | |
| Tumor evaluation (according to RECIST v1.1) | X | X | |||
| Adverse events (CTCAE v4.03) | X | X | X | X | |
| Medication compliance | X | X | X | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ko, M.M.; Jeong, M.-K.; Choi, C.M.; Lee, S.H.; Chun, J.; Yi, J.-M.; Jang, H.; Lee, S.Y. Safety and Efficacy of Bojungikki-Tang in Advanced NSCLC Patients Receiving Treatment with Immune Checkpoint Inhibitors: Protocol for a Multicenter, Double-Blind, Randomized, Placebo-Controlled Pilot Trial. Int. J. Environ. Res. Public Health 2023, 20, 4507. https://doi.org/10.3390/ijerph20054507
Ko MM, Jeong M-K, Choi CM, Lee SH, Chun J, Yi J-M, Jang H, Lee SY. Safety and Efficacy of Bojungikki-Tang in Advanced NSCLC Patients Receiving Treatment with Immune Checkpoint Inhibitors: Protocol for a Multicenter, Double-Blind, Randomized, Placebo-Controlled Pilot Trial. International Journal of Environmental Research and Public Health. 2023; 20(5):4507. https://doi.org/10.3390/ijerph20054507
Chicago/Turabian StyleKo, Mi Mi, Mi-Kyung Jeong, Chang Min Choi, Seung Hyeun Lee, Jaemoo Chun, Jin-Mu Yi, Ho Jang, and Sung Yong Lee. 2023. "Safety and Efficacy of Bojungikki-Tang in Advanced NSCLC Patients Receiving Treatment with Immune Checkpoint Inhibitors: Protocol for a Multicenter, Double-Blind, Randomized, Placebo-Controlled Pilot Trial" International Journal of Environmental Research and Public Health 20, no. 5: 4507. https://doi.org/10.3390/ijerph20054507
APA StyleKo, M. M., Jeong, M.-K., Choi, C. M., Lee, S. H., Chun, J., Yi, J.-M., Jang, H., & Lee, S. Y. (2023). Safety and Efficacy of Bojungikki-Tang in Advanced NSCLC Patients Receiving Treatment with Immune Checkpoint Inhibitors: Protocol for a Multicenter, Double-Blind, Randomized, Placebo-Controlled Pilot Trial. International Journal of Environmental Research and Public Health, 20(5), 4507. https://doi.org/10.3390/ijerph20054507







