Impact of Landfill Gas Exposure on Vegetation in Engineered Landfill Biocover Systems Implemented to Minimize Fugitive Methane Emissions from Landfills
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Location
2.2. Flow-Through Vegetated Biofilter Columns
2.3. Exposure of Vegetated Columns to Methane Gas
2.4. Determination of Plant Growth Characteristics
2.5. Determination of Gas Concentration Profiles in Columns
2.6. Methane Oxidation Assessment
3. Results
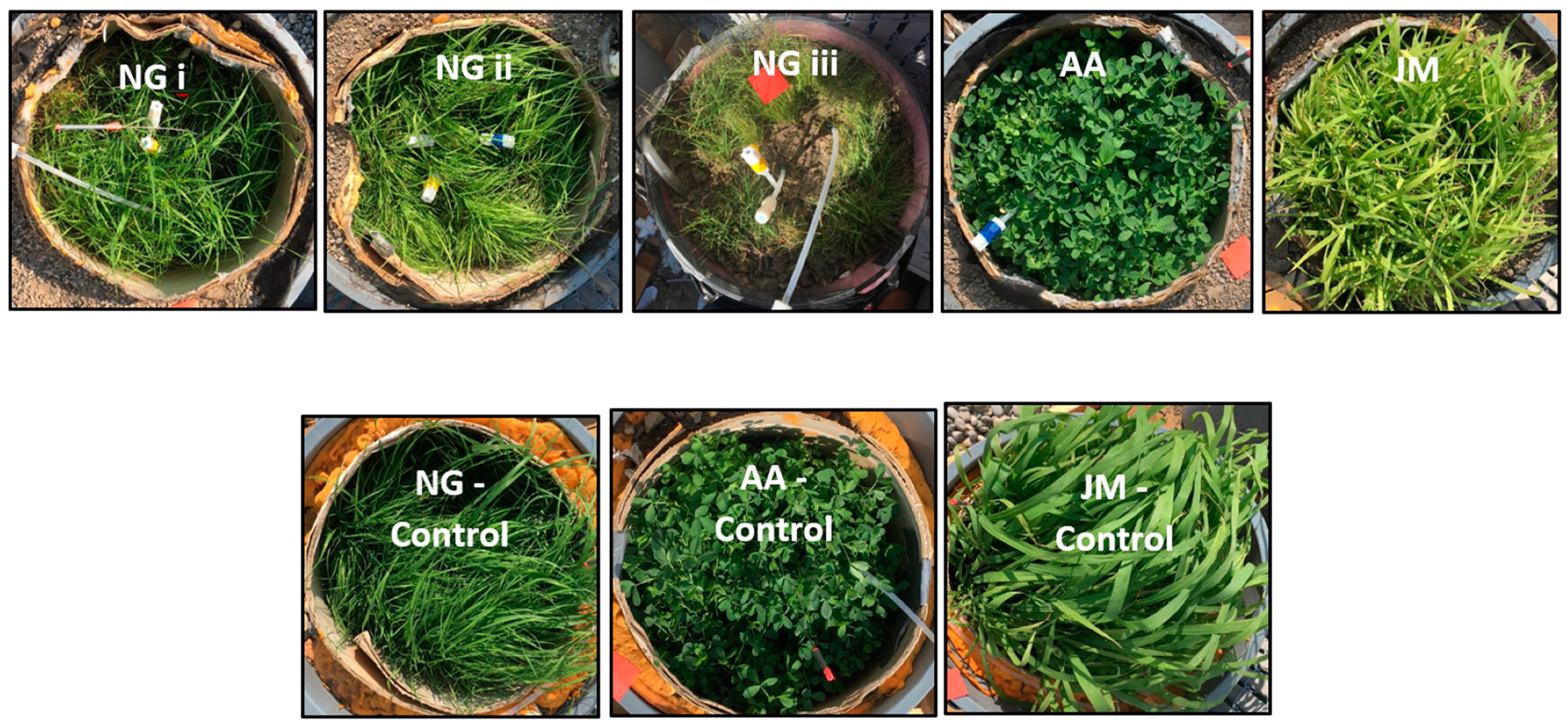
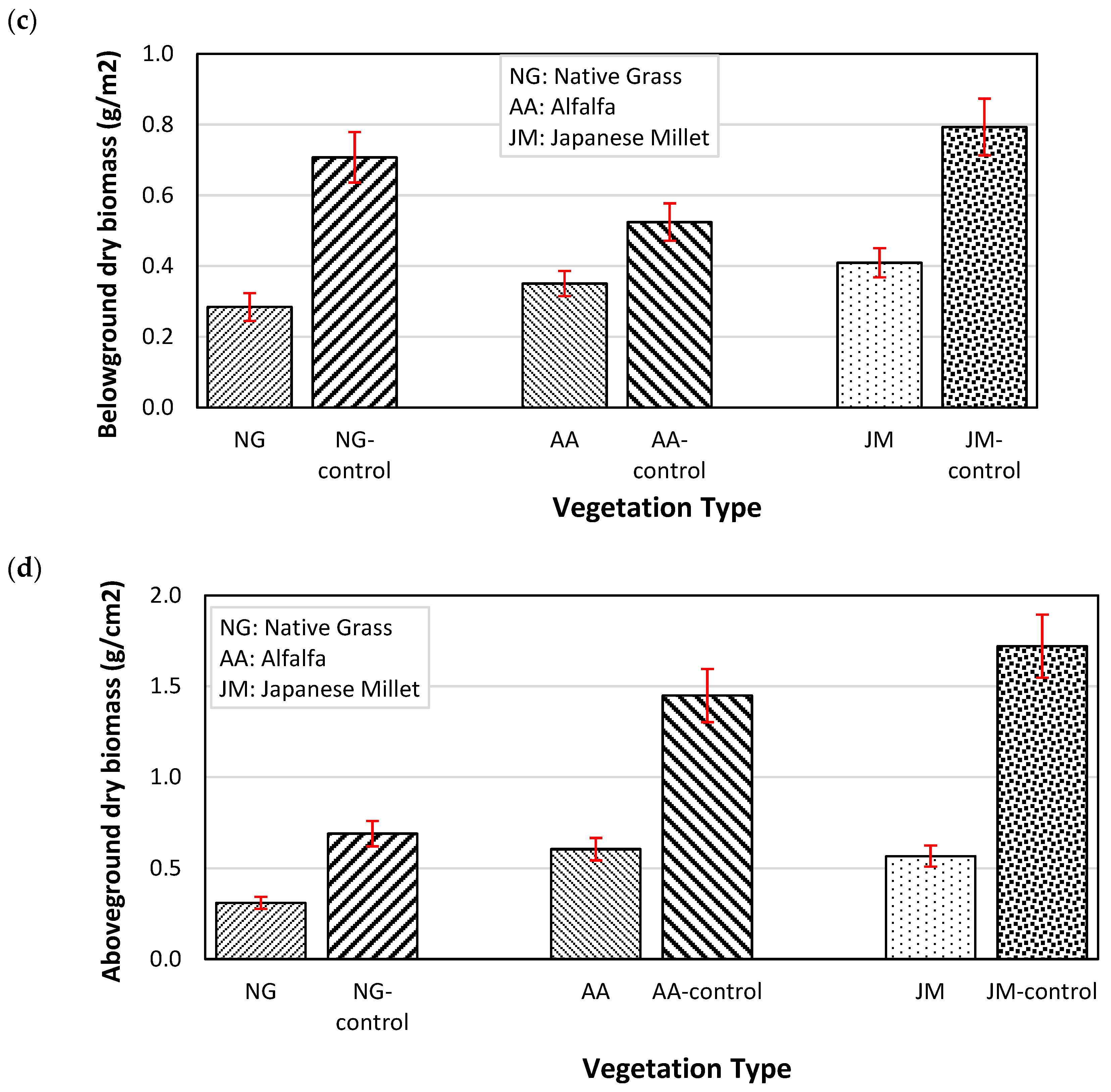
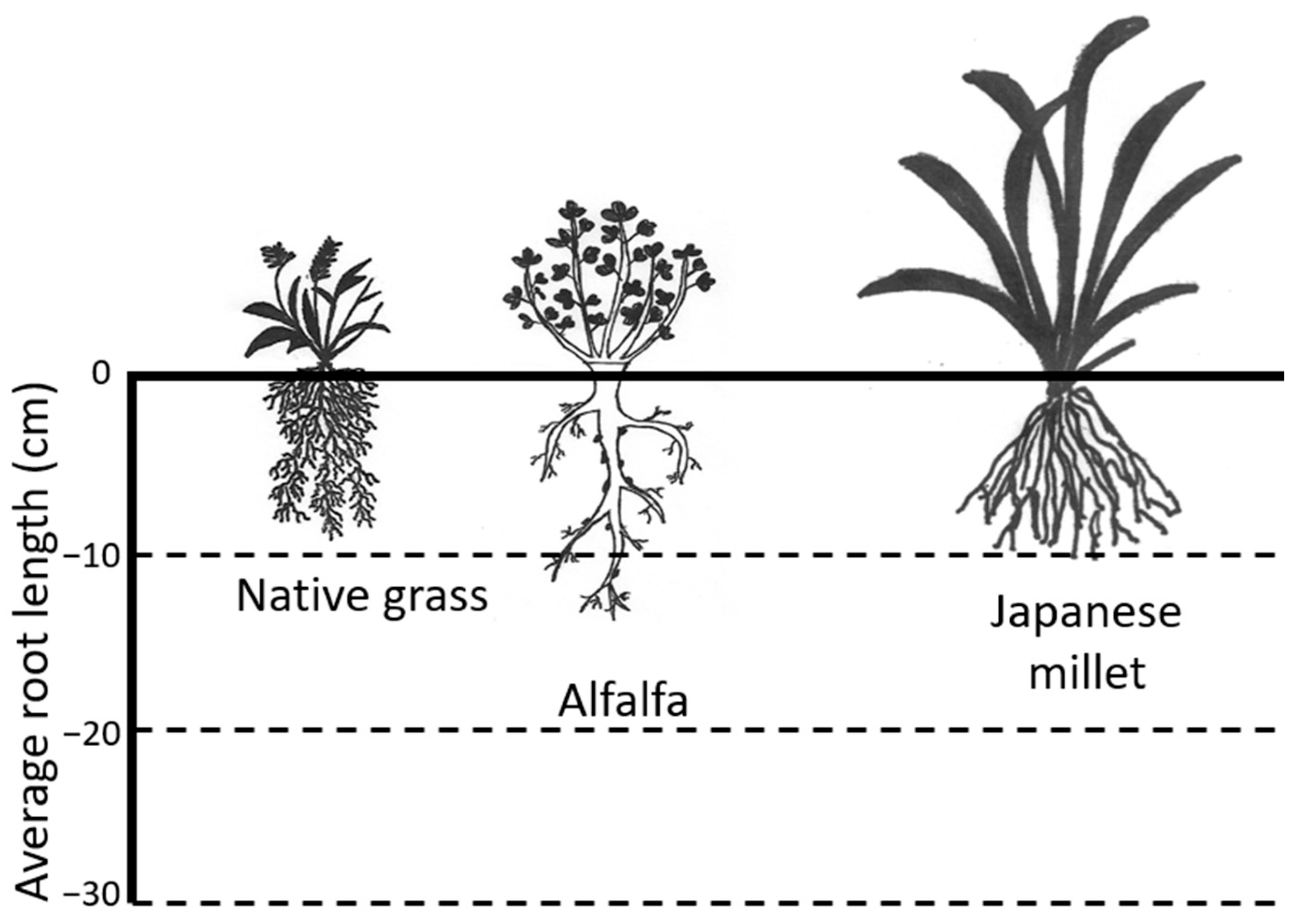
3.1. Vegetation Growth Parameters with and without Gas Exposure
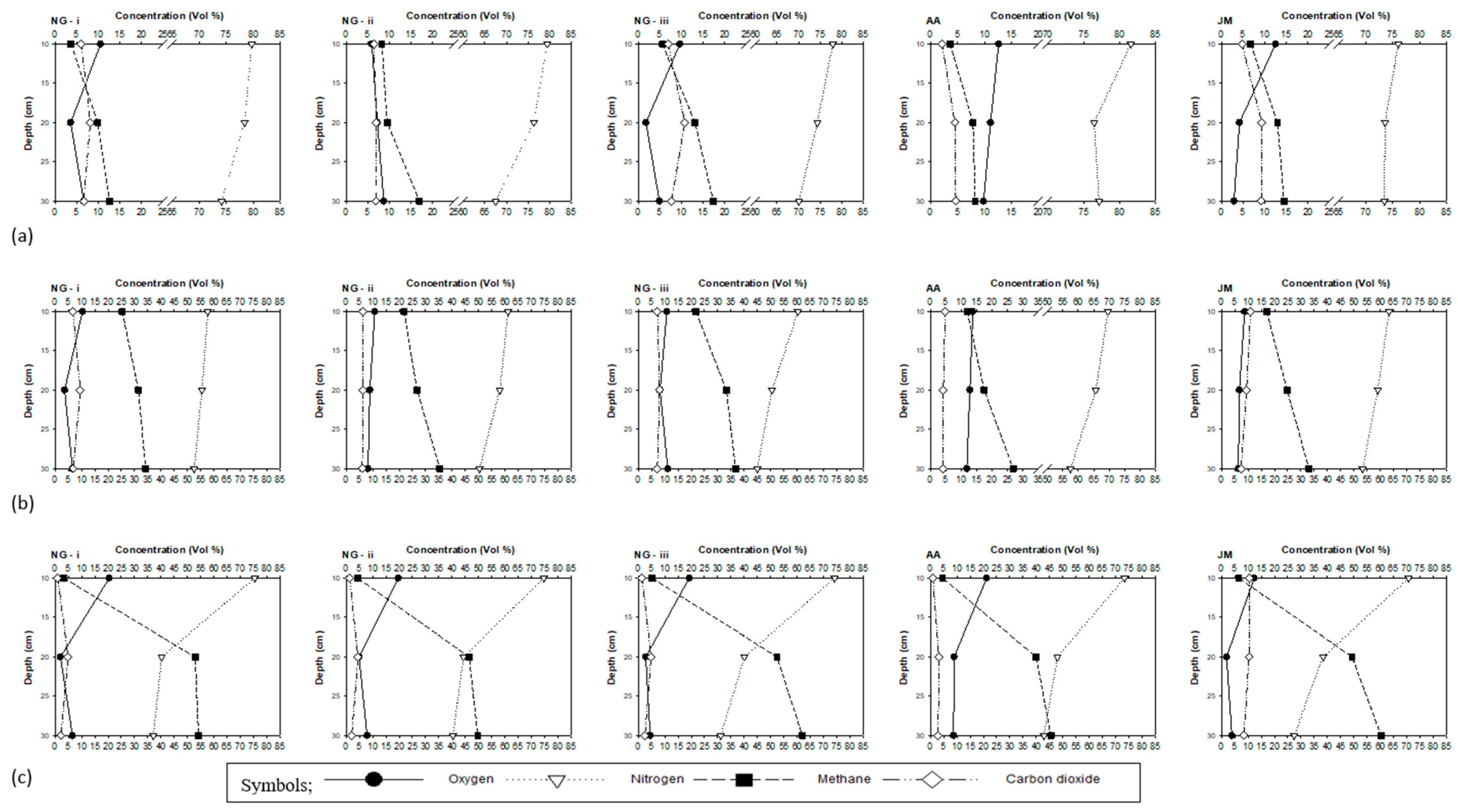
3.2. Gas Concentration Profiles
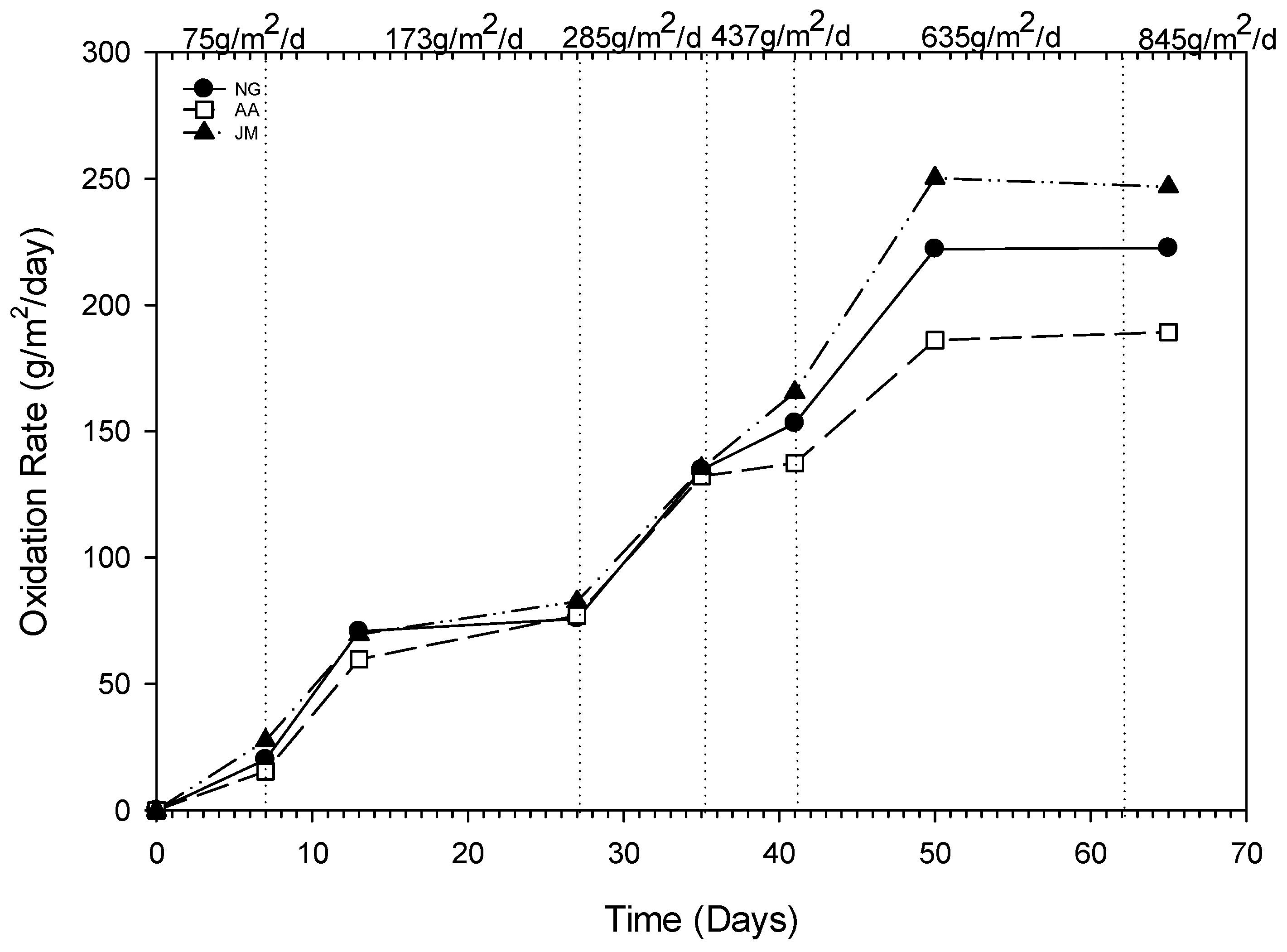
3.3. Methane Oxidation in Vegetated Columns
4. Discussion and Interpretation of Results
4.1. Oxygen Gas Concentrations and Vegetation Impacts
4.2. Methane Oxidation and Vegetation Impacts
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hunte, C.; Hettiaratchi, J.P.A.; Hettiarachchi, H.; Meegoda, J.N. Determination of Waste Properties from Settlement Behaviour of a Full Scale Waste Cell Operated as a Landfill Bioreactor; ASCE GeoFrontiers: Dallas, TX, USA, 2011. [Google Scholar]
- Xiaoli, C.; Xin, Z.; Ziyang, L.; Shimaoka, T.; Nakayama, H.; Xianyan, C.; Youcai, Z. Characteristics of vegetation and its relationship with landfill gas in closed landfill. Biomass Bioenergy 2011, 35, 1295–1301. [Google Scholar] [CrossRef]
- Bogner, J.E.; Spokas, K.A.; Burton, E.A. Temporal variations in greenhouse gas emissions at a midlatitude landfill. J. Environ. Qual. 1999, 28, 278–288. [Google Scholar] [CrossRef]
- Kightley, D.; Nedwell, D.B.; Cooper, M. Capacity for methane oxidation in landfill cover soils measured in laboratory-scale soil microcosms. Appl. Environ. Microbiol. 1995, 61, 592–601. [Google Scholar] [CrossRef]
- La, H.; Hettiaratchi, J.P.A.; Achari, G.; Verbeke, T.J.; Dunfield, P.F. Biofiltration of methane using hybrid mixtures of biochar, lava rock and compost. Environ. Pollut. 2018, 241, 45–54. [Google Scholar] [CrossRef]
- Tao, Z.; Shi, W.; Liu, Y.; Chai, X. Temporal variation of vegetation at two operating landfills and its implications for landfill phytoremediation. Environ. Technol. 2020, 41, 649–657. [Google Scholar] [CrossRef]
- Khapre, A.; Kumar, S.; Rajasekaran, C. Phytocapping: An alternate cover option for municipal solid waste landfills. Environ. Technol. 2019, 40, 2242–2249. [Google Scholar] [CrossRef]
- Reichenauer, T.G.; Watzinger, A.; Riesing, J.; Gerzabek, M.H. Impact of different plants on the gas profile of a landfill cover. Waste Manag. 2011, 31, 843–853. [Google Scholar] [CrossRef]
- Stralis-Pavese, N.; Sessitsch, A.; Weilharter, A.; Reichenauer, T.; Riesing, J.; Csontos, J.; Murrell, J.C.; Bodrossy, L. Optimization of diagnostic microarray for application in analysing landfill methanotroph communities under different plant covers. Environ. Microbiol. 2004, 6, 347–363. [Google Scholar] [CrossRef]
- Attalage, D.S.; Hettiaratchi, P.A.; Jayasinghe, P.; Dunfield, P.F.; Smirnova, A.V.; Rathnavibushana, U.K.; Erkmen, M.; Kumar, S. Field study on the effect of vegetation on the performance of soil methanotrophy-based engineered systems—Column experiments. Soil Biol. Biochem. 2022, 167, 108583. [Google Scholar] [CrossRef]
- Reay, D.S.; Nedwell, D.B.; McNamara, N.; Ineson, P. Effect of tree species on methane and ammonium oxidation capacity in forest soils. Soil Biol. Biochem. 2005, 37, 719–730. [Google Scholar] [CrossRef]
- Chan, Y.S.G.; Chu, L.M.; Wong, M.H. Influence of landfill factors on plants and soil fauna—An ecological perspective. Environ. Pollut. 1997, 97, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Wong, M.H.; Yu, C.T. Monitoring of gin drinkers’ bay landfill, Hong Kong: II. Gas contents, soil properties, and vegetation performance on the side slope. Environ. Manag. 1989, 13, 753–762. [Google Scholar] [CrossRef]
- Wong, M.H.; Yu, C.T. Monitoring of gin drinkers’ bay landfill, Hong Kong: I. landfill gas on top of the landfill. Environ. Manag. 1989, 13, 743–752. [Google Scholar] [CrossRef]
- Leone, I.A.; Flower, F.B.; Arthur, J.J.; Gilman, E.F. Damage to New Jersey crops by landfill gases. Plant Dis. Rep. 1977, 61, 295–299. [Google Scholar]
- Gilman, E.F.; Leone, I.A.; Flower, F.B. The adaptability of 19 woody species in vegetating a former sanitary landfill. For. Sci. 1981, 27, 13–18. [Google Scholar]
- Wong, M.H.; Cheung, Y.H.; Cheung, C.L. The effects of ammonia and ethylene oxide in animal manure and sewage sludge on the seed germination and root elongation of brassica parachinensis. Environ. Pollution. Ser. A Ecol. Biol. 1983, 30, 109–123. [Google Scholar] [CrossRef]
- Gendebien, A.; Pauwels, M.; Ledrut-Damanet, M.J.; Nyns, E.J.; Willumsen, H.C.; Butson, J.; Fabry, R.; Ferrero, G.L. Potential Landfill Gas Damages to Vegetation. In Landfill Gas from Environment to Energy; Commission of the European Communities: Luxembourg, 1992; pp. 35–46. [Google Scholar]
- Kozlowski, T.T. Soil aeration, flooding, and tree growth. J. Arboric. 1985, 11, 85–96. [Google Scholar] [CrossRef]
- Nagendran, R.; Selvam, A.; Joseph, K.; Chiemchaisri, C. Phytoremediation and rehabilitation of municipal solid waste landfills and dumpsites: A brief review. Waste Manag. 2006, 26, 1357–1369. [Google Scholar] [CrossRef]
- Gilman, E.F.; Leone, I.A.; Flower, F.B. Influence of soil gas contamination on tree root growth. Plant Soil 1982, 65, 3–10. [Google Scholar] [CrossRef]
- Danielson, R.E. Physical edaphology—The physics of irrigated and nonirrigated soils. Soil Sci. Soc. Am. J. 1974, 38, iv. [Google Scholar] [CrossRef]
- Bohn, S.; Brunke, P.; Gebert, J.; Jager, J. Improving the aeration of critical fine-grained landfill top cover material by vegetation to increase the microbial methane oxidation efficiency. Waste Manag. 2011, 31, 854–863. [Google Scholar] [CrossRef] [PubMed]
- Jalilzadeh, H. Field Performance and Water Balance Predictions of Evapotranspirative Landfill Biocovers. Master’s Thesis, University of Calgary, Calgary, AB, Canada, 2019. [Google Scholar]
- Adenipekun, C.O.; Oyetunji, O.J.; Kassim, L.S. Effect of spent engine oil on the growth parameters and chlorophyll content of corchorus olitorius linn. Environmentalist 2008, 28, 446–450. [Google Scholar] [CrossRef]
- Cornelissen, J.H.C.; Lavorel, S.; Garnier, E.; Díaz, S.; Buchmann, N.; Gurvich, D.E.; Reich, P.B.; ter Steege, H.; Morgan, H.D.; van der Heijden, M.G.A.; et al. A handbook of protocols for standardised and easy measurement of plant functional traits worldwide. Aust. J. Bot. 2003, 51, 335–380. [Google Scholar] [CrossRef]
- Singh, S.N.; Rao, D.N. Certain responses of wheat plants to cement dust pollution. Environ. Pollut. Ser. A Ecol. Biol. 1981, 24, 75–81. [Google Scholar] [CrossRef]
- Maclachlan, S.; Zalik, S. Plastid structure, chlorophyll concentration, and free amino acid composition of a chlorophyll mutant of barley. Can. J. Bot. 1963, 41, 1053–1062. [Google Scholar] [CrossRef]
- Pihlatie, M.K.; Christiansen, J.R.; Aaltonen, H.; Korhonen, J.F.J.; Nordbo, A.; Rasilo, T.; Benanti, G.; Giebels, M.; Helmy, M.; Sheehy, J.; et al. Comparison of static chambers to measure CH4 emissions from soils. Agric. Meteorol. 2013, 171–172, 124–136. [Google Scholar] [CrossRef]
- Powelson, D.K.; Chanton, J.; Abichou, T.; Morales, J. Methane oxidation in water-spreading and compost biofilters. Waste Manag. Res. J. A Sustain. Circ. Econ. 2006, 24, 528–536. [Google Scholar] [CrossRef]
- Pokhrel, D.; Hettiaratchi, J.; Steele, M. Methane oxidation prediction curves of soil at different organic contents. Curr. Environ. Manag. 2016, 3, 131–143. [Google Scholar] [CrossRef]
- Pokhrel, D. Compost Based Biocap Performance. Ph.D, Thesis, University of Calgary, Calgary, AB, Canada, 2006. [Google Scholar]
- Flower, F.B.; Gilman, E.F.; Leone, I.A. Landfill gas, what it does to trees and how its injurious effects may be prevented. J. Arboric. 1981, 7, 43–52. [Google Scholar] [CrossRef]
- Mi, Y.; Ma, X.; Chen, S. Resistant evaluation of kiwifruit rootstocks to root zone hypoxia stress. Am. J. Plant Sci. 2013, 4, 945–954. [Google Scholar] [CrossRef]
- Cao, F.L.; Conner, W.H. Selection of flood-tolerant populus deltoides clones for reforestation projects in China. For. Ecol. Manag. 1999, 117, 211–220. [Google Scholar] [CrossRef]
- Whalen, S.C.; Reeburgh, W.S.; Sandbeck, K.A. Rapid methane oxidation in a landfill cover soil. Appl. Environ. Microbiol. 1990, 56, 3405–3411. [Google Scholar] [CrossRef]
- Stolwijk, J.A.J.; Thimann, K.V. On the uptake of carbon dioxide and bicarbonate by roots, and its influence on growth. Plant Physiol. 1957, 32, 513–520. [Google Scholar] [CrossRef] [PubMed]
- Jackson, M.B.; Hall, K.C. Early stomatal closure in waterlogged pea plants is mediated by abscisic acid in the absence of foliar water deficits. Plant Cell Environ. 1987, 10, 121–130. [Google Scholar] [CrossRef]
- Milligan, S.P.; Dale, J.E. The effects of root treatments on growth of the primary leaves of phaseolus Vulgaris L.: General features. N. Phytol. 1988, 108, 27–35. [Google Scholar] [CrossRef]
- Chiemchaisri, W.; Visvanathan, C.; Wu, J.S. Biological activities of methane oxidation in tropical landfill cover soils. J. Solid Waste Technol. Manag. 2001, 27, 129–136. [Google Scholar]
- Hanslin, H.M.; Sæbø, A.; Bergersen, O. Estimation of oxygen concentration in the soil gas phase beneath compost mulch by means of a simple method. Urban For. Urban Green 2005, 4, 37–40. [Google Scholar] [CrossRef]
- Geigenberger, P. Response of plant metabolism to too little oxygen. Curr. Opin. Plant Biol. 2003, 6, 247–256. [Google Scholar] [CrossRef]
- Boru, G.; Vantoai, T.; Alves, J.; Hua, D.; Knee, M. Responses of soybean to oxygen deficiency and elevated root-zone carbon dioxide concentration. Ann. Bot. 2003, 91, 447–453. [Google Scholar] [CrossRef]
- Drew, M.C. Oxygen deficiency and root metabolism: Injury and acclimation under hypoxia and anoxia. Annu. Rev. Plant Biol. 1997, 48, 223–250. [Google Scholar] [CrossRef] [PubMed]
- Evans, D.E. Aerenchyma Formation. N. Phytol. 2004, 161, 35–49. [Google Scholar] [CrossRef]
- Joshi, R.; Kumar, P. Lysigenous aerenchyma formation involves non-apoptotic programmed cell death in rice (Oryza Sativa L.) roots. Physiol. Mol. Biol. Plants 2012, 18, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Song, X.F.; Zhou, Z.Q.; Wang, L.K.; Li, J.W.; Deng, X.Y.; Fan, H.Y. Aerenchyma formation: Programmed cell death in adventitious roots of winter wheat (Triticum aestivum) under waterlogging. Funct. Plant Biol. 2010, 37, 748–755. [Google Scholar] [CrossRef]
- Colmer, T.D. Long-distance transport of gases in plants: A perspective on internal aeration and radial oxygen loss from roots. Plant Cell Environ. 2003, 26, 17–36. [Google Scholar] [CrossRef]
- Yamauchi, T.; Tanaka, A.; Mori, H.; Takamure, I.; Kato, K.; Nakazono, M. Ethylene-dependent aerenchyma formation in adventitious roots is regulated differently in rice and maize. Plant Cell Environ. 2016, 39, 2145–2157. [Google Scholar] [CrossRef] [PubMed]
- Matsuura, A.; Kato, Y.; Suzuki, T.; Murata, K.; An, P. Hypoxia tolerance of four millet species is attributable to constitutive aerenchyma formation and root hair development of adventitious roots. Plant Prod. Sci. 2022, 25, 157–171. [Google Scholar] [CrossRef]
- Visvanathan, C.; Pokhrel, D.; Cheimchaisri, W.; Hettiaratchi, J.P.A.; Wu, J.S. Methanotrophic activities in tropical landfill cover soils: Effects of temperature, moisture content and methane concentration. Waste Manag. Res. 1999, 17, 313–323. [Google Scholar] [CrossRef]
- Ponnamperuma, F.N. The chemistry of submerged soils. Adv. Agron. 1972, 24, 29–96. [Google Scholar] [CrossRef]



| Parameters | Units | Topsoil | Residual Compost |
|---|---|---|---|
| Moisture content | % | 22.74 | 28.77 |
| Organic matter | % | 5.89 | 49.53 |
| Dry bulk density | (g/cm3) | 1.21 | 0.95 |
| Field Capacity | % | 45.06 | 164.94 |
| C/N | ratio | 15.30 | 10.57 |
| Column ID | Type of Vegetation | Natural Gas Exposure |
|---|---|---|
| NG-i | Native grass | + |
| NG-ii | Native grass | + |
| NG-iii | Native grass | + |
| AA | Alfalfa | + |
| JM | Japanese Millet | + |
| NG-control | Native grass | − |
| AA-control | Alfalfa | − |
| JM-control | Japanese Millet | − |
| Loading Rate (g/m2/d) | Oxidation Rate (gCH4/m2/d) | Oxidation Efficiency (%) | ||||
|---|---|---|---|---|---|---|
| NG (Average of i, ii and iii) | AA | JM | NG | AA | JM | |
| 75.1 | 20.0 | 15.4 | 27.5 | 26.6 | 20.5 | 36.6 |
| 173.1 | 73.2 | 68.4 | 76.1 | 42.3 | 39.5 | 43.9 |
| 285.1 | 134.8 | 132.3 | 135.4 | 47.3 | 46.4 | 47.5 |
| 437.9 | 153.2 | 137.5 | 165.5 | 35 | 31.4 | 37.8 |
| 635.1 | 222.1 | 186.1 | 250.2 | 34.9 | 29.3 | 39.4 |
| 845.2 | 222.5 | 189.3 | 246.8 | 26.3 | 22.4 | 29.2 |
| Kinetic Parameter | NG | AA | JM | NG-con | AA-con | JM-con |
|---|---|---|---|---|---|---|
| Vmax (µmol/g dw/h) | 3.97 | 2.92 | 6.64 | 0.22 | 0.24 | 0.51 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Attalage, D.S.; Hettiaratchi, J.P.A.; Chu, A.; Pokhrel, D.; Jayasinghe, P.A. Impact of Landfill Gas Exposure on Vegetation in Engineered Landfill Biocover Systems Implemented to Minimize Fugitive Methane Emissions from Landfills. Int. J. Environ. Res. Public Health 2023, 20, 4448. https://doi.org/10.3390/ijerph20054448
Attalage DS, Hettiaratchi JPA, Chu A, Pokhrel D, Jayasinghe PA. Impact of Landfill Gas Exposure on Vegetation in Engineered Landfill Biocover Systems Implemented to Minimize Fugitive Methane Emissions from Landfills. International Journal of Environmental Research and Public Health. 2023; 20(5):4448. https://doi.org/10.3390/ijerph20054448
Chicago/Turabian StyleAttalage, Dinu S., J. Patrick A. Hettiaratchi, Angus Chu, Dinesh Pokhrel, and Poornima A. Jayasinghe. 2023. "Impact of Landfill Gas Exposure on Vegetation in Engineered Landfill Biocover Systems Implemented to Minimize Fugitive Methane Emissions from Landfills" International Journal of Environmental Research and Public Health 20, no. 5: 4448. https://doi.org/10.3390/ijerph20054448
APA StyleAttalage, D. S., Hettiaratchi, J. P. A., Chu, A., Pokhrel, D., & Jayasinghe, P. A. (2023). Impact of Landfill Gas Exposure on Vegetation in Engineered Landfill Biocover Systems Implemented to Minimize Fugitive Methane Emissions from Landfills. International Journal of Environmental Research and Public Health, 20(5), 4448. https://doi.org/10.3390/ijerph20054448







