Metal–Organic Framework (MOF) Derivatives as Promising Chemiresistive Gas Sensing Materials: A Review
Abstract
1. Introduction
2. Sensing Principles of Chemiresistive Gas Sensors
3. Chemiresistive Gas Sensors Using MOF Derivatives
3.1. NO2 Sensors
3.2. Acetone Sensors
3.3. Ethanol Sensors
3.4. H2S Sensors
3.5. Other Gas Sensors
4. Conclusions and Future Perspectives
- (1).
- The MOF derivatives should successfully maintain or inherit the original high porosity and redox activity of pristine MOFs during the high-temperature pyrolysis process so that they can achieve excellent sensitivity and response as a chemiresistive gas sensor;
- (2).
- The sophisticated morphologies and precisely tailored physicochemical properties of the MOF derivatives need to be constructed and established by thermochemical or other methods, avoiding from the unfavorable Ostwald ripening process, in order to increase the active surface areas affording adsorption and catalysis reaction with gaseous molecules, and also the surface electron affinity to enhance their resistance changing signals;
- (3).
- Efficient charge transfer needs to be realized by the construction of a p–n junction and other heterojunction interfaces so that rapid response and recovery times are available for the chemiresistive gas sensors;
- (4).
- The reproducibility and cost control for the preparation of MOF derivatives and the as-resulted chemiresistive sensing devices are still far from satisfactory;
- (5).
- The realization of self-powered, minimized and potable gas sensing devices based on MOF derivatives is another indispensable future research direction, especially with the rapid development of 5G wireless networks currently taking place;
- (6).
- Finally, the rapid and efficient data transmission and establishment of the gas sensors-based IoT system, which are of great significance to safer and cleaner production by avoiding the leakage of toxic, harmful, flammable and explosive gases like methane leakage during the exploitation of oil and natural gas, also require the further utilization and optimization of the MOF derivatives-based chemiresistive gas sensors.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mor, S.; Kumar, S.; Singh, T.; Dogra, S.; Pandey, V.; Ravindra, K. Impact of COVID-19 lockdown on air quality in Chandigarh, India: Understanding the emission sources during controlled anthropogenic activities. Chemosphere 2021, 263, 127978. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Wen, M.; Li, G.; An, T. Recent advances in VOC elimination by catalytic oxidation technology onto various nanoparticles catalysts: A critical review. Appl. Catal. B Environ. 2021, 281, 119447. [Google Scholar] [CrossRef]
- Zhang, L.; Liang, J.; Wang, Y.; Mou, T.; Lin, Y.; Yue, L.; Li, T.; Liu, Q.; Luo, Y.; Li, N.; et al. High-performance electrochemical NO reduction into NH3 by MoS2 nanosheet. Angew. Chem. Int. Ed. 2021, 133, 25467–25472. [Google Scholar] [CrossRef]
- Xu, C.; Zhao, W.; Zhang, M.; Cheng, B. Pollution haven or halo? The role of the energy transition in the impact of FDI on SO2 emissions. Sci. Total Environ. 2021, 763, 143002. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Wan, L.; Jian, Y.; Ren, C.; Jin, K.; Su, X.; Bai, X.; Haick, H.; Yao, M.; Wu, W. Electronic noses: From advanced materials to sensors aided with data processing. Adv. Mater. Technol. 2019, 4, 1800488. [Google Scholar] [CrossRef]
- Cao, Q.; Che, R.; Chen, N. Facile and rapid growth of Ag2S microrod arrays as efficient substrates for both SERS detection and photocatalytic degradation of organic dyes. Chem. Commun. 2014, 50, 4931–4933. [Google Scholar] [CrossRef]
- Zhang, D.; Fang, Z.; Wang, L.; Yu, H.; Lu, X.; Song, K.; Teng, J.; Yang, W. Controllable growth of single-crystalline zinc oxide nanosheets under ambient condition toward ammonia sensing with ultrahigh selectivity and sensitivity. J. Adv. Ceram. 2022, 11, 1187–1195. [Google Scholar] [CrossRef]
- Cao, Q.; Liu, X.; Yuan, K.; Yu, J.; Liu, Q.; Delaunay, J.J.; Che, R. Gold nanoparticles decorated Ag(Cl, Br) micro-necklaces for efficient and stable SERS detection and visible-light photocatalytic degradation of Sudan I. Appl. Catal. B Environ. 2017, 201, 607–616. [Google Scholar] [CrossRef]
- Cheng, Y.F.; Cao, Q.; Zhang, J.; Wu, T.; Che, R. Efficient photodegradation of dye pollutants using a novel plasmonic AgCl microrods array and photo-optimized surface-enhanced Raman scattering. Appl. Catal. B Environ. 2017, 217, 37–47. [Google Scholar] [CrossRef]
- Cao, Q.; Yuan, K.; Yu, J.; Delaunay, J.J.; Che, R. Ultrafast self-assembly of silver nanostructures on carbon-coated copper grids for surface-enhanced Raman scattering detection of trace melamine. J. Colloid Interface Sci. 2017, 490, 23–28. [Google Scholar] [CrossRef]
- Li, L.; Zou, J.; Han, Y.; Liao, Z.; Lu, P.; Nezamzadeh-Ejhieh, A.; Liu, J.; Peng, Y. Recent advances in Al(III)/In(III)-based MOFs for the detection of pollutants. New J. Chem. 2022, 46, 19577–19592. [Google Scholar] [CrossRef]
- Zhou, S.; Lu, L.; Liu, D.; Wang, J.; Sakiyama, H.; Muddassir, M.; Nezamzadeh-Ejhieh, A.; Liu, J. Series of highly stable Cd(II)-based MOFs as sensitive and selective sensors for detection of nitrofuran antibiotic. CrystEngComm 2021, 23, 8043–8052. [Google Scholar] [CrossRef]
- Kimmel, D.W.; LeBlanc, G.; Meschievitz, M.E.; Cliffel, D.E. Electrochemical sensors and biosensors. Anal. Chem. 2012, 84, 685–707. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.J.; Lu, P.; Culp, J.T.; Ohodnicki, P.R. Metal-organic framework thin film coated optical fiber sensors: A novel waveguide based chemical sensing platform. ACS Sens. 2018, 3, 386–394. [Google Scholar] [CrossRef]
- Surya, S.G.; Bhanoth, S.; Majhi, S.M.; More, Y.D.; Teja, V.M.; Chappanda, K.N. A silver nanoparticle-anchored UiO-66 (Zr) metal-organic framework (MOF)-based capacitive gas sensor. CrystEngComm 2019, 21, 7303–7312. [Google Scholar] [CrossRef]
- Cao, Q.; Yuan, K.; Liu, Q.; Liang, C.; Wang, X.; Cheng, Y.F.; Li, Q.; Wang, M.; Che, R. Porous Au–Ag alloy particles inlaid AgCl membranes as versatile plasmonic catalytic interfaces with simultaneous, in situ SERS monitoring. ACS Appl. Mater. Interfaces 2015, 7, 18491–18500. [Google Scholar] [CrossRef]
- Cao, Q.; Che, R. Tailoring Au–Ag–S composite microstructures in one-pot for both SERS detection and photocatalytic degradation of plasticizers DEHA and DEHP. ACS Appl. Mater. Interfaces 2014, 6, 7020–7027. [Google Scholar] [CrossRef]
- Yao, M.S.; Li, W.H.; Xu, G. Metal–organic frameworks and their derivatives for electrically-transduced gas sensors. Coord. Chem. Rev. 2021, 426, 213479. [Google Scholar] [CrossRef]
- Wang, X.F.; Song, X.Z.; Sun, K.M.; Cheng, L.; Ma, W. MOFs-derived porous nanomaterials for gas sensing. Polyhedron 2018, 152, 155–163. [Google Scholar] [CrossRef]
- Cao, Q.; Zhang, J.; Zhang, H.; Xu, J.; Che, R. Dual-surfactant templated hydrothermal synthesis of CoSe2 hierarchical microclews for dielectric microwave absorption. J. Adv. Ceram. 2022, 11, 504–514. [Google Scholar] [CrossRef]
- Cao, Q.; Hao, S.; Wu, Y.; Pei, K.; You, W.; Che, R. Interfacial charge redistribution in interconnected network of Ni2P–Co2P boosting electrocatalytic hydrogen evolution in both acidic and alkaline conditions. Chem. Eng. J. 2021, 424, 130444. [Google Scholar] [CrossRef]
- Cao, Q.; Yu, J.; Yuan, K.; Zhong, M.; Delaunay, J.J. Facile and large-area preparation of porous Ag3PO4 photoanodes for enhanced photoelectrochemical water oxidation. ACS Appl. Mater. Interfaces 2017, 9, 19507–19512. [Google Scholar] [CrossRef] [PubMed]
- Bian, Y.; Zhang, C.; Wang, H.; Cao, Q. Degradable nanofiber for eco-friendly air filtration: Progress and perspectives. Sep. Purif. Technol. 2023, 306, 122642. [Google Scholar] [CrossRef]
- Lee, J.; Choi, Y.; Park, B.J.; Han, J.W.; Lee, H.S.; Park, J.H.; Lee, W. Precise control of surface oxygen vacancies in ZnO nanoparticles for extremely high acetone sensing response. J. Adv. Ceram. 2022, 11, 769–783. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, C. Oxygen vacancy engineering on cerium oxide nanowires for room-temperature linalool detection in rice aging. J. Adv. Ceram. 2022, 11, 1559–1570. [Google Scholar] [CrossRef]
- Hao, S.; Liu, J.; Cao, Q.; Zhao, Y.; Zhao, X.; Pei, K.; Zhang, J.; Chen, G.; Che, R. In-situ electrochemical pretreatment of hierarchical Ni3S2-based electrocatalyst towards promoted hydrogen evolution reaction with low overpotential. J. Colloid Interface Sci. 2020, 559, 282–290. [Google Scholar] [CrossRef]
- Yu, J.; Cao, Q.; Li, Y.; Long, X.; Yang, S.; Clark, J.K.; Nakabayashi, M.; Shibata, N.; Delaunay, J.J. Defect-rich NiCeOx electrocatalyst with ultrahigh stability and low overpotential for water oxidation. ACS Catal. 2019, 9, 1605–1611. [Google Scholar] [CrossRef]
- Yu, J.; Wang, J.; Long, X.; Chen, L.; Cao, Q.; Wang, J.; Qiu, C.; Lim, J.; Yang, S. Formation of FeOOH nanosheets induces substitutional doping of CeO2−x with high-valence Ni for efficient water oxidation. Adv. Energy Mater. 2021, 11, 2002731. [Google Scholar] [CrossRef]
- Yuan, K.; Cao, Q.; Lu, H.L.; Zhong, M.; Zheng, X.; Chen, H.Y.; Wang, T.; Delaunay, J.J.; Luo, W.; Zhang, L.; et al. Oxygen-deficient WO3−x@TiO2−x core-shell nanosheets for efficient photoelectrochemical oxidation of neutral water solutions. J. Mater. Chem. A 2017, 5, 14697–14706. [Google Scholar] [CrossRef]
- Zeng, X.; Zhao, Y.; Hu, X.; Stucky, G.D.; Moskovits, M. Rational component and structure design of noble-metal composites for optical and catalytic applications. Small Struct. 2021, 2, 2000138. [Google Scholar] [CrossRef]
- Wu, Y.; Pang, H.; Liu, Y.; Wang, X.; Yu, S.; Fu, D.; Chen, J.; Wang, X. Environmental remediation of heavy metal ions by novel-nanomaterials: A review. Environ. Pollut. 2019, 246, 608–620. [Google Scholar] [CrossRef] [PubMed]
- Tchinsa, A.; Hossain, M.F.; Wang, T.; Zhou, Y. Removal of organic pollutants from aqueous solution using metal organic frameworks (MOFs)-based adsorbents: A review. Chemosphere 2021, 284, 131393. [Google Scholar] [PubMed]
- Koo, W.T.; Jang, J.S.; Kim, I.D. Metal-organic frameworks for chemiresistive sensors. Chem 2019, 5, 1938–1963. [Google Scholar] [CrossRef]
- Zeng, X.; Nie, T.; Zhao, C.; Zhu, G.; Zhang, X.; Yu, R.; Stucky, G.D.; Che, R. Coupling between the 2D “ligand” and 2D “host” and their assembled hierarchical heterostructures for electromagnetic wave absorption. ACS Appl. Mater. Interfaces 2022, 14, 41235–41245. [Google Scholar] [CrossRef]
- Liu, C.; Bai, Y.; Li, W.; Yang, F.; Zhang, G.; Pang, H. In situ growth of three-dimensional MXene/metal–organic framework composites for high performance supercapacitors. Angew. Chem. Int. Ed. 2022, 61, e2021162822022. [Google Scholar]
- Qin, L.; Liang, F.; Li, Y.; Wu, J.; Guan, S.; Wu, M.; Xie, S.; Luo, M.; Ma, D. A 2D porous zinc-organic framework platform for loading of 5-fluorouracil. Inorganics 2022, 10, 202. [Google Scholar] [CrossRef]
- Qin, L.; Li, Y.; Liang, F.; Li, L.; Lan, Y.; Li, Z.; Lu, X.; Yang, M.; Ma, D. A microporous 2D cobalt-based MOF with pyridyl sites and open metal sites for selective adsorption of CO2. Microporous Mesoporous Mater. 2022, 341, 112098. [Google Scholar] [CrossRef]
- Dong, X.; Li, D.; Li, Y.; Sakiyama, H.; Muddassir, M.; Pan, Y.; Srivastava, D.; Kumar, A. A 3,8-connected Cd(II)-based metal-organic framework as an appropriate luminescent sensor for the antibiotic sulfasalazine. CrystEngComm 2022, 24, 7157–7165. [Google Scholar]
- Cao, Q.; Li, Q.; Pi, Z.; Zhang, J.; Sun, L.W.; Xu, J.; Cao, Y.; Cheng, J.; Bian, Y. Metal–organic-framework-derived ball-flower-like porous Co3O4/Fe2O3 heterostructure with enhanced visible-light-driven photocatalytic activity. Nanomaterials 2022, 12, 904. [Google Scholar] [CrossRef]
- Lee, C.S.; Li, H.Y.; Kim, B.Y.; Jo, Y.M.; Byun, H.G.; Hwang, I.S.; Abdel-Hady, F.; Wazzan, A.A.; Lee, J.H. Discriminative detection of indoor volatile organic compounds using a sensor array based on pure and Fe-doped In2O3 nanofibers. Sens. Actuators B Chem. 2019, 285, 193–200. [Google Scholar] [CrossRef]
- Wei, D.; Jiang, W.; Gao, H.; Chuai, X.; Liu, F.; Liu, F.; Sun, P.; Liang, X.; Gao, Y.; Yan, X.; et al. Facile synthesis of La-doped In2O3 hollow microspheres and enhanced hydrogen sulfide sensing characteristics. Sens. Actuators B Chem. 2018, 276, 413–420. [Google Scholar] [CrossRef]
- Shen, J.; Li, F.; Yin, B.; Sun, L.; Chen, C.; Wen, S.; Chen, Y.; Ruan, S. Enhanced ethyl acetate sensing performance of Al-doped In2O3 microcubes. Sens. Actuators B Chem. 2017, 253, 461–469. [Google Scholar] [CrossRef]
- Bai, S.; Guo, T.; Zhao, Y.; Sun, J.; Li, D.; Chen, A.; Liu, C.C. Sensing performance and mechanism of Fe-doped ZnO microflowers. Sens. Actuators B Chem. 2014, 195, 657–666. [Google Scholar] [CrossRef]
- Du, W.; Si, W.; Zhao, J.; Wang, F.; Han, Z.; Wang, Z.; Liu, W.; Lu, G.; Liu, J.; Wu, L. Mesoporous Fe-doped In2O3 nanorods derived from metal organic frameworks for enhanced nitrogen dioxide detection at low temperature. Ceram. Int. 2020, 46, 20385–20394. [Google Scholar] [CrossRef]
- Yu, J.; Cao, Q.; Qiu, C.; Chen, L.; Delaunay, J.J. Modulating Ni/Ce ratio in NiyCe100−yOx electrocatalysts for enhanced water oxidation. Nanomaterials 2021, 11, 437. [Google Scholar] [CrossRef]
- Cao, Q.; Yu, J.; Cao, Y.; Delaunay, J.J.; Che, R. Unusual effects of vacuum annealing on large-area Ag3PO4 microcrystalline film photoanode boosting cocatalyst- and scavenger-free water splitting. J. Mater. 2021, 7, 929–939. [Google Scholar] [CrossRef]
- Li, Q.; Huang, Z.; Guan, P.; Su, R.; Cao, Q.; Chao, Y.; Shen, W.; Guo, J.; Xu, H.; Che, R. Simultaneous Ni doping at atom scale in ceria and assembling into well-defined lotuslike structure for enhanced catalytic performance. ACS Appl. Mater. Interfaces 2017, 9, 16243–16251. [Google Scholar] [CrossRef]
- Zhang, Y.; Han, S.; Wang, M.; Liu, S.; Liu, G.; Meng, X.; Xu, Z.; Wang, M.; Qiao, G. Electrospun Cu-doped In2O3 hollow nanofibers with enhanced H2S gas sensing performance. J. Adv. Ceram. 2022, 11, 427–442. [Google Scholar] [CrossRef]
- Zhang, C.; Huan, Y.; Li, Y.; Luo, Y.; Debliquy, M. Low concentration isopropanol gas sensing properties of Ag nanoparticles decorated In2O3 hollow spheres. J. Adv. Ceram. 2022, 11, 379–391. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, K.; Zhang, X.; Wang, B.; Xu, X.; Du, X.; Yang, C.; Zhang, K. Interfacial energy barrier tuning of hierarchical Bi2O3/WO3 heterojunctions for advanced triethylamine sensor. J. Adv. Ceram. 2022, 11, 1860–1872. [Google Scholar] [CrossRef]
- Yuan, K.; Wang, C.Y.; Zhu, L.Y.; Cao, Q.; Yang, J.H.; Li, X.X.; Huang, W.; Wang, Y.Y.; Lu, H.L.; Zhang, D.W. Fabrication of a micro-electromechanical system-based acetone gas sensor using CeO2 nanodot-decorated WO3 nanowires. ACS Appl. Mater. Interfaces 2020, 12, 14095–14104. [Google Scholar] [CrossRef] [PubMed]
- Lonergan, M.; Severin, E.; Doleman, B.; Beaber, S.A.; Grubbs, R.H.; Lewis, N.S. Array-based vapor sensing using chemically sensitive carbon black-polymer resistors. Chem. Mater. 1996, 8, 2298–2312. [Google Scholar] [CrossRef]
- Spitalsky, Z.; Tasis, D.; Papagelis, K.; Galiotis, C. Carbon nanotube-polymer composites: Chemistry, processing, mechanical and electrical properties. Prog. Polym. Sci. 2010, 35, 357–401. [Google Scholar] [CrossRef]
- Yuan, K.; Zhu, L.Y.; Cao, Q.; Ma, H.P.; Tao, J.J.; Huang, W.; Lu, H.L. ALD-based hydrothermal facile synthesis of a dense WO3@TiO2-Fe2O3 nanodendrite array with enhanced photoelectrochemical properties. J. Mater. Chem C 2020, 8, 6756–6762. [Google Scholar] [CrossRef]
- Siahroudi, M.G.; Daryakenari, A.A.; Molamahaleh, Y.B.; Cao, Q.; Daryakenari, M.A.; Delaunay, J.J.; Siavoshi, H.; Molaei, F. Ethylene glycol assisted solvo-hydrothermal synthesis of NGr-Co3O4 nanostructures for ethanol electrooxidation. Int. J. Hydrogen Energy 2020, 45, 30357–30366. [Google Scholar] [CrossRef]
- Liu, Q.; Cao, Q.; Bi, H.; Liang, C.; Yuan, K.; She, W.; Yang, Y.; Che, R. CoNi@SiO2@TiO2 and CoNi@air@TiO2 microspheres with strong wideband microwave absorption. Adv. Mater. 2016, 28, 486–490. [Google Scholar] [CrossRef]
- Hao, S.; Cao, Q.; Yang, L.; Che, R. Morphology-optimized interconnected Ni3S2 nanosheets coupled with Ni(OH)2 nanoparticles for enhanced hydrogen evolution reaction. J. Alloys Compd. 2020, 827, 154163. [Google Scholar] [CrossRef]
- Yuan, K.; Cao, Q.; Li, X.; Chen, H.Y.; Deng, Y.; Wang, Y.Y.; Luo, W.; Lu, H.L.; Zhang, D.W. Synthesis of WO3@ZnWO4@ZnO-ZnO hierarchical nanocactus arrays for efficient photoelectrochemical water splitting. Nano Energy 2017, 41, 543–551. [Google Scholar] [CrossRef]
- Yin, Z.; Wan, S.; Yang, J.; Kurmoo, M.; Zeng, M.H. Recent advances in post-synthetic modification of metal-organic frameworks: New types and tandem reactions. Coord. Chem. Rev. 2019, 378, 500–512. [Google Scholar] [CrossRef]
- Agrawal, A.V.; Kumar, N.; Kumar, M. Strategy and future prospects to develop room-temperature recoverable NO2 gas sensor based on two-dimensional molybdenum sulfide. Nano-Micro Lett. 2021, 13, 38. [Google Scholar] [CrossRef]
- Bauwens, M.; Compernolle, S.; Stavrakou, T.; Müller, J.F.; van Gent, J.; Eskes, H.; Levelt, P.F.; van der A, R.; Veefkind, J.P.; Vlietinck, J.; et al. Impact of coronavirus outbreak on NO2 pollution assessed using TROPOMI and OMI observations. Geophys. Res. Lett. 2020, 47, e2020GL087978. [Google Scholar] [CrossRef] [PubMed]
- Muhammad, S.; Long, X.; Salman, M. COVID-19 pandemic and environmental pollution: A blessing in disguise? Sci. Total Environ. 2020, 728, 138820. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; Hussain, S.; Ge, C.; Wang, M.; Shah, S.; Liu, G.; Qiao, G. ZIF-67 MOF-derived unique double-shelled Co3O4/NiCo2O4 nanocages for superior gas-sensing performances. Sens. Actuators B Chem. 2020, 303, 127251. [Google Scholar] [CrossRef]
- Drobek, M.; Kim, J.H.; Bechelany, M.; Vallicari, C.; Julbe, A.; Kim, S.S. MOF-based membrane encapsulated ZnO nanowires for enhanced gas sensor selectivity. ACS Appl. Mater. Interfaces 2016, 8, 8323–8328. [Google Scholar] [CrossRef]
- Ren, X.; Xu, Z.; Liu, D.; Li, Y.; Zhang, Z.; Tang, Z. Conductometric NO2 gas sensors based on MOF-derived porous ZnO nanoparticles. Sens. Actuators B Chem. 2022, 357, 131384. [Google Scholar] [CrossRef]
- Volkringer, C.; Meddouri, M.; Loiseau, T.; Guillou, N.; Marrot, J.; Férey, G.; Haouas, M.; Taulelle, F.; Audebrand, N.; Latroche, M. The Kagomé topology of the gallium and indium metal-organic framework types with a MIL-68 structure: Synthesis, XRD, solid-state NMR characterizations, and hydrogen adsorption. Inorg. Chem. 2008, 47, 11892–11901. [Google Scholar] [CrossRef]
- Bag, A.; Kumar, M.; Moon, D.B.; Hanif, A.; Sultan, M.J.; Yoon, D.H.; Lee, N.E. A room-temperature operable and stretchable NO2 gas sensor composed of reduced graphene oxide anchored with MOF-derived ZnFe2O4 hollow octahedron. Sens. Actuators B Chem. 2021, 346, 130463. [Google Scholar] [CrossRef]
- Li, C.; Cao, Q.; Wang, F.; Xiao, Y.; Li, Y.; Delaunay, J.J.; Zhu, H. Engineering graphene and TMDs based van der Waals heterostructures for photovoltaic and photoelectrochemical solar energy conversion. Chem. Soc. Rev. 2018, 47, 4981–5037. [Google Scholar] [CrossRef]
- Liu, M.; Wang, Z.; Song, P.; Yang, Z.; Wang, Q. Flexible MXene/rGO/CuO hybrid aerogels for high performance acetone sensing at room temperature. Sens. Actuators B Chem. 2021, 340, 129946. [Google Scholar] [CrossRef]
- Parmar, B.; Bisht, K.K.; Rachuri, Y.; Suresh, E. Zn(II)/Cd(II) based mixed ligand coordination polymers as fluorosensors for aqueous phase detection of hazardous pollutants. Inorg. Chem. Front. 2020, 7, 1082–1107. [Google Scholar] [CrossRef]
- Ma, X.; Zhou, X.; Gong, Y.; Han, N.; Liu, H.; Chen, Y. MOF-derived hierarchical ZnO/ZnFe2O4 hollow cubes for enhanced acetone gas-sensing performance. RSC Adv. 2017, 7, 34609–34617. [Google Scholar] [CrossRef]
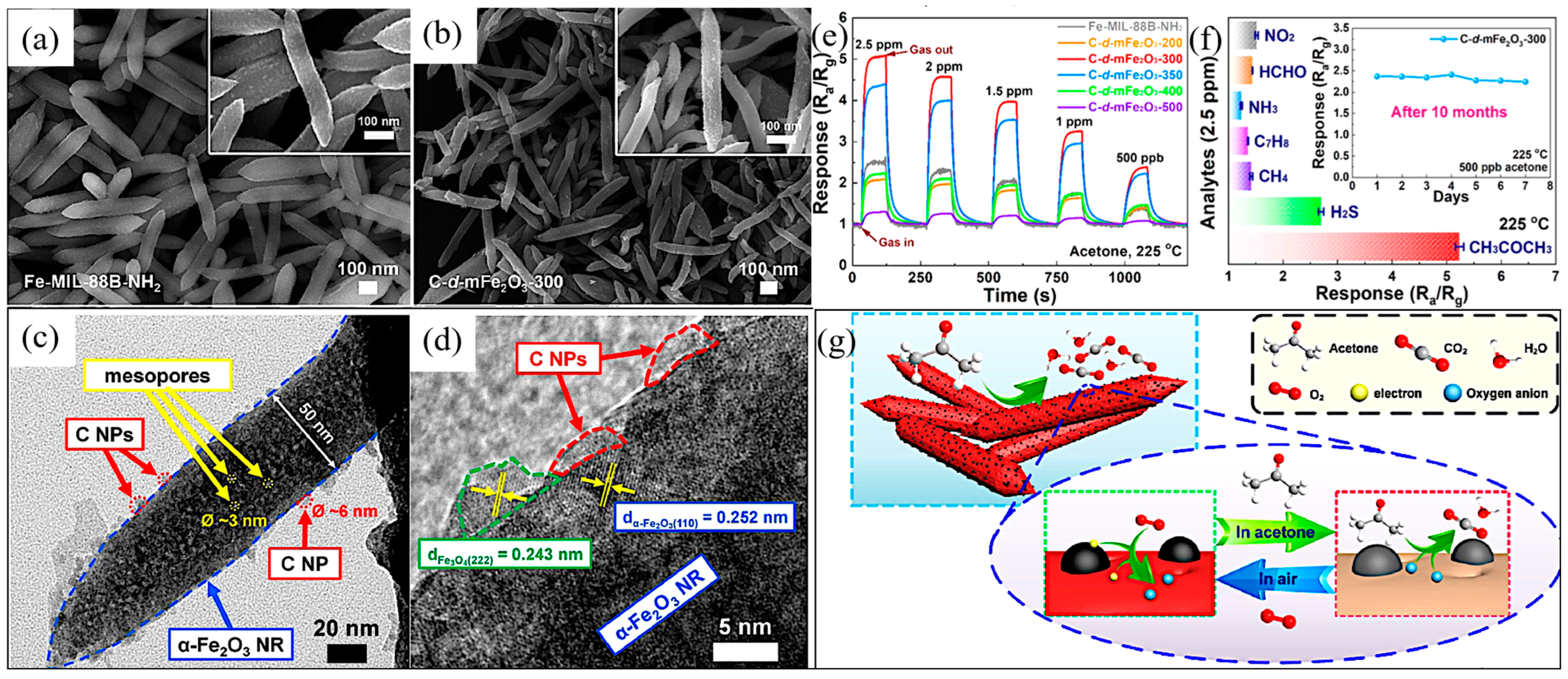
- Zhu, L.Y.; Yuan, K.; Li, Z.C.; Miao, X.Y.; Wang, J.C.; Sun, S.; Devi, A.; Lu, H.L. Highly sensitive and stable MEMS acetone sensors based on well-designed α-Fe2O3/C mesoporous nanorods. J. Colloid Interface Sci. 2022, 622, 156–168. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.Y.; Miao, X.Y.; Ou, L.X.; Mao, L.W.; Yuan, K.; Sun, S.; Devi, A.; Lu, H.L. Heterostructured α-Fe2O3@ZnO@ZIF-8 core-shell nanowires for a highly selective MEMS-based ppb-level H2S gas sensor system. Small 2022, 18, 2204828. [Google Scholar] [CrossRef]
- Zhang, D.; Yang, Z.; Wu, Z.; Dong, G. Metal-organic frameworks-derived hollow zinc oxide/cobalt oxide nanoheterostructure for highly sensitive acetone sensing. Sens. Actuators B Chem. 2019, 283, 42–51. [Google Scholar] [CrossRef]
- Bayan, E.M.; Petrov, V.V.; Volkova, M.G.; Storozhenko, V.Y.; Chernyshev, A.V. SnO2–ZnO nanocomposite thin films: The influence of structure, composition and crystallinity on optical and electrophysical properties. J. Adv. Dielectr. 2021, 11, 2160008. [Google Scholar] [CrossRef]
- Uddin, M.F.; Ullah, M.S.; Hoque, S.M.; Khan, F.A.; Momin, A.A.; Islam, S.R.; Salehin, F.; Hakim, M.A. Electrical transport properties of V2O5-added Ni–Co–Zn ferrites. J. Adv. Dielectr. 2021, 11, 2150025. [Google Scholar] [CrossRef]
- Guerra, J.D.S.; Guarany, C.A.; Lima, E.C.; Araújo, E.B.; Garcia, J.E. Exploring the electromechanical response and electric field-induced dielectric anomalies in PMN–PT electroceramics. J. Adv. Dielectr. 2021, 11, 2140005. [Google Scholar] [CrossRef]
- Manan, A.; Rehman, M.U.; Ullah, A.; Ahmad, A.S.; Iqbal, Y.; Qazi, I.; Khan, M.A.; Shah, H.U.; Wazir, A.H. High energy storage density with ultra-high efficiency and fast charging–discharging capability of sodium bismuth niobate lead-free ceramics. J. Adv. Dielectr. 2021, 11, 2150018. [Google Scholar] [CrossRef]
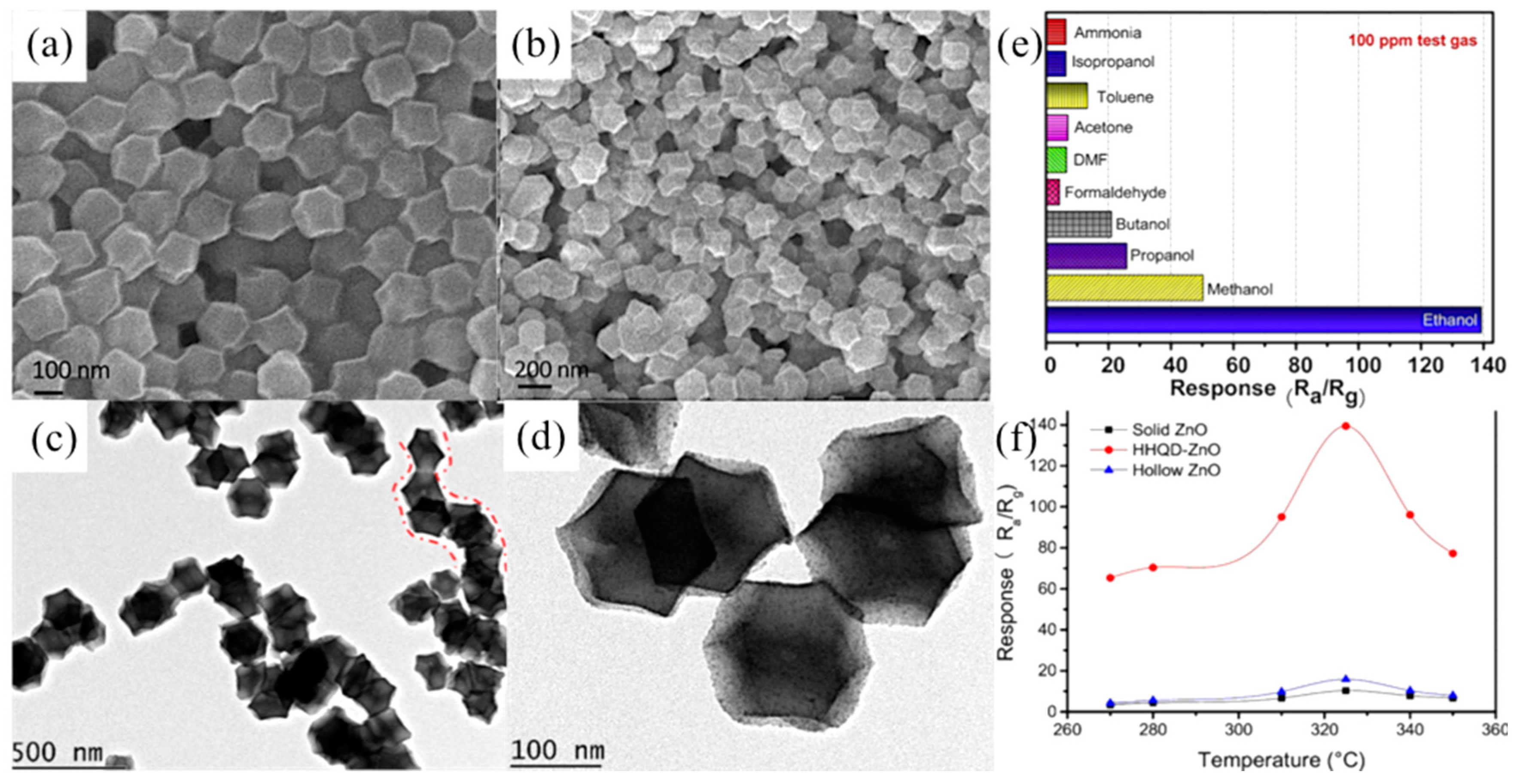
- Zhang, X.; Lan, W.; Xu, J.; Luo, Y.; Pan, J.; Liao, C.; Yang, L.; Tan, W.; Huang, X. ZIF-8 derived hierarchical hollow ZnO nanocages with quantum dots for sensitive ethanol gas detection. Sens. Actuators B Chem. 2019, 289, 144–152. [Google Scholar] [CrossRef]
- Wang, X.; Li, S.; Xie, L.; Li, X.; Lin, D.; Zhu, Z. Low-temperature and highly sensitivity H2S gas sensor based on ZnO/CuO composite derived from bimetal metal-organic frameworks. Ceram. Int. 2020, 46, 15858–15866. [Google Scholar] [CrossRef]
- Pan, W.; Zhang, Y.; Yu, S.; Liu, X.; Zhang, D. Hydrogen sulfide gas sensing properties of metal organic framework derived α-Fe2O3 hollow nanospheres decorated with MoSe2 nanoflowers. Sens. Actuators B Chem. 2021, 344, 130221. [Google Scholar] [CrossRef]
- Ngoc, T.M.; Van Duy, N.; Hung, C.M.; Hoa, N.D.; Nguyen, H.; Tonezzer, M.; Hieu, N.V. Self-heated Ag-decorated SnO2 nanowires with low power consumption used as a predictive virtual multisensor for H2S-selective sensing. Anal. Chim. Acta 2019, 1069, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Cao, Q.; Che, R.; Chen, N. Scalable synthesis of Cu2S double-superlattice nanoparticle systems with enhanced UV/visible-light-driven photocatalytic activity. Appl. Catal. B Environ. 2015, 162, 187–195. [Google Scholar] [CrossRef]
- Cao, Q.; Cheng, Y.F.; Bi, H.; Zhao, X.; Yuan, K.; Liu, Q.; Li, Q.; Wang, M.; Che, R. Crystal defect-mediated band-gap engineering: A new strategy for tuning the optical properties of Ag2Se quantum dots toward enhanced hydrogen evolution performance. J. Mater. Chem. A 2015, 3, 20051–20055. [Google Scholar] [CrossRef]
- Cheng, J.; Li, C.; Xiong, Y.; Zhang, H.; Raza, H.; Ullah, S.; Wu, J.; Zhang, G.; Cao, Q.; Zhang, D.; et al. Recent advances in design strategies and multifunctionality of flexible electromagnetic interference shielding materials. Nano-Micro Lett. 2022, 14, 80. [Google Scholar] [CrossRef] [PubMed]
- Wan, X.; Wu, L.; Zhang, L.; Song, H.; Lv, Y. Novel metal-organic frameworks-based hydrogen sulfide cataluminescence sensors. Sens. Actuators B Chem. 2015, 220, 614–621. [Google Scholar] [CrossRef]
- Huang, C.; Liu, D.; Wang, D.; Guo, H.; Thomas, T.; Attfield, J.P.; Qu, F.; Ruan, S.; Yang, M. Mesoporous Ti0.5Cr0.5N for trace H2S detection with excellent long-term stability. J. Hazard. Mater. 2022, 423, 127193. [Google Scholar] [CrossRef]
- Li, S.; Xie, L.; He, M.; Hu, X.; Luo, G.; Chen, C.; Zhu, Z. Metal-organic frameworks-derived bamboo-like CuO/In2O3 heterostructure for high-performance H2S gas sensor with low operating temperature. Sens. Actuators B Chem. 2020, 310, 127828. [Google Scholar] [CrossRef]
- Karuppasamy, K.; Sharma, B.; Vikraman, D.; Lee, J.H.; Islam, M.; Santhoshkumar, P.; Kim, H.S. Metal-organic framework-derived Ni4Mo/MoO2@C composite nanospheres as the sensing materials for hydrogen sulfide detection. J. Alloys Compd. 2022, 900, 163421. [Google Scholar] [CrossRef]
- Lyapunov, N.; Suen, C.H.; Wong, C.M.; Tang, X.; Ho, Z.L.; Zhou, K.; Chen, X.X.; Liu, H.M.; Zhou, X.; Dai, J.Y. Ultralow switching voltage and power consumption of GeS2 thin film resistive switching memory. J. Adv. Dielectr. 2021, 11, 2150004. [Google Scholar] [CrossRef]
- Khatun, N.; Hossain, M.S.; Begum, M.H.A.; Islam, S.; Tanvir, N.I.; Bhuiyan, R.H.; Al-Mamun, M. Effect of sintering temperature on structural, magnetic, dielectric and optical properties of Ni–Mn–Zn ferrites. J. Adv. Dielectr. 2021, 11, 2150028. [Google Scholar] [CrossRef]
- Wang, C.L. Photocatalytic degradations of JWS-type kinetics. J. Adv. Dielectr. 2021, 11, 2150029. [Google Scholar] [CrossRef]
- Zhang, Y.; Jia, C.; Wang, Q.; Kong, Q.; Chen, G.; Guan, H.; Dong, C. MOFs-derived porous NiFe2O4 nano-octahedrons with hollow interiors for an excellent toluene gas sensor. Nanomaterials 2019, 9, 1059. [Google Scholar] [CrossRef]
- Sun, J.; Wang, Y.; Song, P.; Yang, Z.; Wang, Q. Metal-organic framework-derived Cr-doped hollow In2O3 nanoboxes with excellent gas-sensing performance toward ammonia. J. Alloys Compd. 2021, 879, 160472. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, H.; Zhang, H.; Song, B.; Yuan, K.; Xiao, H.; Cao, Y.; Cao, Q. Metal–Organic Framework (MOF) Derivatives as Promising Chemiresistive Gas Sensing Materials: A Review. Int. J. Environ. Res. Public Health 2023, 20, 4388. https://doi.org/10.3390/ijerph20054388
Wei H, Zhang H, Song B, Yuan K, Xiao H, Cao Y, Cao Q. Metal–Organic Framework (MOF) Derivatives as Promising Chemiresistive Gas Sensing Materials: A Review. International Journal of Environmental Research and Public Health. 2023; 20(5):4388. https://doi.org/10.3390/ijerph20054388
Chicago/Turabian StyleWei, Huijie, Huiyan Zhang, Bing Song, Kaiping Yuan, Hongbin Xiao, Yunyi Cao, and Qi Cao. 2023. "Metal–Organic Framework (MOF) Derivatives as Promising Chemiresistive Gas Sensing Materials: A Review" International Journal of Environmental Research and Public Health 20, no. 5: 4388. https://doi.org/10.3390/ijerph20054388
APA StyleWei, H., Zhang, H., Song, B., Yuan, K., Xiao, H., Cao, Y., & Cao, Q. (2023). Metal–Organic Framework (MOF) Derivatives as Promising Chemiresistive Gas Sensing Materials: A Review. International Journal of Environmental Research and Public Health, 20(5), 4388. https://doi.org/10.3390/ijerph20054388





