Dissolved Oxygen and Water Temperature Drive Vertical Spatiotemporal Variation of Phytoplankton Community: Evidence from the Largest Diversion Water Source Area
Abstract
1. Introduction
2. Materials and Methods
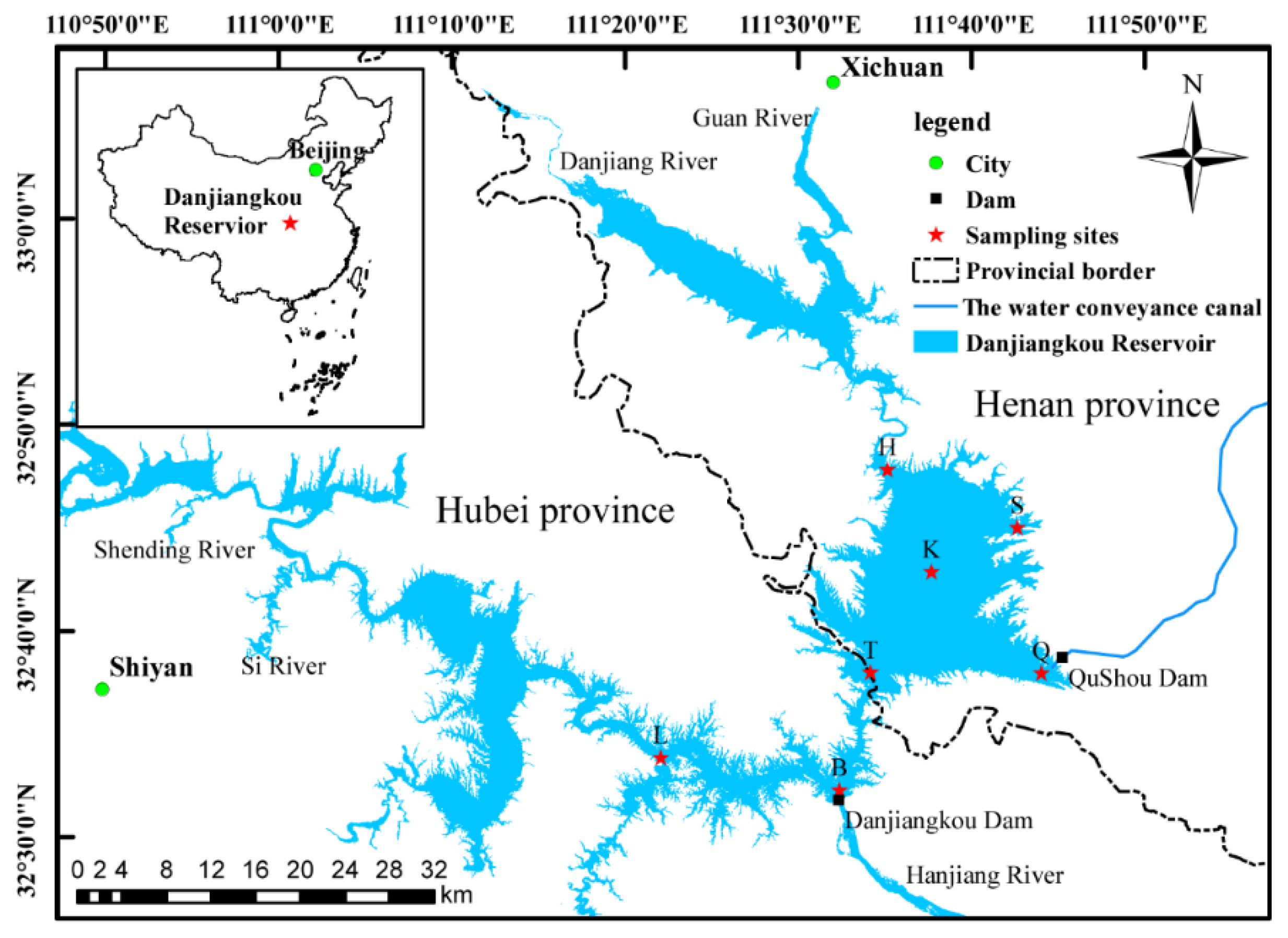
2.1. Study Area
2.2. Sample Collection and Determination
2.3. Data Processing
3. Results
3.1. Hydrological and Environmental Characteristics of Danjiangkou Reservoir
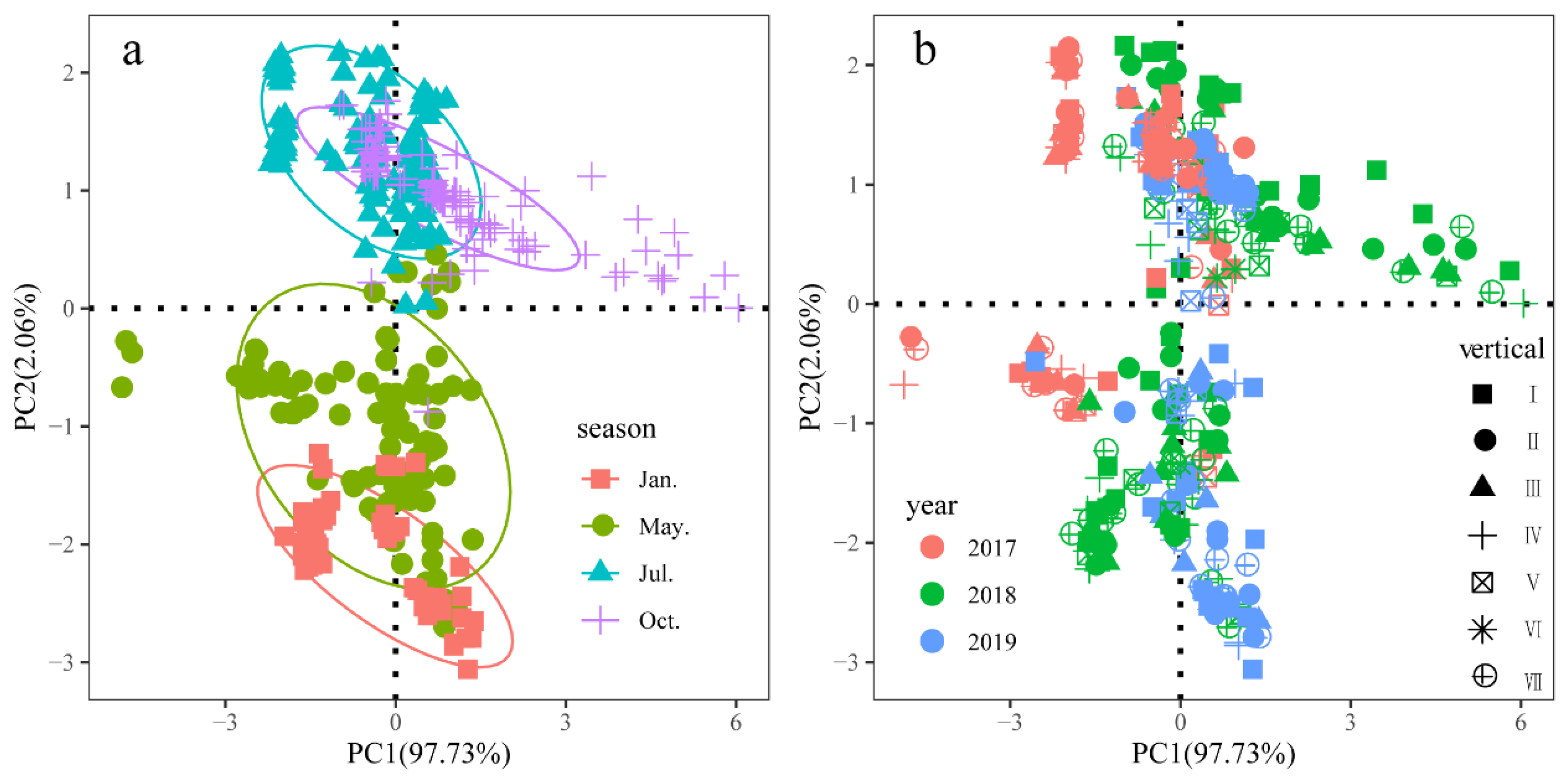
3.1.1. Principal Component Analysis of Environmental Factors
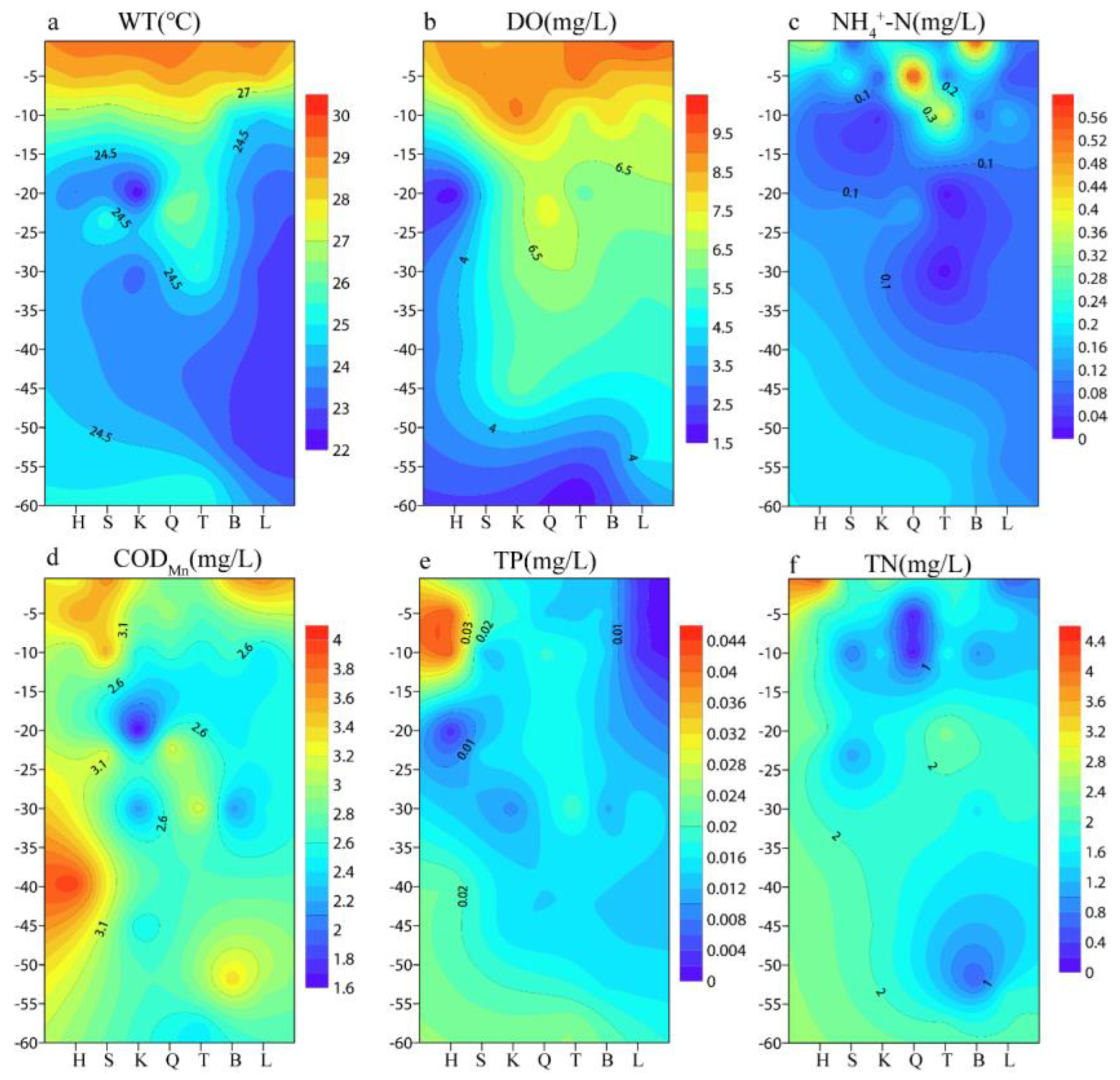
3.1.2. Physical and Chemical Factor Analysis
3.2. Community Characteristics of Phytoplankton Species in Danjiangkou Reservoir
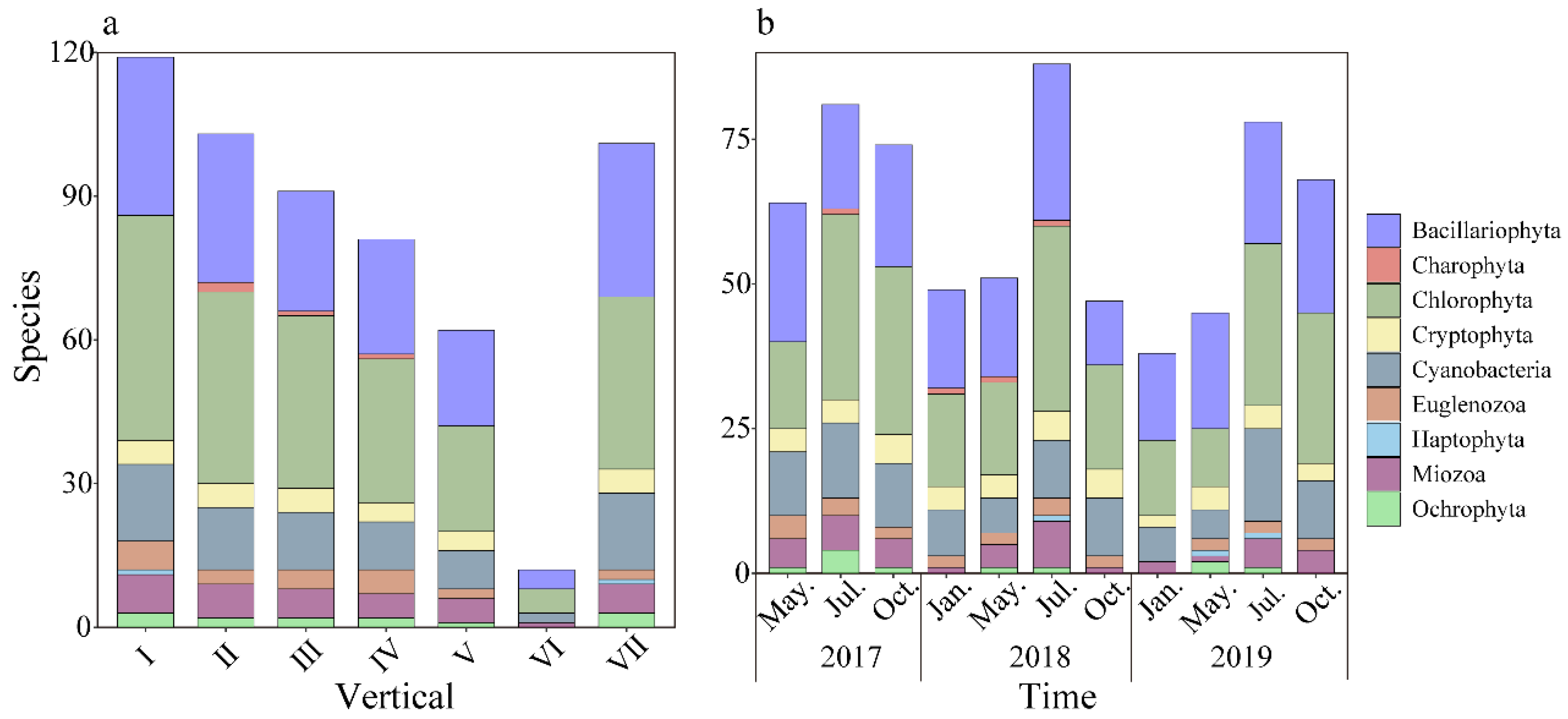
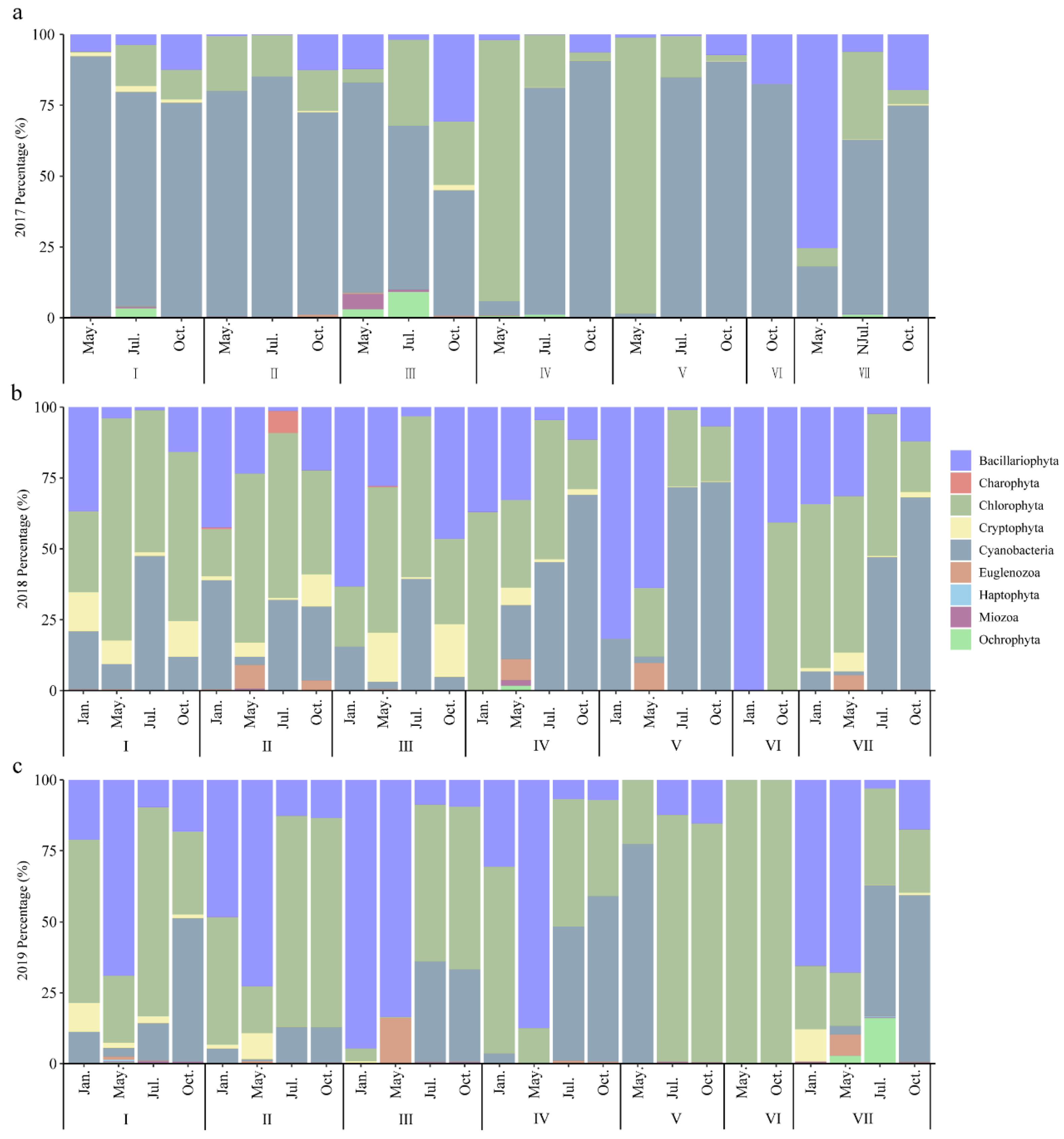
3.2.1. Phytoplankton Species Composition
3.2.2. Changes in Phytoplankton Abundance
3.2.3. Dominant Species of Phytoplankton
3.3. Relationship between Phytoplankton Community Structure and Environmental Factors
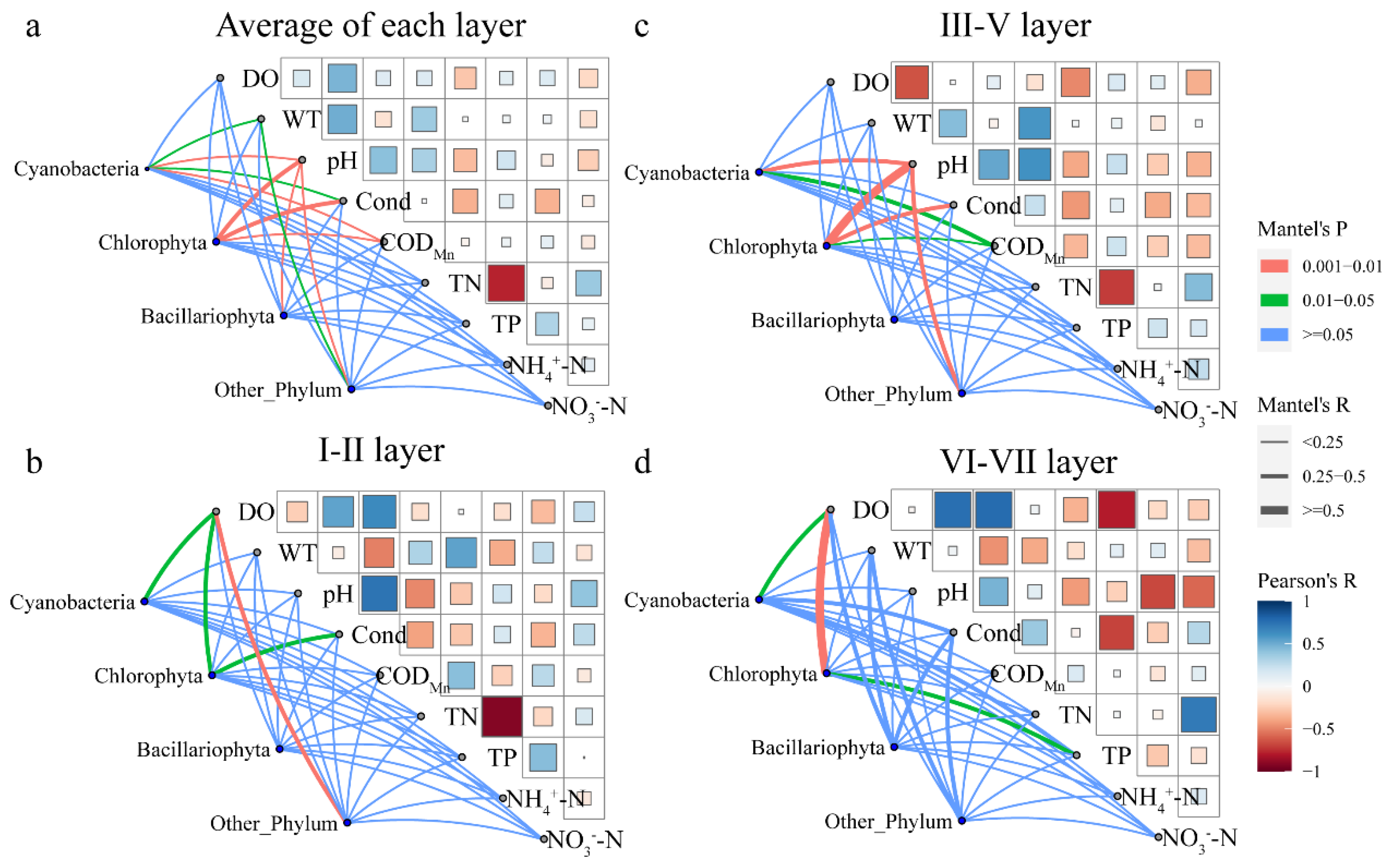
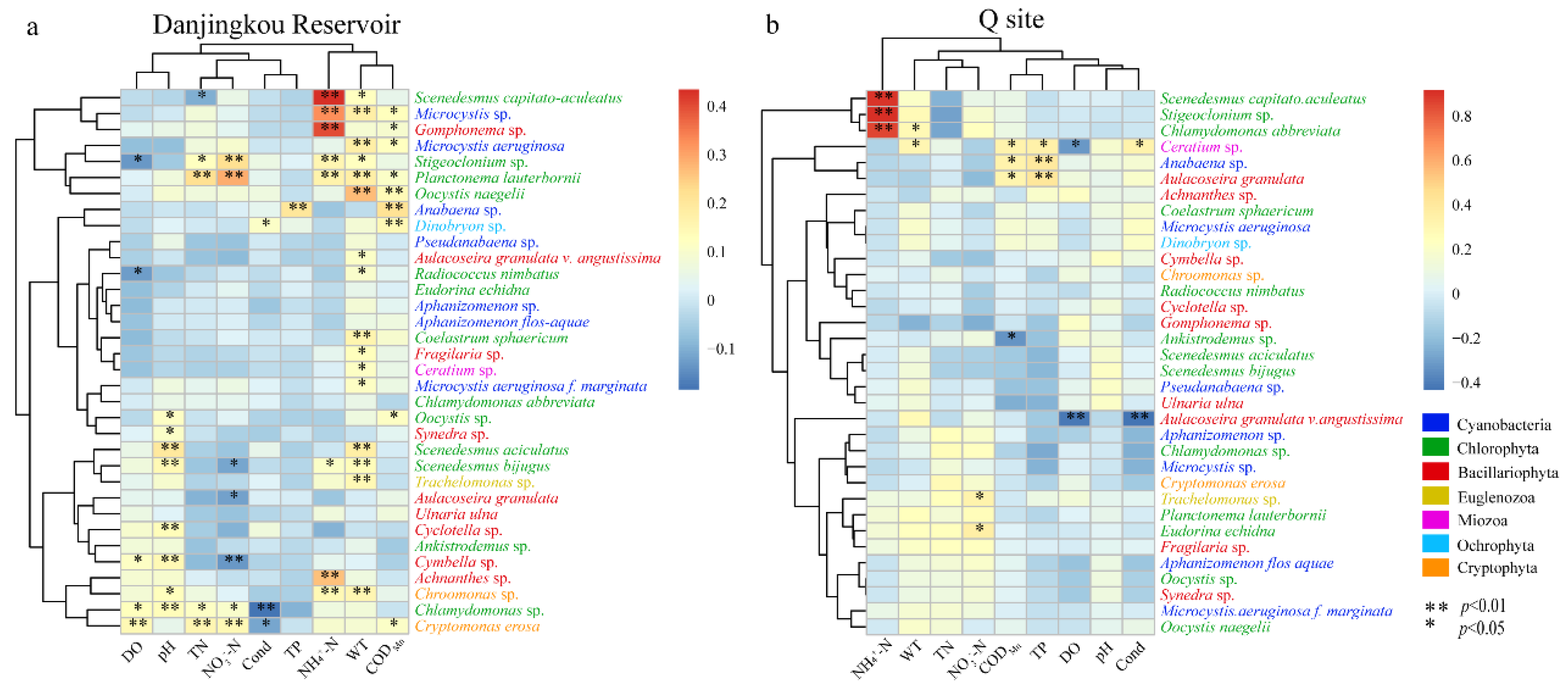
3.3.1. Relationship between Community Structure and Environmental Factors
3.3.2. Relationship between Community Structure and Environmental Factors of Dominant Species
4. Discussion
4.1. Vertical Distribution and Succession of Phytoplankton Communities
4.2. Effects of Environmental Factors on Phytoplankton Communities
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shen, H.; Xu, Y.; Wang, L.; Zhang, M.; Kong, L.; Cai, Q. Spatial and Temporal Variations of Phytoplankton in Danjiangkou Reservoir and its Affecting Factors. Plant Sci. 2011, 29, 683–690. [Google Scholar] [CrossRef]
- Zhou, G.; Kuang, Q.; Hu, Z.; Cai, Q. Study on Succession of Algae and the Trend of Water-Blooms Occurred in XiangXi Bay. Acta Hydrobiol. Sin. 2006, 30, 42–46. [Google Scholar] [CrossRef]
- Fang, T.; Li, D.; Yu, L.; Gao, L.; Zhang, L. Effects of irradiance and phosphate on growth of nanophytoplankton and picophytoplankton. Acta Ecol. Sin. 2006, 26, 2783–2789. [Google Scholar] [CrossRef]
- Padisák, J.; Borics, G.; Grigorszky, I.; Soróczki-Pintér, É. Use of Phytoplankton Assemblages for Monitoring Ecological Status of Lakes within the Water Framework Directive: The Assemblage Index. Hydrobiologia 2006, 553, 1–14. [Google Scholar] [CrossRef]
- Beaver, J.R.; Kirsch, J.E.; Tausz, C.E.; Samples, E.E.; Renicker, T.R.; Scotese, K.C.; McMaster, H.A.; Blasius-Wert, B.J.; Zimba, P.V.; Casamatta, D.A. Long-term trends in seasonal plankton dynamics in Lake Mead (Nevada-Arizona, USA) and implications for climate change. Hydrobiologia 2018, 822, 85–109. [Google Scholar] [CrossRef]
- Guo, F.; Jiang, G.; Zhao, H.; Polk, J.; Liu, S. Physicochemical parameters and phytoplankton as indicators of the aquatic environment in karstic springs of South China. Sci. Total. Environ. 2019, 659, 74–83. [Google Scholar] [CrossRef]
- He, Y.; Mai, S.; Ren, Y.; Li, W.; Zhao, T.; Ma, Y. Characteristics of eukaryotic phytoplankton community structure and its relationship with environmental factors in Danjiangkou Reservoir. Environ. Sci. 2022, 43, 5096–5105. [Google Scholar] [CrossRef]
- Li, P.; Yao, Y.; Lian, J.; Ma, C. Effect of thermal stratified flow on algal blooms in a tributary bay of the Three Gorges reservoir. J. Hydrol. 2021, 601, 126648. [Google Scholar] [CrossRef]
- Yang, M.; Bi, Y.; Hu, J.; Hu, Z. Diel vertical migration and distribution of phytoplankton during spring blooms in Xiangxi Bay, Three Gorges Reservoir. J. Lake Sci. 2011, 23, 375–382. [Google Scholar] [CrossRef]
- Long, L.; Chen, P.; Xu, H.; Ji, D.; Liu, L.; Yang, Z.; Lorke, A. Recent changes of the thermal structure in Three Gorges Reservoir, China and its impact on algal bloom in tributary bays. Ecol. Indic. 2022, 144, 109465. [Google Scholar] [CrossRef]
- Li, R.; Chen, G.-J.; Kang, W.-G.; Chen, L.; Wang, J.-Y.; Chen, X.-L.; Liu, Y.-Y.; Feng, Z.; Zhang, T. [Spatio-temporal Variations of Diatom Community and Their Relationship with Water Environment in Fuxian Lake]. Environ. Sci. 2018, 39, 3168–3178. [Google Scholar] [CrossRef]
- Liu, M.; Zhang, Y.; Shi, K.; Melack, J.; Zhang, Y.; Zhou, Y.; Zhu, M.; Zhu, G.; Wu, Z.; Liu, M. Spatial Variations of Subsurface Chlorophyll Maxima During Thermal Stratification in a Large, Deep Subtropical Reservoir. J. Geophys. Res. Biogeosci. 2020, 125, e2019JG005480. [Google Scholar] [CrossRef]
- An, R.; Pan, C.; Taba, L.; Yang, X.; Ba, S. Vertical distribution characteristics of phytoplankton functional groups and their relationships with environmental factors in Lake Basomtso, Tibet, China. J. Lake Sci. 2021, 33, 86–101. [Google Scholar] [CrossRef]
- Domingues, R.B.; Barbosa, A.; Galvão, H. Nutrients, light and phytoplankton succession in a temperate estuary (the Guadiana, South-Western Iberia). Estuar. Coast. Shelf Sci. 2005, 64, 249–260. [Google Scholar] [CrossRef]
- Coale, K.H.; Bruland, K.W. Oceanic stratified euphotic zone as elucidated by 234Th: 238U disequilibrial. Limnol. Oceanogr. 1987, 32, 189–200. [Google Scholar] [CrossRef]
- Mignot, A.; Claustre, H.; Uitz, J.; Poteau, A.; D’Ortenzio, F.; Xing, X. Understanding the seasonal dynamics of phytoplankton biomass and the deep chlorophyll maximum in oligotrophic environments: A Bio-Argo float investigation. Glob. Biogeochem. Cycles 2014, 28, 856–876. [Google Scholar] [CrossRef]
- Wu, H.; Peng, J.; Han, D.; Jian, D.; Zhou, Q. Composition and Ecological Changes of Phytoplankton in Danjiangkou Reservoir. J. Lake Sci. 1996, 8, 43–50. [Google Scholar] [CrossRef]
- Jiang, Y.-J.; He, W.; Liu, W.-X.; Qin, N.; Ouyang, H.-L.; Wang, Q.-M.; Kong, X.-Z.; He, Q.-S.; Yang, C.; Yang, B.; et al. The seasonal and spatial variations of phytoplankton community and their correlation with environmental factors in a large eutrophic Chinese lake (Lake Chaohu). Ecol. Indic. 2014, 40, 58–67. [Google Scholar] [CrossRef]
- Hoyer, A.B.; Ostos, E.M.; Vidal, J.; Blanco, J.M.; Palomino-Torres, R.L.; Basanta, A.; Escot, C.; Rueda, F.J. The influence of external perturbations on the functional composition of phytoplankton in a Mediterranean reservoir. Hydrobiologia 2009, 636, 49–64. [Google Scholar] [CrossRef]
- Zhang, X.; Xiong, J.; Cheng, J.; Yao, Z.; Chen, Y. Application of Fuzzy Mathematics for Evaluation of Eutrophication in Danjiangkou Reservoir. Environ. Monit. China 2017, 33, 99–105. [Google Scholar] [CrossRef]
- Gao, W.; Chen, Z.; Li, Y.; Pan, Y.; Zhu, J.; Guo, S.; Hu, L.; Huang, J. Bioassessment of a Drinking Water Reservoir Using Plankton: High Throughput Sequencing vs. Traditional Morphological Method. Water 2018, 10, 82. [Google Scholar] [CrossRef]
- Pan, Y.; Guo, S.; Li, Y.; Yin, W.; Qi, P.; Shi, J.; Hu, L.; Li, B.; Bi, S.; Zhu, J. Effects of Water Level Increase on Phytoplankton Assemblages in a Drinking Water Reservoir. Water 2018, 10, 256. [Google Scholar] [CrossRef]
- Zhu, Y.X.; Zhang, J.; Liu, R.; Wang, Y. Study on Remote Sensing Monitoring of Water Quality for Danjiangkou Reservoir by HJ-1Satellite Data. Environ. Sci. Technol. 2014, 27, 52–58. [Google Scholar] [CrossRef]
- Qu, Y.; Cai, Q.; Shen, H.; Li, B. Variation and Influencing Factors of Euphotic Depth iin Danjiangkou Reservoir in Different Hydrological Periods. Resour. Environ. Yangtze Basin 2014, 23, 53–59. [Google Scholar] [CrossRef]
- Gao, X.; Chen, H.; Gu, B.; Jeppesen, E.; Xue, Y.; Yang, J. Particulate organic matter as causative factor to eutrophication of subtropical deep freshwater: Role of typhoon (tropical cyclone) in the nutrient cycling. Water Res. 2021, 188, 116470. [Google Scholar] [CrossRef]
- Administration, S.E.P. Monitoring and Analysis Methods of Water Waste Water, 4th ed.; Environmental Science Press: Beijing, China, 2002. [Google Scholar]
- Qian, K.; Liu, X.; Chen, Y. A review on methods of cell enumeration and quantification of freshwater phytoplankton. J. Lake Sci. 2015, 27, 767–775. [Google Scholar] [CrossRef]
- Editorial Committee of Chinese Spore Flora; C.A.O.S. Flora Algarum Sinicarum Aquae Dulcis; Science Press: Wuhan, China, 2013. [Google Scholar]
- Hydrological Bureau, M.O.W.R.; Center, Y.R.B.W. Map of Common Algae in Chinese Inland Waters; Changjiang Press: Wuhan, China, 2012. [Google Scholar]
- Hu, H.; Wei, Y. The Freshwater Algae of China-Systematics, Taxonomy and Ecology; Beijing Science Press: Beijing, China, 2006. [Google Scholar]
- Wang, X.; Sun, M.; Wang, J.; Yang, L.; Luo, L.; Li, P.; Kong, F. Microcystis Genotype Succession and Related Environmental Factors in Lake Taihu during Cyanobacterial Blooms. Microb. Ecol. 2012, 64, 986–999. [Google Scholar] [CrossRef]
- Xing, B.; Xu, J.; Cao, Y.; Deng, G.; Pang, W.; Wang, Q. Phytoplankton community structure and ecological evaluation in summer, Lake Changhai of Jiuzhaigou National Nature Reserve. J. Lake Sci. 2020, 32, 1088–1099. [Google Scholar] [CrossRef]
- Da, W.-Y.; Zhu, G.-W.; Li, Y.-X.; Wu, Z.-X.; Zheng, W.-T.; Lan, J.; Wang, Y.-C.; Xu, H.; Zhu, M.-Y. [High-Frequency Dynamics of Water Quality and Phytoplankton Community in Inflowing River Mouth of Xin’anjiang Reservoir, China]. Environ. Sci. 2020, 41, 713–727. [Google Scholar] [CrossRef]
- Wang, Q.; Huang, W.; Chen, K.; Yi, Q.; Li, H. Phytoplankton community structure and trophic status evaluation in Reservior Daxi. Acta Sci. Circum. 2020, 40, 1286–1297. [Google Scholar] [CrossRef]
- Yan, G.; Xue-Yan, Y.; Wang, X.; Wang, L.; Ying-Jie, L.; Li, H.; Chen, W. Effects of environmental factors on the composition of phytoplankton community in Sankou of the Yangtze River and the Western Dongting Lake. China Environ. Sci. 2019, 39, 2532–2540. [Google Scholar] [CrossRef]
- Cai, Y.; Tang, C.; Cao, Y. Seasonal variation of physical and chemical factors and distribution characteristics of phytoplankton in a subtropical reservoir: A case study from Lianhe Reservoir, South China. Acta Sci. Nat. Univ. Sunyatseni 2020, 59, 59–72. [Google Scholar] [CrossRef]
- Zeng, H.; Song, L.; Yu, Z.; Chen, H. Distribution of phytoplankton in the Three-Gorge Reservoir during rainy and dry seasons. Sci. Total. Environ. 2006, 367, 999–1009. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wu, Z.; Liu, M.; He, J.; Shi, K.; Zhou, Y.; Wang, M.; Liu, X. Dissolved oxygen stratification and response to thermal structure and long-term climate change in a large and deep subtropical reservoir (Lake Qiandaohu, China). Water Res. 2015, 75, 249–258. [Google Scholar] [CrossRef]
- Jones, J.R.; Knowlton, M.F.; Obrecht, D.V.; Graham, J.L. Temperature and oxygen in Missouri reservoirs. Lake Reserv. Manag. 2011, 27, 173–182. [Google Scholar] [CrossRef]
- Carey, C.C.; Ibelings, B.W.; Hoffmann, E.P.; Hamilton, D.P.; Brookes, J.D. Eco-physiological adaptations that favour freshwater cyanobacteria in a changing climate. Water Res. 2012, 46, 1394–1407. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, L.; Niu, Y.; Yu, H.; Luo, M. Spatio-temporal variation in phytoplankton community and its influencing factors in Danjingkou Reservoir. J. Lake Sci. 2016, 28, 1057–1065. [Google Scholar] [CrossRef]
- Feng, Y.; Ma, C.; Yu, H. Changes in Phytoplankton Community and Water Quality after Expansion of Haohanpo Reservoir. Chin. J. Fish. 2013, 26, 41–46. [Google Scholar] [CrossRef]
- Li, X.; Dong, S.; Zhao, Q.; Liu, S. Impacts of Manwan Dam construction on aquatic habitat and community in Middle Reach of Lancang River. Procedia Environ. Sci. 2010, 2, 706–712. [Google Scholar] [CrossRef]
- Nürnberg, G.K. Quantification of Oxygen Depletion in Lakes and Reservoirs with the Hypoxic Factor. Lake Reserv. Manag. 2002, 18, 299–306. [Google Scholar] [CrossRef]
- Marcé, R.; Moreno-Ostos, E.; García-Barcina, J.M.; Armengol, J. Tailoring dam structures to water quality predictions in new reservoir projects: Assisting decision-making using numerical modeling. J. Environ. Manag. 2010, 91, 1255–1267. [Google Scholar] [CrossRef] [PubMed]
- Niu, Y.; Kong, X.; Yu, H.; Liu, Q. Spatial distribution of phytoplankton community during summer stratification in Lake Fuxian. Chin. J. Ecol. 2016, 35, 1865–1871. [Google Scholar] [CrossRef]
- Yan, X.; Zhang, Y.; Li, Y.; Jiang, Y.; Cui, Z.; Gao, X.; Wu, N.; Fohrer, N.; Xuemei, H. Hydrologic and physicochemical factors co-drive seasonal changes of phytoplankton during dynamic water diversion processes in the Danjiangkou Reservoir. J. Lake Sci. 2021, 33, 1350–1363. [Google Scholar] [CrossRef]
- Guo, S.; Wang, X.; Han, P.; Zheng, B.; Jiang, Y.; Guo, K.; Han, X.; Li, B.; Gao, X.; Li, Y. Spatiotemporal characteristics of layered chlorophyll-a concentration and influencing factors in Danjiangkou Reservoir. J. Lake Sci. 2021, 33, 366–376. [Google Scholar] [CrossRef]
- Yang, J.; Lv, H.; Liu, L.; Yu, X.; Chen, H. Decline in water level boosts cyanobacteria dominance in subtropical reservoirs. Sci. Total. Environ. 2016, 557–558, 445–452. [Google Scholar] [CrossRef]
- Powley, H.R.; Krom, M.D.; Van Cappellen, P. Understanding the unique biogeochemistry of the Mediterranean Sea: Insights from a coupled phosphorus and nitrogen model. Glob. Biogeochem. Cycles 2017, 31, 1010–1031. [Google Scholar] [CrossRef]
- Burford, M.A.; Green, S.A.; Cook, A.J.; Johnson, S.A.; Kerr, J.G.; O’Brien, K.R. Sources and fate of nutrients in a subtropical reservoir. Aquat. Sci. 2012, 74, 179–190. [Google Scholar] [CrossRef]
- Pérez, V.; Fernández, E.; Marañón, E.; Morán, X.A.G.; Zubkov, M.V. Vertical distribution of phytoplankton biomass, production and growth in the Atlantic subtropical gyres. Deep. Sea Res. Part I Oceanogr. Res. Pap. 2006, 53, 1616–1634. [Google Scholar] [CrossRef]
- Latasa, M.; Cabello, A.M.; Morán, X.A.G.; Massana, R.; Scharek, R. Distribution of phytoplankton groups within the deep chlorophyll maximum. Limnol. Oceanogr. 2017, 62, 665–685. [Google Scholar] [CrossRef]
- Mouriño-Carballido, B.; Hojas, E.; Cermeño, P.; Chouciño, P.; Fernández-Castro, B.; Latasa, M.; Marañón, E.; Morán, X.; Vidal, M. Nutrient supply controls picoplankton community structure during three contrasting seasons in the northwestern Mediterranean Sea. Mar. Ecol. Prog. Ser. 2016, 543, 1–19. [Google Scholar] [CrossRef]
- Mella-Flores, D.; Six, C.; Ratin, M.; Partensky, F.; Boutte, C.; Le Corguillé, G.; Marie, D.; Blot, N.; Gourvil, P.; Kolowrat, C.; et al. Prochlorococcus and Synechococcus have Evolved Different Adaptive Mechanisms to Cope with Light and UV Stress. Front. Microbiol. 2012, 3, 285. [Google Scholar] [CrossRef] [PubMed]
- Miloslavić, M.; Lučić, D.; Žarić, M.; Gangai, B.; Onofri, I. The importance of vertical habitat gradients on zooplankton distribution in an enclosed marine environment (South Adriatic Sea). Mar. Biol. Res. 2015, 11, 462–474. [Google Scholar] [CrossRef]
- Guo, C.; Liu, H.; Zheng, L.; Song, S.; Chen, B.; Huang, B. Seasonal and spatial patterns of picophytoplankton growth, grazing and distribution in the East China Sea. Biogeosciences 2014, 11, 1847–1862. [Google Scholar] [CrossRef]

| Parameters | I Layer | II Layer | III Layer | IV Layer | V Layer | VI Layer | VII Layer |
|---|---|---|---|---|---|---|---|
| WT (°C) | 21.44 ± 6.08 a | 21.09 ± 6.06 ab | 20.71 ± 5.70 ab | 20.32 ± 5.54 ab | 21.10 ± 5.11 ab | 20.98 ± 5.22 ab | 19.28 ± 5.81 b |
| pH | 8.25 ± 0.44 a | 8.21 ± 0.44 ac | 8.14 ± 0.45 ad | 8.08 ± 0.43 bcd | 7.98 ± 0.43 bd | 7.80 ± 0.53 b | 8.07 ± 0.48 bcd |
| DO (mg/L) | 8.98 ± 1.67 a | 9.00 ± 2.22 a | 8.55 ± 2.11 ac | 7.82 ± 2.42 bc | 7.34 ± 1.86 b | 6.84 ± 2.02 b | 7.18 ± 2.56 b |
| Cond (μS/cm) | 265.82 ± 41.12 | 264.63 ± 41.15 | 266.12 ± 41.34 | 269.91 ± 42.49 | 263.04 ± 35.73 | 259.63 ± 15.43 | 266.94 ± 43.52 |
| NO3−-N (mg/L) | 0.937 ± 0.378 | 0.916 ± 0.278 | 0.908 ± 0.284 | 0.923 ± 0.321 | 0.983 ± 0.295 | 0.978 ± 0.189 | 0.931 ± 0.345 |
| NH4+-N (mg/L) | 0.068 ± 0.084 | 0.070 ± 0.080 | 0.070 ± 0.071 | 0.052 ± 0.037 | 0.055 ± 0.031 | 0.068 ± 0.067 | 0.061 ± 0.043 |
| TN (mg/L) | 1.30 ± 0.55 a | 1.18 ± 0.48 ab | 1.15 ± 0.44 bc | 1.32 ± 0.40 a | 1.32 ± 0.41 ac | 1.27 ± 0.35 ac | 1.28 ± 0.41 ac |
| TP (mg/L) | 0.018 ± 0.012 | 0.018 ± 0.010 | 0.018 ± 0.011 | 0.018 ± 0.010 | 0.017 ± 0.009 | 0.019 ± 0.010 | 0.018 ± 0.010 |
| CODMn (mg/L) | 2.82 ± 0.59 a | 2.71 ± 0.52 ab | 2.63 ± 0.41 b | 2.63 ± 0.54 b | 2.52 ± 0.47 b | 2.44 ± 0.24 b | 2.66 ± 0.53 b |
| Phylum | Dominant Species | I | II | III | IV | V | VI | VII |
|---|---|---|---|---|---|---|---|---|
| Cyanobacteria | Aphanizomenon sp. | - | - | 0.033 | - | - | - | - |
| Microcystis aeruginosa Kützing | - | - | - | - | 0.029 | - | - | |
| Chlorophyta | Chlamydomonas sp. | 0.130 | 0.105 | 0.074 | 0.103 | 0.100 | 0.169 | 0.121 |
| Eudorina echidna Svirenko | - | - | - | - | - | 0.025 | - | |
| Oocystis naegelii | 0.040 | 0.031 | 0.039 | 0.045 | 0.050 | 0.089 | 0.046 | |
| Planctonema lauterbornii | 0.192 | 0.229 | 0.260 | 0.213 | 0.224 | - | 0.127 | |
| Scenedesmus sp. | - | - | - | - | - | - | - | |
| S. aciculatus | - | 0.021 | 0.033 | - | - | 0.039 | - | |
| S. bijugus (Turpin) Lagerheim | - | 0.023 | - | 0.020 | 0.042 | - | - | |
| Stigeoclonium sp. | - | 0.022 | 0.024 | - | - | - | 0.022 | |
| Bacillariophyta | Aulacoseira granulata | 0.109 | 0.117 | 0.140 | 0.148 | 0.116 | 0.184 | 0.126 |
| A. var. angustissima (O.Müller) Simonsen | 0.040 | 0.037 | 0.042 | 0.050 | 0.061 | 0.181 | 0.046 | |
| Cyclotella sp. | 0.186 | 0.177 | 0.170 | 0.201 | 0.118 | 0.275 | 0.173 | |
| Fragilaria sp. | 0.036 | 0.022 | 0.034 | 0.049 | 0.040 | - | 0.061 | |
| Ulnaria ulna (Nitzsch) Compère | - | - | - | - | 0.038 | - | - | |
| Ochrophyta | Dinobryon sp. | - | 0.022 | 0.028 | - | - | - | 0.021 |
| Cryptophyta | Chroomonas sp. | 0.021 | 0.025 | 0.023 | - | - | - | - |
| Cryptomonas erosa Ehrenberg | 0.021 | - | - | - | - | - | - |
| Layer | Jan. | May. | Jul. | Oct. |
|---|---|---|---|---|
| I | 1.795 ad | 1.011 bd | 2.117 a | 1.997 af |
| II | 0.998 be | 1.346 bd | 1.87 a | 1.95 af |
| III | 0.755 be | 1.128 bd | 1.827 a | 1.786 a |
| IV | 0.607 ce | 1.249 bd | 1.846 a | 1.477 ab |
| V | 0.366 be | 1.288 ad | 1.64 a | 1.324 a |
| VI | 0.693 de | / | / | 0.901 f |
| VII | 1.272 bd | 1.223 bd | 1.785 a | 1.734 af |
| Water Layer | H | S | K | Q | T | B | L | |
|---|---|---|---|---|---|---|---|---|
| I–II layers | DO | 0.031 | 0.081 | −0.015 | 0.051 | 0.056 | 0.005 | 0.013 |
| WT | −0.031 | 0.084 | −0.008 | 0.123 * | 0.058 | 0.096 | 0.061 | |
| pH | −0.010 | −0.086 | −0.016 | −0.031 | −0.038 | 0.058 | −0.027 | |
| Cond | −0.089 | −0.092 | −0.038 | −0.051 | −0.014 | −0.117 | −0.081 | |
| CODMn | 0.153 * | 0.029 | −0.038 | 0.045 | 0.158 * | 0.007 | −0.019 | |
| TN | −0.038 | 0.047 | 0.013 | 0.076 | −0.118 | −0.144 | −0.008 | |
| TP | 0.078 | −0.040 | −0.030 | −0.054 | 0.103 | 0.006 | 0.070 | |
| NH4+-N | 0.018 | 0.089 | −0.019 | 0.158 ** | −0.049 | −0.014 | 0.027 | |
| NO3−-N | −0.033 | −0.018 | −0.059 | −0.096 | −0.205 | −0.038 | −0.076 | |
| III–V layers | DO | 0.082 | 0.267 * | 0.187 ** | 0.120 | 0.269 ** | 0.159 ** | 0.211 ** |
| WT | 0.142 * | 0.442 ** | 0.240 ** | 0.216 * | 0.297 ** | 0.249 ** | 0.138 * | |
| pH | 0.095 | −0.109 | 0.057 | −0.082 | 0.116 * | 0.123 * | 0.119 * | |
| Cond | −0.004 | −0.085 | 0.070 | −0.077 | 0.053 | −0.019 | 0.068 | |
| CODMn | 0.142 * | 0.045 | −0.072 | 0.214 * | 0.122 * | −0.005 | −0.018 | |
| TN | 0.106 | −0.027 | 0.092 | 0.005 | −0.026 | −0.020 | 0.187 ** | |
| TP | 0.195 ** | 0.116 | −0.077 | 0.226 * | 0.014 | 0.072 | −0.010 | |
| NH4+-N | −0.034 | 0.014 | 0.034 | 0.128 | 0.031 | 0.025 | −0.035 | |
| NO3−-N | 0.097 | −0.076 | 0.041 | −0.062 | −0.030 | 0.029 | 0.129 * | |
| VI–VII layers | DO | 0.017 | 0.295 ** | 0.069 | 0.323 * | 0.097 | 0.211 * | 0.002 |
| WT | 0.110 | 0.538 ** | 0.357 * | 0.523 ** | 0.450 * | 0.218 * | 0.325 ** | |
| pH | 0.304 | −0.061 | 0.001 | −0.146 | 0.181 | 0.041 | −0.067 | |
| Cond | 0.312 | 0.035 | −0.084 | 0.029 | −0.122 | −0.005 | −0.024 | |
| CODMn | 0.285 | −0.073 | −0.145 | 0.070 | 0.232 * | 0.220 * | 0.096 | |
| TN | −0.148 | 0.087 | −0.095 | −0.091 | −0.072 | 0.281 ** | 0.065 | |
| TP | 0.070 | 0.015 | 0.023 | 0.072 | 0.212 * | 0.098 | 0.077 | |
| NH4+-N | 0.153 | 0.020 | 0.036 | −0.201 | −0.067 | 0.080 | −0.176 | |
| NO3−-N | 0.055 | −0.240 | 0.060 | −0.048 | −0.139 | 0.378 ** | 0.176 | |
| Average of each layer | DO | 0.035 | 0.179 ** | 0.062 * | 0.143 ** | 0.170 ** | 0.080 ** | 0.011 |
| WT | 0.094 ** | 0.306 ** | 0.188 ** | 0.223 ** | 0.268 ** | 0.204 ** | 0.168 ** | |
| pH | 0.091 * | 0.019 | 0.024 | −0.060 | 0.072 * | 0.114 ** | 0.058 * | |
| Cond | 0.041 | 0.034 | 0.056 | −0.019 | 0.003 | 0.010 | 0.033 | |
| CODMn | 0.107 ** | 0.073 * | −0.021 | 0.070 * | 0.186 ** | 0.066 * | 0.072 * | |
| TN | 0.060 | 0.022 | 0.075 * | −0.016 | −0.003 | 0.032 | 0.124 ** | |
| TP | 0.144 ** | 0.061 | 0.013 | 0.042 | 0.039 | 0.068 * | 0.046 | |
| NH4+-N | 0.083 * | −0.002 | 0.031 | 0.046 | 0.009 | 0.053 | −0.011 | |
| NO3−-N | 0.072 * | 0.019 | 0.051 | 0.042 | −0.027 | 0.039 | 0.124 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cui, Z.; Gao, W.; Li, Y.; Wang, W.; Wang, H.; Liu, H.; Fan, P.; Fohrer, N.; Wu, N. Dissolved Oxygen and Water Temperature Drive Vertical Spatiotemporal Variation of Phytoplankton Community: Evidence from the Largest Diversion Water Source Area. Int. J. Environ. Res. Public Health 2023, 20, 4307. https://doi.org/10.3390/ijerph20054307
Cui Z, Gao W, Li Y, Wang W, Wang H, Liu H, Fan P, Fohrer N, Wu N. Dissolved Oxygen and Water Temperature Drive Vertical Spatiotemporal Variation of Phytoplankton Community: Evidence from the Largest Diversion Water Source Area. International Journal of Environmental Research and Public Health. 2023; 20(5):4307. https://doi.org/10.3390/ijerph20054307
Chicago/Turabian StyleCui, Zhenzhen, Wanli Gao, Yuying Li, Wanping Wang, Hongtian Wang, Han Liu, Panpan Fan, Nicola Fohrer, and Naicheng Wu. 2023. "Dissolved Oxygen and Water Temperature Drive Vertical Spatiotemporal Variation of Phytoplankton Community: Evidence from the Largest Diversion Water Source Area" International Journal of Environmental Research and Public Health 20, no. 5: 4307. https://doi.org/10.3390/ijerph20054307
APA StyleCui, Z., Gao, W., Li, Y., Wang, W., Wang, H., Liu, H., Fan, P., Fohrer, N., & Wu, N. (2023). Dissolved Oxygen and Water Temperature Drive Vertical Spatiotemporal Variation of Phytoplankton Community: Evidence from the Largest Diversion Water Source Area. International Journal of Environmental Research and Public Health, 20(5), 4307. https://doi.org/10.3390/ijerph20054307








