Effects of PM2.5 Exposure on the ACE/ACE2 Pathway: Possible Implication in COVID-19 Pandemic
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. PM Sources and Characterization
2.3. Dose
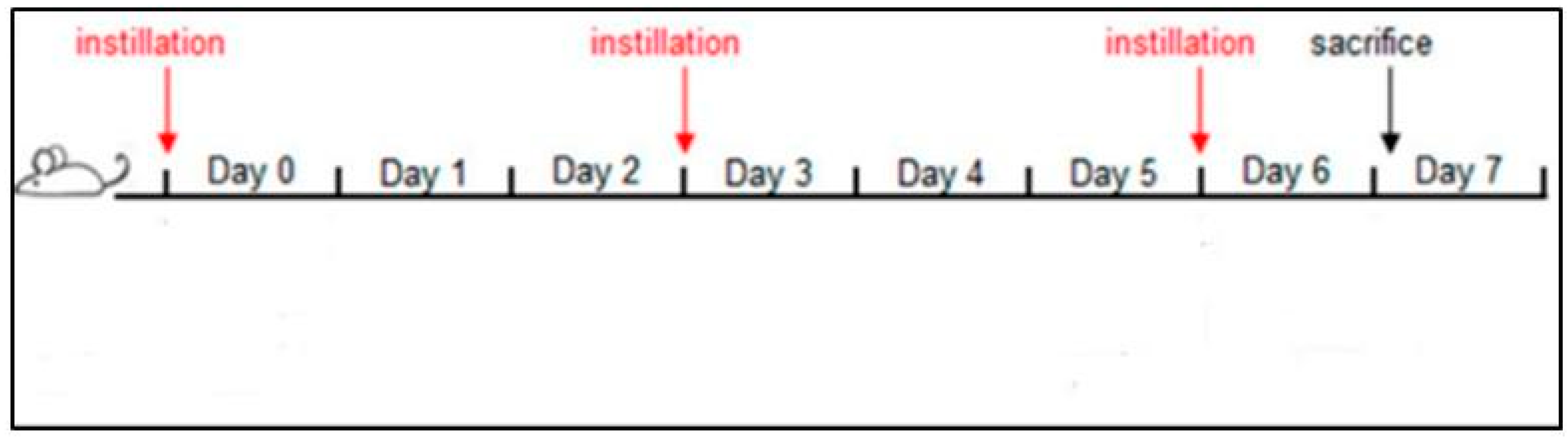
2.4. Intratracheal PM2.5 Instillation
2.5. Organ Homogenization
2.6. Electrophoresis and Immunoblotting
2.7. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mishra, R.; Krishnamoorthy, P.; Gangamma, S.; Raut, A.A.; Kumar, H. Particulate matter (PM10) enhances RNA virus infection through modulation of innate immune responses. Environ. Pollut. 2020, 266, 115148. [Google Scholar] [CrossRef] [PubMed]
- Deng, Q.; Deng, L.; Miao, Y.; Guo, X.; Li, Y. Particle deposition in the human lung: Health implications of particulate matter from different sources. Environ. Res. 2019, 169, 237–245. [Google Scholar] [CrossRef] [PubMed]
- Idani, E.; Geravandi, S.; Akhzari, M.; Goudarzi, G.; Alavi, N.; Yari, A.R.; Mehrpour, M.; Khavasi, M.; Bahmaei, J.; Bostan, H.; et al. Characteristics, sources, and health risks of atmospheric PM10-bound heavy metals in a populated middle eastern city. Toxin Rev. 2020, 39, 266–274. [Google Scholar] [CrossRef]
- Brook, R.D.; Franklin, B.; Cascio, W.; Hong, Y.; Howard, G.; Howard, G.; Lipsett, M.; Luepker, R.; Mittleman, M.; Samet, J.; et al. Expert Panel on Population and Prevention Science of the American Heart Association. Air pollution and cardiovascular disease: A statement for healthcare professionals from the expert panel on population and prevention science of the American heart association. Circulation 2004, 109, 2655–2671. [Google Scholar] [CrossRef] [PubMed]
- Ito, T.; Suzuki, T.; Tamura, K.; Nezu, T.; Honda, K.; Kobayashi, T. Examination of mRNA expression in rat hearts and lungs for analyses of effects of exposure to concentrated ambient particles on cardiovascular function. Toxicology 2008, 243, 271–283. [Google Scholar] [CrossRef] [PubMed]
- Gualtieri, M.; Mantecca, P.; Corvaja, V.; Longhin, E.; Perrone, M.G.; Bolzacchini, E.; Camatini, M. Winter fine particulate matter from Milan induces morphological and functional alterations in human pulmonary epithelial cells (A549). Toxicol. Lett. 2009, 188, 52–62. [Google Scholar] [CrossRef] [PubMed]
- Happo, M.S.; Salonen, R.O.; Hälinen, A.I.; Jalava, P.I.; Pennanen, A.S.; Dormans, J.A.M.A.; Gerlofs-Nijland, M.E.; Cassee, F.R.; Kosma, V.-M.; Sillanpää, M.; et al. Inflammation and tissue damage in mouse lung by single and repeated dosing of urban air coarse and fine particles collected from six European cities. Inhal. Toxicol. 2010, 22, 402–416. [Google Scholar] [CrossRef] [PubMed]
- Perrone, M.G.; Gualtieri, M.; Ferrero, L.; Lo Porto, C.; Udisti, R.; Bolzacchini, E.; Camatini, M. Seasonal variations in chemical composition and in vitro biological effects of fine PM from Milan. Chemosphere 2010, 78, 1368–1377. [Google Scholar] [CrossRef]
- Mossman, B.T.; Borm, P.J.; Castranova, V.; Costa, D.L.; Donaldson, K.; Kleeberger, S.R. Mechanisms of action of inhaled fibers, particles and nanoparticles in lung and cardiovascular diseases. Part. Fibre Toxicol. 2007, 4, 4–10. [Google Scholar] [CrossRef]
- Cao, Y.; Chen, M.; Dong, D.; Xie, S.; Liu, M. Environmental pollutants damage airway epithelial cell cilia: Implications for the prevention of obstructive lung diseases. Thorac. Cancer 2020, 11, 505–510. [Google Scholar] [CrossRef] [PubMed]
- Conticini, E.; Frediani, B.; Caro, D. Can atmospheric pollution be considered a co-factor in extremely high level of SARS-CoV-2 lethality in Northern Italy? Environ. Pollut. 2020, 261, 114465. [Google Scholar] [CrossRef] [PubMed]
- Lu, R.; Zhao, X.; Li, J.; Niu, P.; Yang, B.; Wu, H.; Wang, W.; Song, H.; Huang, B.; Zhu, N.; et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: Implications for virus origins and receptor binding. Lancet 2020, 395, 565–574. [Google Scholar] [CrossRef] [PubMed]
- Marquès, M.; Domingo, J.L. Positive association between outdoor air pollution and the incidence and severity of COVID-19. A review of the recent scientific evidences. Environ. Res. 2021, 203, 111930. [Google Scholar] [CrossRef]
- Gulletta, E.; Foti, D.P.; Galliera, E.; Corsi, M.M. Caleidoscopio Italiano. Citochine e Chemochine; Medical Systems SpA: Genova, Italy, 2008; Volume 217, Available online: www.medicalsystems.it (accessed on 15 May 2020).
- Pan, T.; Xiao, Z.H. Expression of P38 MAPK and MMP-2 mRNA in neonatal rats with hyperoxia-induced lung injury. Chin. J. Contemp. Pediatr. 2013, 15, 383–386. [Google Scholar]
- Gent, J.F.; Triche, E.W.; Holford, T.R.; Belanger, K.; Bracken, M.B.; Beckett, W.S.; Leaderer, B.P. Association of low-level ozone and fine particles with respiratory symptoms in children with asthma. JAMA 2003, 290, 1859–1867. [Google Scholar] [CrossRef]
- Gong, H.J.; Linn, W.S.; Clark, K.W.; Anderson, K.R.; Geller, M.D.; Sioutas, C. Respiratory responses to exposures with fine particulates and nitrogen dioxide in the elderly with and without COPD. Inhal. Toxicol. 2005, 17, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Tong, Y.; Zhang, G.; Li, Y.; Tan, M.; Wang, W.; Chen, J.; Hwu, Y.; Hsu, P.-C.; Je, J.H.; Margaritondo, G.; et al. Synchrotron microradiography study on acute lung injury of mouse caused by PM (2.5) aerosols. Eur. J. Radiol. 2006, 58, 266–272. [Google Scholar] [CrossRef]
- Lin, C.I.; Tsai, C.H.; Sun, Y.L.; Hsieh, W.-J.; Lin, Y.-C.; Chen, C.-J.; Lin, C.-S. Instillation of particulate matter 2.5 induced acute lung injury and attenuated the injury recovery in ACE2 knockout mice. Int. J. Biol. Sci. 2018, 14, 253–265. [Google Scholar] [CrossRef]
- Marshall, R.P. The pulmonary renin-angiotensin system. Curr. Pharm. Des. 2003, 9, 715–722. [Google Scholar] [CrossRef] [PubMed]
- Hung, Y.H.; Hsieh, W.Y.; Hsieh, J.S.; Liu, F.C.; Tsai, C.H.; Lu, L.C.; Huang, C.Y.; Wu, C.L.; Lin, C.S. Alternative Roles of STAT3 and MAPK Signaling Pathways in the MMPs Activation and Progression of Lung Injury Induced by Cigarette Smoke Exposure in ACE2 Knockout Mice. Int. J. Biol. Sci. 2016, 12, 454–465. [Google Scholar] [CrossRef]
- Parajuli, N.; Ramprasath, T.; Patel, V.B.; Wang, W.; Putko, B.; Mori, J.; Oudit, G.Y. Targeting angiotensin-converting enzyme 2 as a new therapeutic target for cardiovascular diseases. Can. J. Physiol. Pharmacol. 2014, 92, 558–565. [Google Scholar] [CrossRef]
- Lee, Y.B.; Nagai, A.; Kim, S.U. Cytokines, chemokines and cytokine receptors in human microglia. J. Neurosci. Res. 2002, 69, 94–103. [Google Scholar] [CrossRef]
- Dagenais, N.J.; Jamali, F. Protective effects of angiotensin II interruption: Evidence for antiinflammatory actions. Pharmacotherapy 2005, 25, 1213–1229. [Google Scholar] [CrossRef]
- Patel, V.B.; Basu, R.; Oudit, G.Y. ACE2/Ang 1-7 axis: A critical regulator of epicardial adipose tissue inflammation and cardiac dysfunction in obesity. Adipocyte 2016, 5, 306–311. [Google Scholar] [CrossRef] [PubMed]
- Lan, J.; Ge, J.; Yu, J.; Shan, S.; Zhou, H.; Fan, S.; Zhang, Q.; Shi, X.; Wang, Q.; Zhang, L. Structure of the SARS-CoV-2 spike receptor-binding domain bound to the ACE2 receptor. Nature 2020, 581, 215–220. [Google Scholar] [CrossRef] [PubMed]
- South, A.M.; Diz, D.; Chappell, M.C. COVID-19, ACE2 and the Cardiovascular Consequences. Am. J. Physiol. Heart Circ. Physiol. 2020, 1318, H1084–H1090. [Google Scholar] [CrossRef]
- Vaduganathan, M.; Vardeny, O.; Michel, T.; McMurray, J.J.; Pfeffer, M.A.; Solomon, S.D. Renin–Angiotensin–Aldosterone System Inhibitors in Patients with COVID-19. N. Engl. J. Med. 2020, 382, 1653–1659. [Google Scholar] [CrossRef] [PubMed]
- Bm, H. COVID-19 induced Renin-Angiotensin System (RAS) imbalance may drive acute lung injury: The evidence and therapeutic options. BMJ 2020, 368, m406. [Google Scholar]
- Gheblawi, M.; Wang, K.; Viveiros, A.; Nguyen, Q.; Zhong, J.-C.; Turner, A.J.; Raizada, M.K.; Grant, M.B.; Oudit, G.Y. Angiotensin Converting Enzyme 2: SARSCoV-2 Receptor and Regulator of the Renin-Angiotensin System. Circ. Res. 2020, 126, 1456–1474. [Google Scholar] [CrossRef]
- Mahmudpour, M.; Roozbeh, J.; Keshavarz, M.; Farrokhi, S.; Nabipour, I. COVID-19 cytokine storm: The anger of inflammation. Cytokine 2020, 133, 155151. [Google Scholar] [CrossRef] [PubMed]
- Farina, F.; Lonati, E.; Milani, C.; Massimino, L.; Ballarini, E.; Donzelli, E.; Crippa, L.; Marmiroli, P.; Botto, L.; Corsetto, P.A.; et al. In Vivo Comparative Study on Acute and Sub-acute Biological Effects Induced by Ultrafine Particles of Different Anthropogenic Sources in BALB/c Mice. Int. J. Mol. Sci. 2019, 20, 2805. [Google Scholar] [CrossRef]
- Milani, C.; Farina, F.; Botto, L.; Massimino, L.; Lonati, E.; Donzelli, E.; Ballarini, E.; Crippa, L.; Marmiroli, P.; Bulbarelli, A.; et al. Systemic Exposure to Air Pollution Induces Oxidative Stress and Inflammation in Mouse Brain, Contributing to Neurodegeneration Onset. Int. J. Mol. Sci. 2020, 21, 3699. [Google Scholar] [CrossRef] [PubMed]
- Mehta, P.; McAuley, D.F.; Brown, M.; Sanchez, E.; Tattersall, R.S.; Manson, J.J.; HLH Across Speciality Collaboration, UK. COVID-19: Consider cytokine storm syndromes and immunosuppression. Lancet 2020, 395, 1033–1034. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Wu, D.; Chen, H.; Yan, W.; Yang, D.; Chen, G.; Ma, K.; Xu, D.; Yu, H.; Wang, H.; et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: Retrospective study. BMJ 2019, 368, m1091. [Google Scholar] [CrossRef]
- Arif, H.; Aggarwal, S. Salicylic Acid (Aspirin). In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2020. [Google Scholar]
- Grosser, N.; Abate, A.; Oberle, S.; Vreman, H.J.; Dennery, P.A.; Becker, J.C.; Pohle, T.; Seidman, D.S.; Schröder, H. Heme oxygenase-1 induction may explain the antioxidant profile of aspirin. Biochem. Biophys. Res. Commun. 2003, 308, 956–960. [Google Scholar] [CrossRef] [PubMed]
- Taubert, D.; Berkels, R.; Grosser, N.; Schröder, H.; Gründemann, D.; Schömig, E. Aspirin induces nitric oxide release from vascular endothelium: A novel mechanism of action. Br. J. Pharmacol. 2004, 143, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Perrone, M.G.; Larsen, B.R.; Ferrero, L.; Sangiorgi, G.; De Gennaro, G.; Udisti, R.; Zangrando, R.; Gambaro, A.; Bolzacchini, E. Sources of high PM2.5 concentrations in Milan, Northern Italy: Molecular marker data and CMB modelling. Sci. Total. Environ. 2012, 414, 343–355. [Google Scholar] [CrossRef]
- Sancini, G.; Farina, F.; Battaglia, C.; Cifola, I.; Mangano, E.; Mantecca, P.; Camatini, M.; Palestini, P. Health Risk Assessment for Air Pollutants: Alterations in Lung and Cardiac Gene Expression in Mice Exposed to Milano Winter Fine Particulate Matter (PM2.5). PLoS ONE 2014, 9, e109685. [Google Scholar] [CrossRef]
- Farina, F.; Sancini, G.; Battaglia, C.; Tinaglia, V.; Mantecca, P.; Camatini, M.; Palestini, P. Milano summer particulate matter (PM10) triggers lung inflammation and extra pulmonary adverse events in mice. PLoS ONE 2013, 8, e56636. [Google Scholar] [CrossRef]
- Mantecca, P.; Sancini, G.; Moschini, E.; Farina, F.; Gualtieri, M.; Rohr, A.; Miserocchi, G.; Palestini, P.; Camatini, M. Lung toxicity induced by intratracheal instillation of size-fractionated tire particles. Toxicol. Lett. 2009, 189, 206–214. [Google Scholar] [CrossRef]
- Mantecca, P.; Farina, F.; Moschini, E.; Gallinotti, D.; Gualtieri, M.; Rohr, A.; Sancini, G.; Palestini, P.; Camatini, M. Comparative acute lung inflammation induced by atmospheric PM and sizefractionated tire particles. Toxicol. Lett. 2010, 198, 244–254. [Google Scholar] [CrossRef] [PubMed]
- Farina, F.; Sancini, G.; Mantecca, P.; Gallinotti, D.; Camatini, M.; Palestini, P. The acute toxic effects of particulate matter in mouse lung are related to size and season of collection. Toxicol. Lett. 2011, 202, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Shen, Y. An old method facing a new challenge: Re-visiting housekeeping proteins as internal reference control for neuroscience research. Life Sci. 2013, 92, 747–751. [Google Scholar] [CrossRef] [PubMed]
- Moritz, C.P. Tubulin or Not Tubulin: Heading Toward Total Protein Staining as Loading Control in Western Blots. Proteomics 2017, 17, 1600189. [Google Scholar] [CrossRef] [PubMed]
- Daffara, R.; Botto, L.; Beretta, E.; Conforti, E.; Faini, A.; Palestini, P.; Miserocchi, G. Endothelial cells as early sensors of pulmonary interstitial edema. J. Appl. Physiol. 2004, 97, 1575–1583. [Google Scholar] [CrossRef] [PubMed]
- Zhu, P.; Zhang, W.; Feng, F.; Qin, L.; Ji, W.; Li, D.; Liang, R.; Zhang, Y.; Wang, Y.; Li, M.; et al. Role of angiotensin-converting enzyme 2 in fine particulate matter-induced acute lung injury. Sci. Total Environ. 2022, 825, 153964. [Google Scholar] [CrossRef]
- Wrapp, D.; Wang, N.; Corbett, K.S.; Goldsmith, J.A.; Hsieh, C.L.; Abiona, O.; Graham, B.S.; McLellan, J.S. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science 2020, 367, 1260–1263. [Google Scholar] [CrossRef]
- Khan, Z.; Ualiyeva, D.; Khan, A.; Zaman, N.; Sapkota, S.; Khan, A.; Ali, B.; Ghafoor, D. A Correlation among the COVID-19 Spread, Particulate Matters, and Angiotensin-Converting Enzyme 2: A Review. J. Environ. Public Health 2021, 2021, 5524098. [Google Scholar] [CrossRef]
- Paital, B.; Agrawal, P.K. Air pollution by NO2 and PM2.5 explains COVID-19 infection severity by overexpression of angiotensin-converting enzyme 2 in respiratory cells: A review. Environ. Chem. Lett. 2021, 19, 25–42. [Google Scholar] [CrossRef]
- Passos, F.R.S.; Heimfarth, L.; Monteiro, B.S.; Corrêa, C.B.; Moura, T.R.; Araújo, A.A.S.; Martins-Filho, P.R.; Quintans-Júnior, L.J.; Quintans, J.S.S. Oxidative stress and inflammatory markers in patients with COVID-19: Potential role of RAGE, HMGB1, GFAP and COX-2 in disease severity. Int. Immunopharmacol. 2022, 104, 108502. [Google Scholar] [CrossRef] [PubMed]
- Fakhouri, E.W.; Peterson, S.J.; Kothari, J.; Alex, R.; Shapiro, J.I.; Abraham, N.G. Genetic Polymorphisms Complicate COVID-19 Therapy: Pivotal Role of HO-1 in Cytokine Storm. Antioxidants 2020, 9, 636. [Google Scholar] [CrossRef] [PubMed]
- Vassiliou, A.G.; Zacharis, A.; Keskinidou, C.; Jahaj, E.; Pratikaki, M.; Gallos, P.; Dimopoulou, I.; Kotanidou, A.; Orfanos, S.E. Soluble Angiotensin Converting Enzyme 2 (ACE2) Is Upregu-lated and Soluble Endothelial Nitric Oxide Synthase (eNOS) is Downregulated in COVID-19-induced Acute Respiratory Distress Syndrome (ARDS). Pharmaceuticals 2021, 14, 695. [Google Scholar] [CrossRef] [PubMed]
- Santos, R.A.S.; Sampaio, W.O.; Alzamora, A.C.; Motta-Santos, D.; Alenina, N.; Bader, M.; Campagnole-Santos, M.J. The ACE2/Angiotensin-(1–7)/MAS Axis of the Renin-Angiotensin System: Focus on Angiotensin-(1–7). Physiol. Rev. 2018, 98, 505–553. [Google Scholar] [CrossRef]
- Akboga, S.A.; Gokce, A.; Hatipoglu, M.; Beyoglu, M.A.; Inan, K.; Sezen, A.; Cankar Dal, H.; Akkas, Y.; Turan, S.; Kocer, B. The relationship between mortality and inflammatory markers and the systemic immune inflammatory index in patients in the intensive care unit with a pneumothorax as a complication of COVID-19 disease. Ir. J. Med. Sci. 2021, 191, 1931–1936. [Google Scholar] [CrossRef] [PubMed]
- Çakırca, G.; Damar Çakırca, T.; Üstünel, M.; Torun, A.; Koyuncu, I. Thiol level and total oxidant/antioxidant status in patients with COVID-19 infection. Ir. J. Med. Sci. 2021, 191, 1925–1930. [Google Scholar] [CrossRef] [PubMed]
- Hammock, B.D.; Wang, W.; Gilligan, M.M.; Panigrahy, D. Eicosanoids: The Overlooked Storm in Coronavirus Disease 2019 (COVID-19)? Am. J. Pathol. 2020, 190, 1782–1788. [Google Scholar] [CrossRef]
- Ferreira, A.J.; Santos, R.A.; Almeida, A.P. Angiotensin-(1-7): Cardioprotective effect in myocardial ischemia/reperfusion. Hypertension 2001, 38, 665–668. [Google Scholar] [CrossRef]
- Lubel, J.S.; Herath, C.B.; Burrell, L.M.; Angus, P.W. Liver disease and the renin-angiotensin system: Recent discoveries and clinical implications. J. Gastroenterol. Hepatol. 2008, 23, 1327–1338. [Google Scholar] [CrossRef]
- Doobay, M.F.; Talman, L.S.; Obr, T.D.; Tian, X.; Davisson, R.L.; Lazartigues, E. Differential expression of neuronal ACE2 in transgenic mice with overexpression of the brain renin-angiotensin system. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007, 292, R373–R381. [Google Scholar] [CrossRef]
- Bianconi, V.; Violi, F.; Fallarino, F.; Pignatelli, P.; Sahebkar, A.; Pirro, M. Is Acetylsalicylic Acid a Safe and Potentially Useful Choice for Adult Patients with COVID-19? Drugs 2020, 80, 1383–1396. [Google Scholar] [CrossRef]
- Exner, M.; Minar, E.; Wagner, O.; Schillinger, M. The role of heme oxygenase-1 promoter polymorphisms in human disease. Free Radic. Biol. Med. 2004, 37, 1097–1104. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Huang, Y.; Quan, J.; Liu, J.; Wang, H.; Billiar, T.R.; Lotze, M.T.; Zeh, H.J.; Kang, R.; Tang, D. HMGB1 as a potential biomarker and therapeutic target for severe COVID-19. Heliyon 2020, 6, e05672. [Google Scholar] [CrossRef] [PubMed]

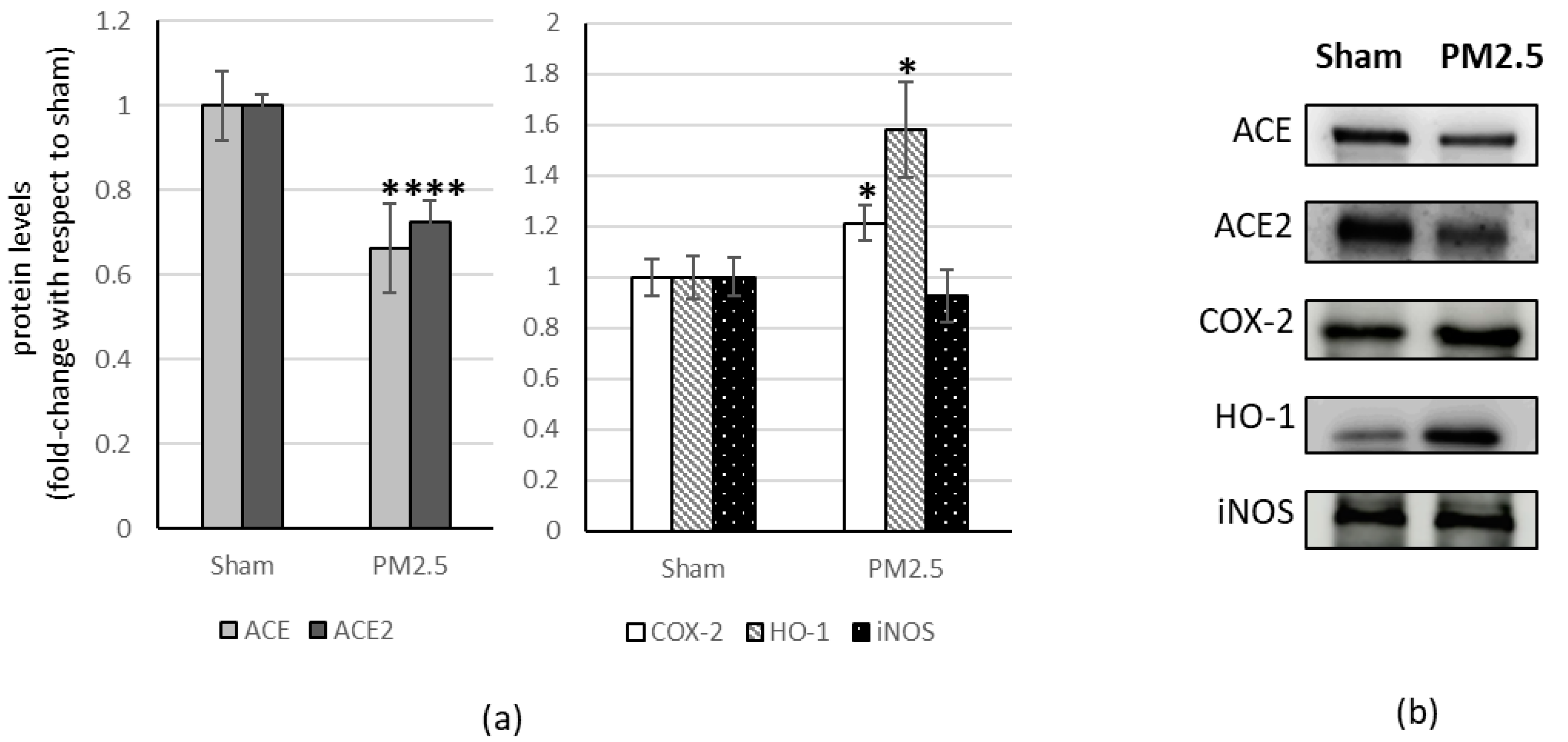
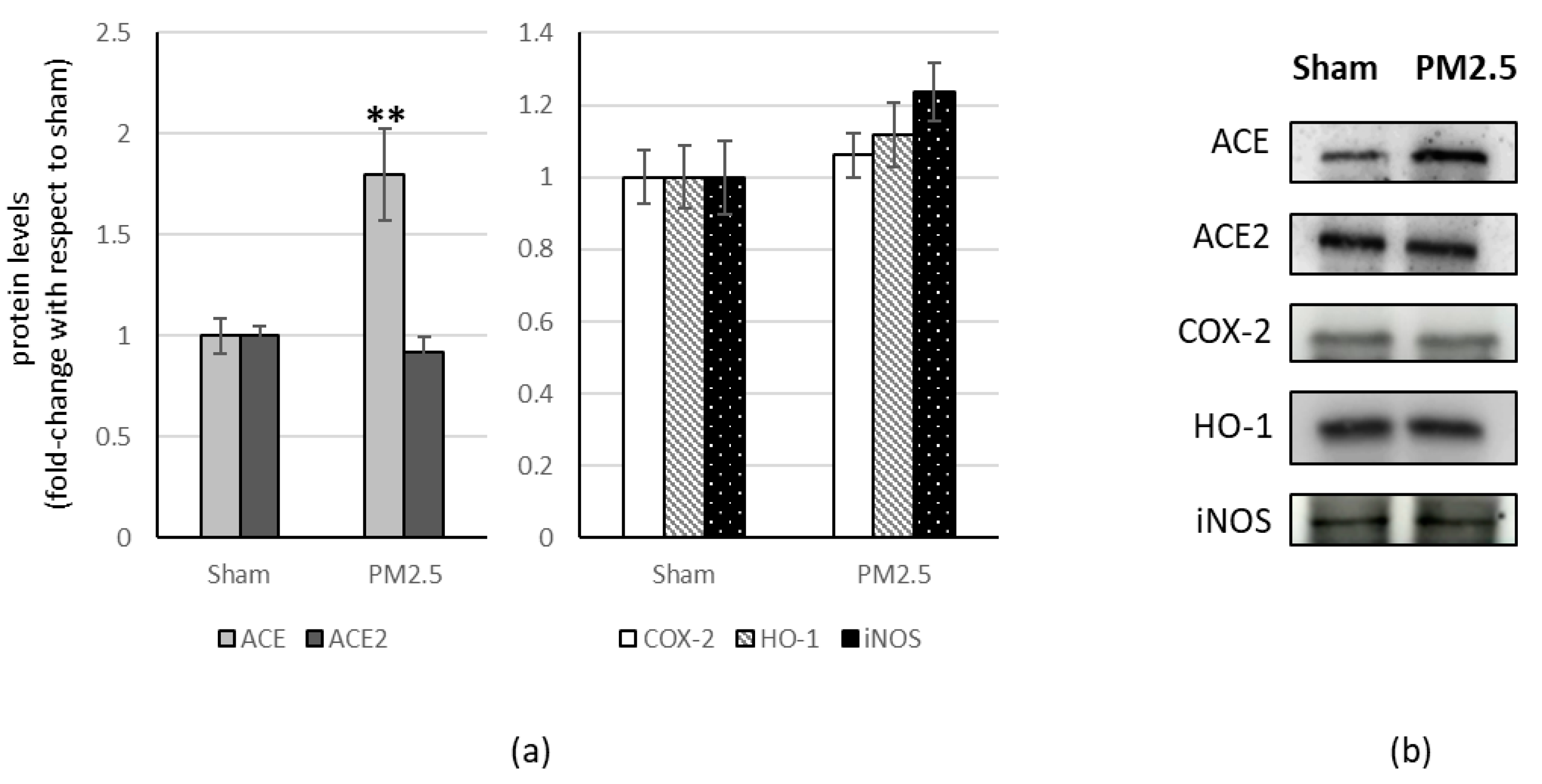

| Organ | ACE/ACE2 | ±S.E. | p |
|---|---|---|---|
| lung | 0.82 | ±0.087 | |
| heart | 0.90 | ±0.143 | |
| liver | 1.83 | ±0.187 | *** |
| brain | 1.40 | ±0.127 | * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Botto, L.; Lonati, E.; Russo, S.; Cazzaniga, E.; Bulbarelli, A.; Palestini, P. Effects of PM2.5 Exposure on the ACE/ACE2 Pathway: Possible Implication in COVID-19 Pandemic. Int. J. Environ. Res. Public Health 2023, 20, 4393. https://doi.org/10.3390/ijerph20054393
Botto L, Lonati E, Russo S, Cazzaniga E, Bulbarelli A, Palestini P. Effects of PM2.5 Exposure on the ACE/ACE2 Pathway: Possible Implication in COVID-19 Pandemic. International Journal of Environmental Research and Public Health. 2023; 20(5):4393. https://doi.org/10.3390/ijerph20054393
Chicago/Turabian StyleBotto, Laura, Elena Lonati, Stefania Russo, Emanuela Cazzaniga, Alessandra Bulbarelli, and Paola Palestini. 2023. "Effects of PM2.5 Exposure on the ACE/ACE2 Pathway: Possible Implication in COVID-19 Pandemic" International Journal of Environmental Research and Public Health 20, no. 5: 4393. https://doi.org/10.3390/ijerph20054393
APA StyleBotto, L., Lonati, E., Russo, S., Cazzaniga, E., Bulbarelli, A., & Palestini, P. (2023). Effects of PM2.5 Exposure on the ACE/ACE2 Pathway: Possible Implication in COVID-19 Pandemic. International Journal of Environmental Research and Public Health, 20(5), 4393. https://doi.org/10.3390/ijerph20054393






