Arsenic in Mining Areas: Environmental Contamination Routes
Abstract
1. Introduction
2. Materials and Methods
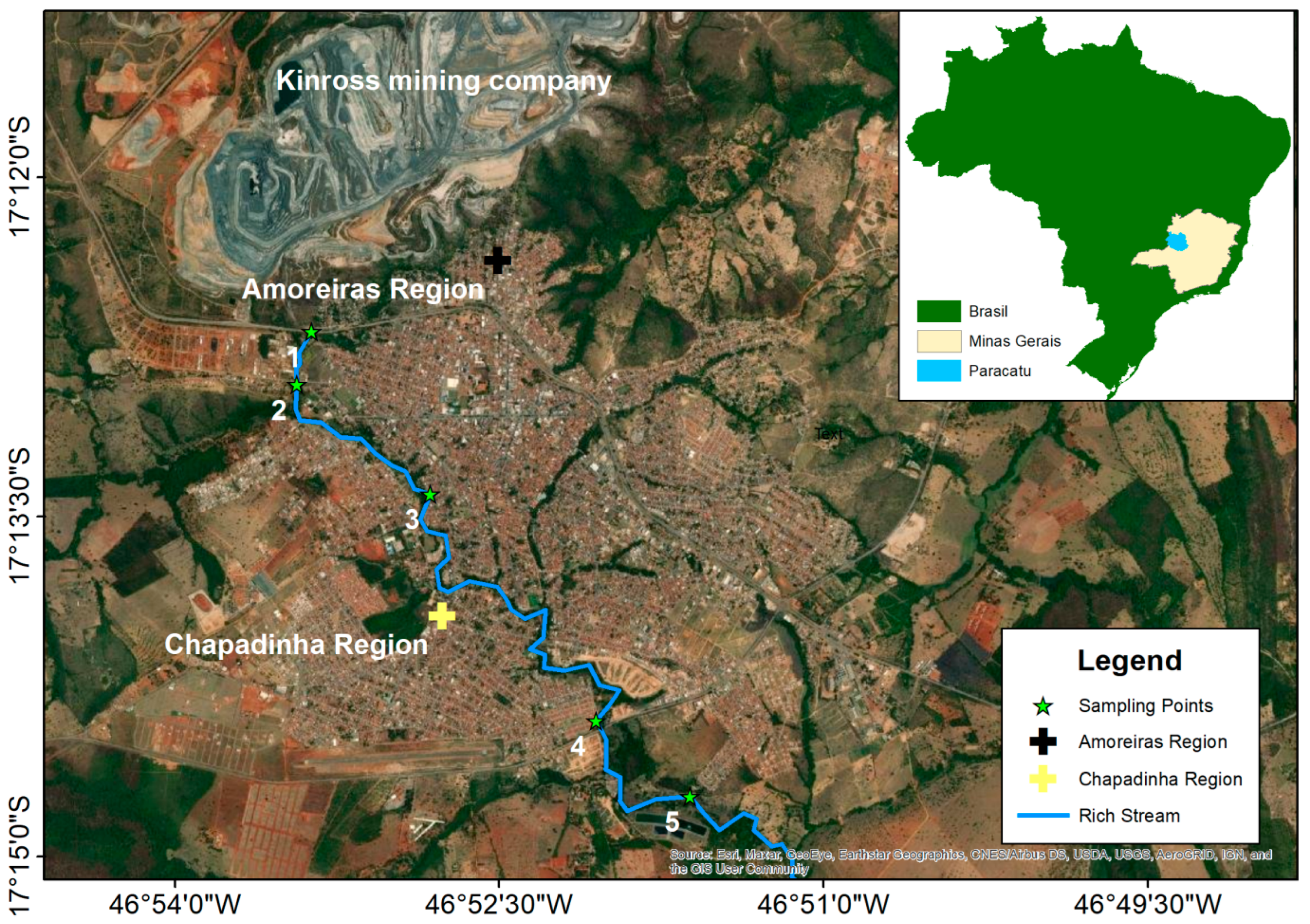
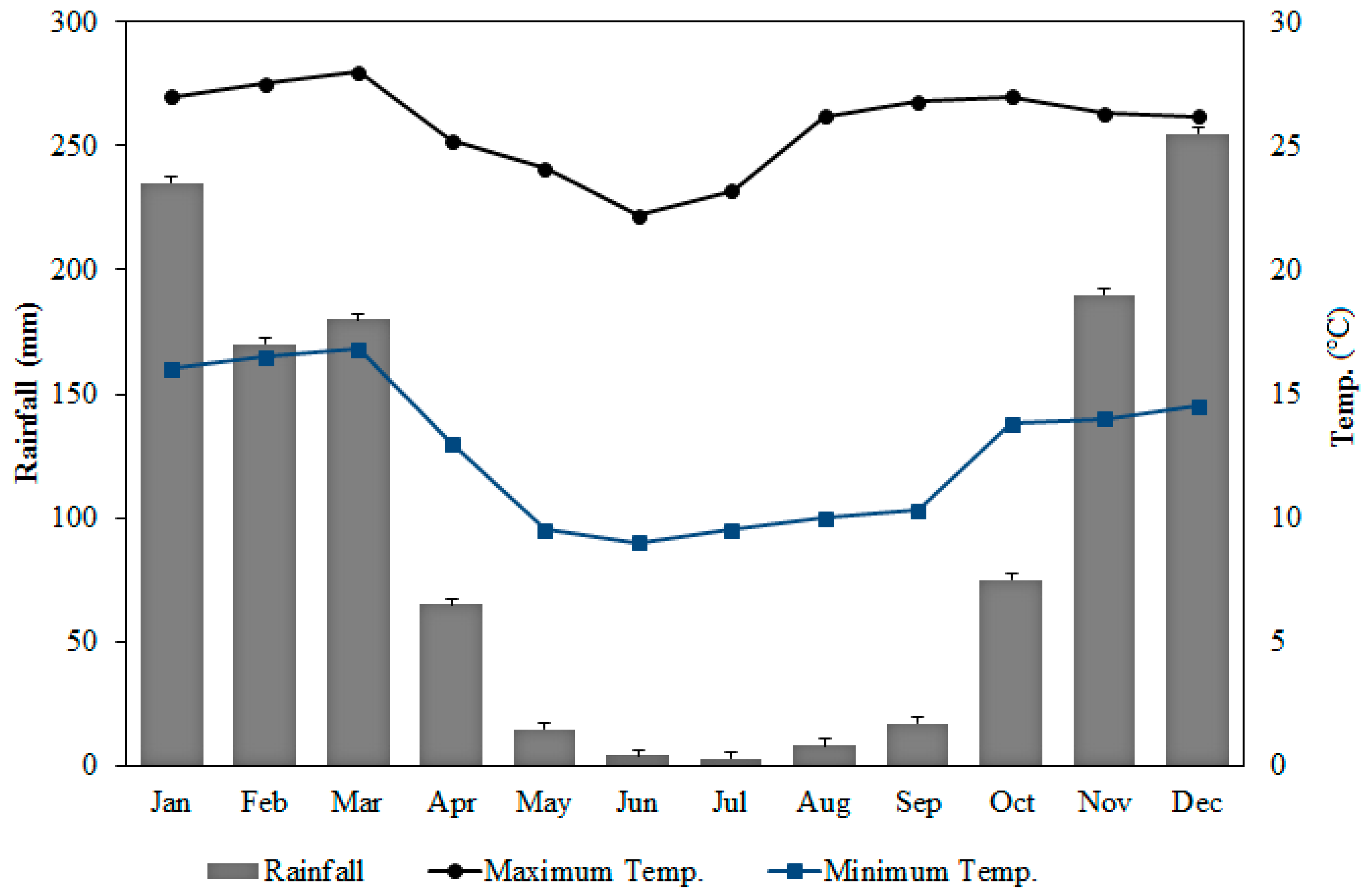
2.1. Study Location and Collection Points
2.2. Materials and Chemicals
2.3. Analysis of Arsenic from Environmental Samples
2.3.1. Quantification of Arsenic in the Samples
2.3.2. Soil Samples
2.3.3. Water Samples
2.3.4. Particulate Material Samples
2.4. Determination of Hydrogen Potential (pH), Water Hardness, and Turbidity
2.5. Quantification of As Species in Maize, Cassava, and Fish
2.6. Allium Cepa Test
3. Results and Discussion
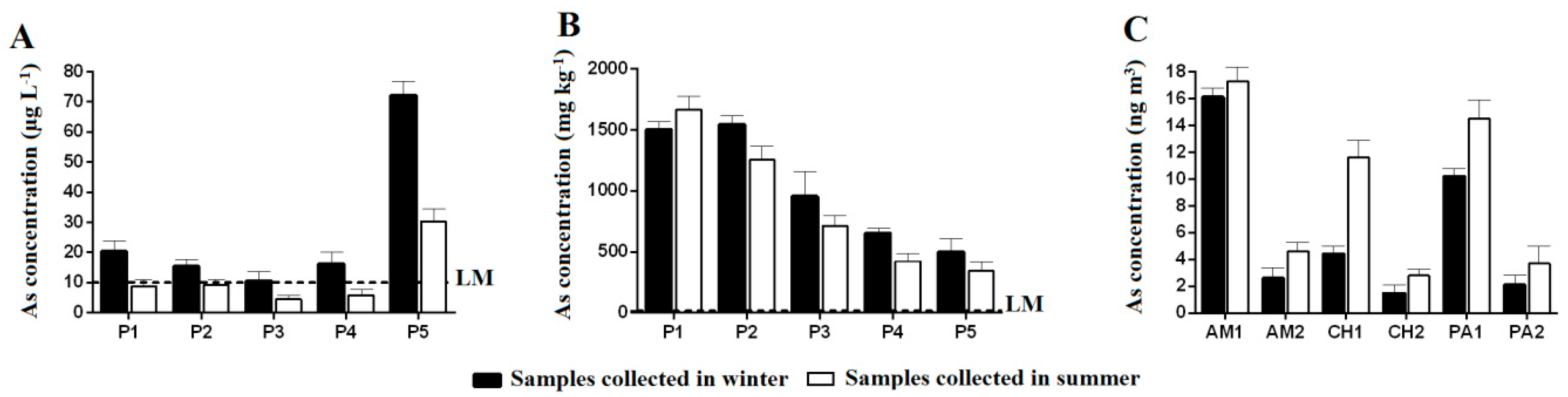
3.1. Physicochemical Parameters and Arsenic Concentration in Surface Water and Soil on the Banks of the Rico Stream
3.2. Determination of As Concentration in Particulate Matter (PM) of Atmospheric Air in the City of Paracatu, MG
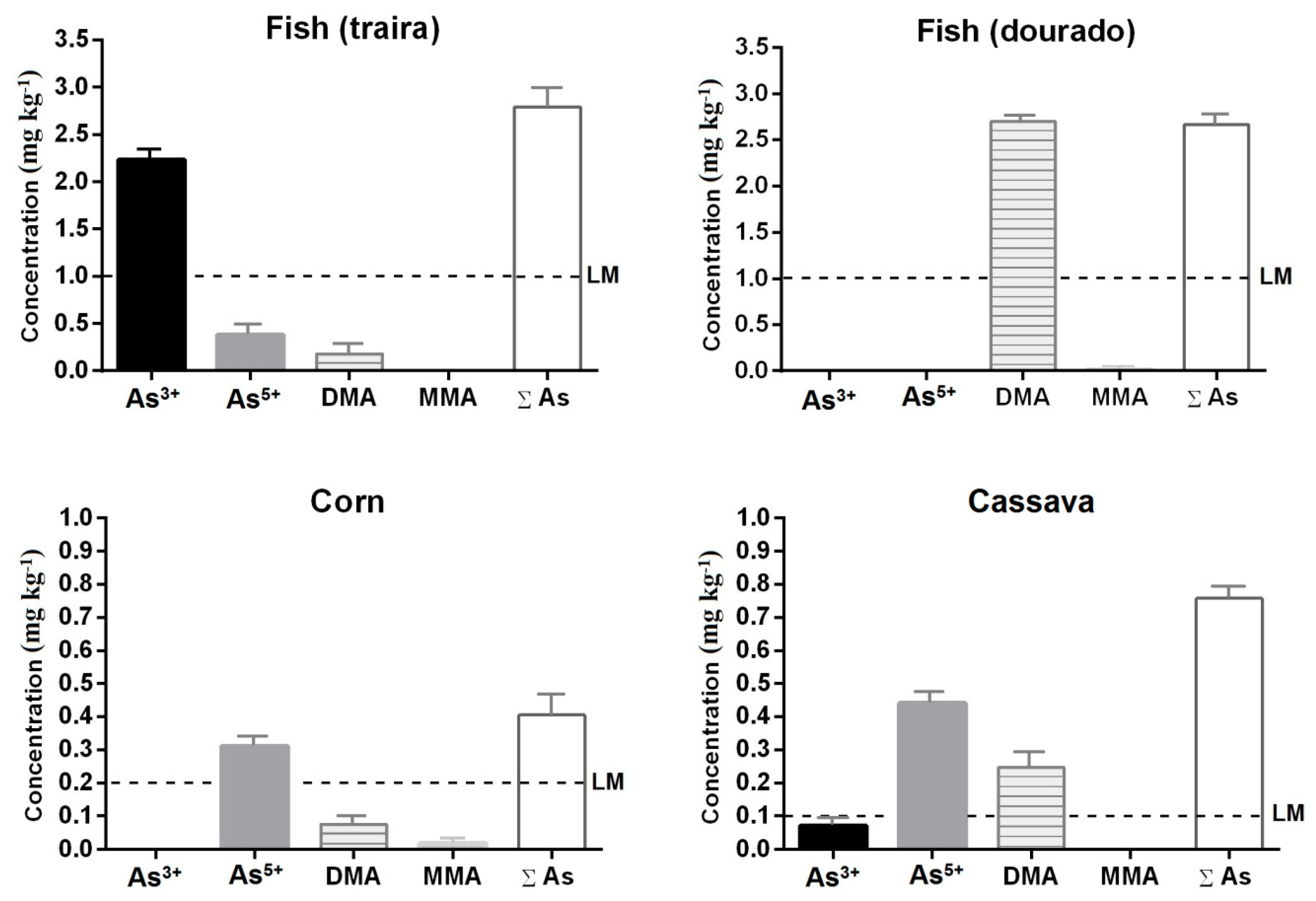
3.3. Quantification of As Species (As3+, As5+, MMA, and DMA) in Samples of Corn, Cassava, and Fish Exposed to Water and Soil on the Banks of the Rico Stream
3.4. Allium Cepa Test
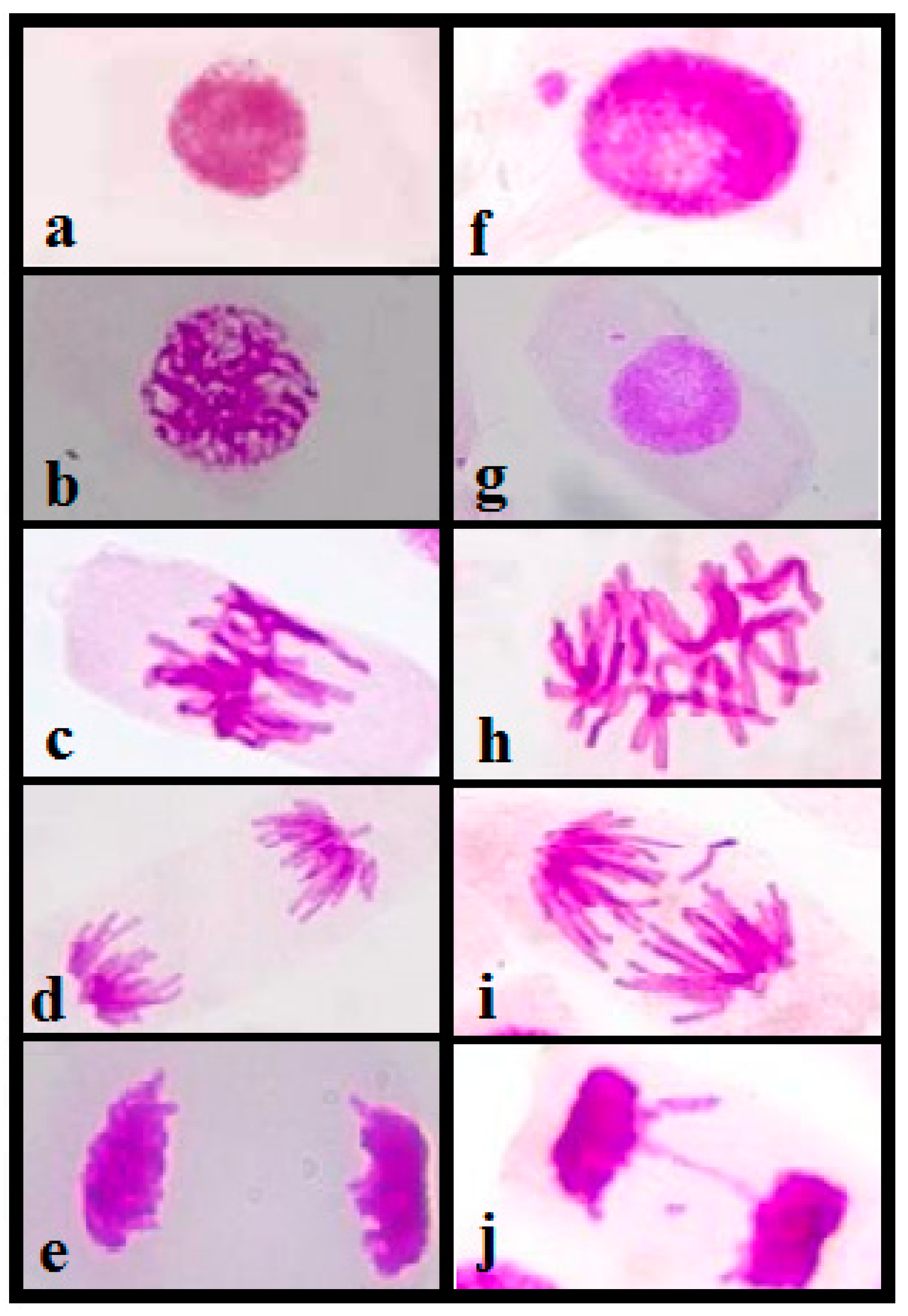
3.4.1. Analysis of Toxicity of Rico Stream Waters
3.4.2. Mitotic Index
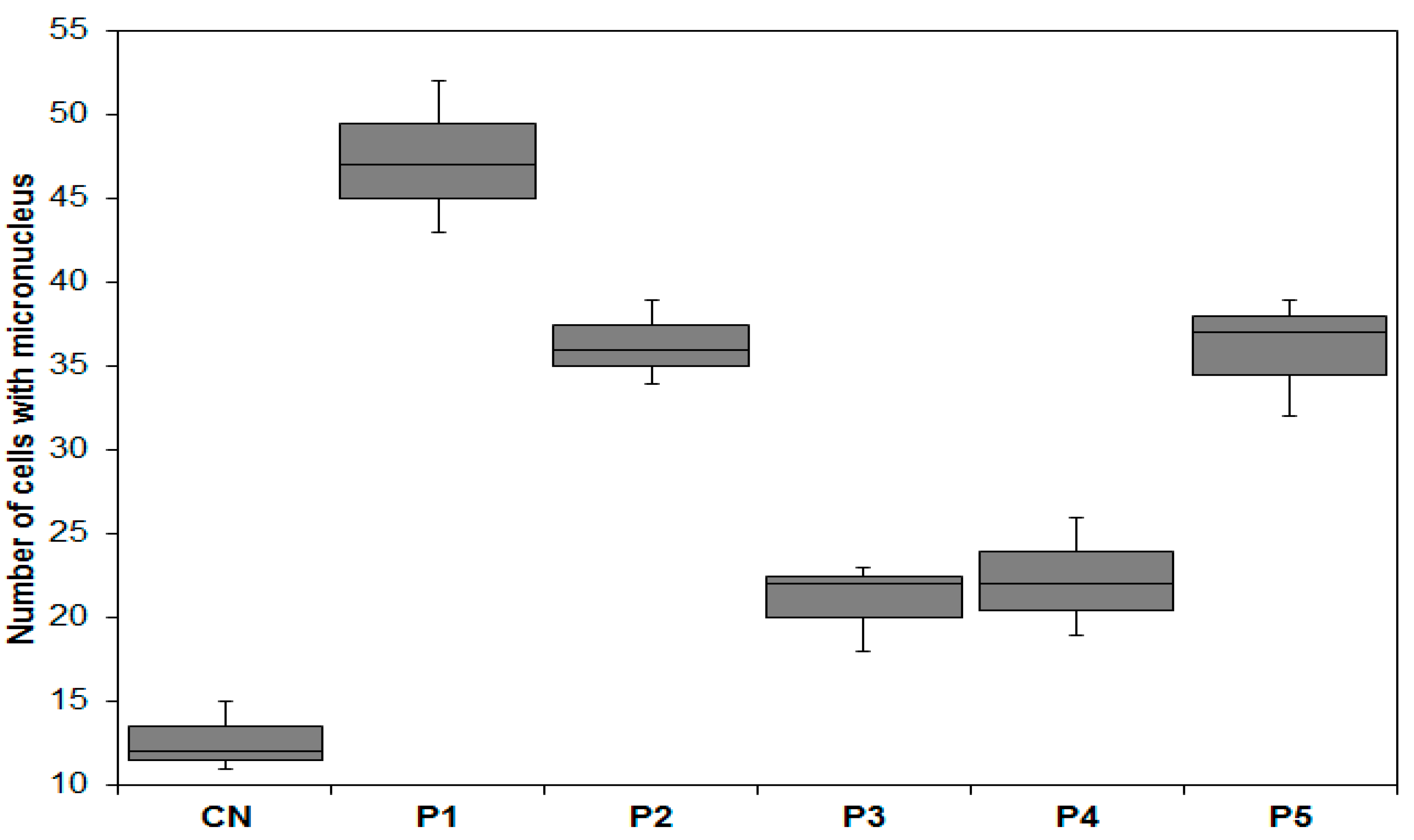
3.4.3. Genotoxicity Analysis
3.4.4. Mutagenicity Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bhattacharya, P.; Welch, A.H.; Stollenwerk, K.G.; McLaughlin, M.J.; Bundschuh, J.; Panaullah, G. Arsenic in the environment: Biology and Chemistry. Sci. Total Environ. 2007, 379, 109–120. [Google Scholar] [CrossRef] [PubMed]
- ATSDR. ATSDR’s Substance Priority List. Agency for Toxic Substances and Disease Registry. 2019. Available online: https://www.atsdr.cdc.gov/ (accessed on 1 December 2022).
- Wang, M.; Bera, G.; Mitra, K.; Wade, T.L.; Knap, A.H.; Phillips, T.D. Tight sorption of arsenic, cadmium, mercury, and lead by edible activated carbon and acid-processed montmorillonite clay. Environ. Sci. Pollut. Res. 2020, 28, 6758–6770. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Ballesta, R.; Bravo, S.; Pérez-de-los-Reyes, C.; Amorós, J.A.; Garcia-Navarro, F.J. Contents and Spatial Distribution of Arsenic in Vineyard Soils in Mediterranean Environment. Water Air Soil Pollut. 2023, 234, 83. [Google Scholar] [CrossRef]
- Marchant, B.P.; Saby, N.; Arrouays, D. A survey of topsoil arsenic and mercury concentrations across France. Chemosphere 2017, 181, 635–644. [Google Scholar] [CrossRef]
- Yang, Q.; Li, Z.; Lu, X.; Duan, Q.; Huang, L.; Bi, J. A review of soil heavy metal pollution from industrial and agricultural regions in china: Pollution and risk assessment. Sci. Total Environ. 2018, 642, 690–700. [Google Scholar] [CrossRef]
- Zuzolo, D.; Cicchella, D.; Demetriades, A.; Birke, M.; Albanese, S.; Dinelli, E.; Lima, A.; Valera, P.; De Vivo, B. Arsenic: Geochemical distribution and age-related health risk in Italy. Environ. Res. 2020, 182, 109076. [Google Scholar] [CrossRef]
- Dos Santos Farias, J.; Milani, M.R.; Niencheski, L.F.H.; De Paiva, M.L. Especiação química De arsênio inorgânico no Estuário Da Laguna Dos Patos (RS, Brasil). Química Nova 2012, 35, 1401–1406. [Google Scholar] [CrossRef]
- Borba, R.P.; Coscione, A.R.; Figueiredo, B.R.; Zambello, F. Estudo da especiação de arsênio inorgânico e determinação de arsênio total no monitoramento ambiental da qualidade de águas subterrâneas. Química Nova 2009, 32, 970–975. [Google Scholar] [CrossRef]
- Styblo, M.; Del Razo, L.M.; Vega, L.; Germolec, D.R.; LeCluyse, E.L.; Hamilton, G.A.; Reed, W.; Wang, C.; Cullen, W.R.; Thomas, D.J. Comparative toxicity of trivalent and pentavalent inorganic and methylated arsenicals in rat and human cells. Arch. Toxicol. 2000, 74, 289–299. [Google Scholar] [CrossRef]
- Rezende, P.S.; Costa, L.M.; Windmöller, C.C. Arsenic Mobility in Sediments from Paracatu River Basin, MG, Brazil. Arch. Environ. Contam Toxicol. 2015, 68, 588–602. [Google Scholar] [CrossRef]
- Mandal, B.K.; Suzuki, K.T. Arsenic round the world: A review. Talanta 2002, 58, 201–235. [Google Scholar] [CrossRef]
- De Andrade, R.P.; Filho, S.S.; De Mello, J.W.V.; De Figueiredo, B.R.; Dussin, T.M. Arsenic mobilization from sulfidic materials from gold mines in Minas Gerais State. Química Nova 2008, 31, 1127–1130. [Google Scholar] [CrossRef]
- De Andrade, R.P.; De Mello, J.W.V.; Windmöller, C.C.; Da Silva, J.B.B.; Figueiredo, B.R. Evaluation of arsenic availability in sulfidic materials from gold mining areas in Brazil. Water Air Soil Pollut. 2012, 223, 4679–4686. [Google Scholar] [CrossRef]
- Thompson, D.J. A chemical hypothesis for arsenic methylation in mammals. Chem. Biol. Interact. 1993, 88, 89–114. [Google Scholar] [CrossRef] [PubMed]
- Klaasen, C.D. Casarett and Doull’s Toxicology: The Basic Science of Poison, 7th ed.; McGraw-Hill: New York, NY, USA, 2007. [Google Scholar]
- Jasrotia, S.; Kansal, A.; Kishore, V.V.N. Application of solar energy for water supply and sanitation in arsenic affected rural areas: A study for Kaudikasa village, India. J. Clean. Prod. 2013, 60, 102–106. [Google Scholar] [CrossRef]
- Jang, Y.-C.; Somanna, Y.; Kim, H. Source, Distribution, Toxicity and Remediation of Arsenic in the Environment—A review. Int. J. Appl. Environ. Sci. 2016, 11, 559–581. [Google Scholar]
- Ng, J.C.; Ciminelli, V.; Gasparon, M.; Caldeira, C. Health risk apportionment of arsenic from multiple exposure pathways in Paracatu, a gold mining town in Brazil. Sci. Total Environ. 2019, 673, 36–43. [Google Scholar] [CrossRef]
- Brewer, R.; Belzer, W. Assessment of metal concentrations in atmospheric particles from Burnaby Lake, British Columbia, CAN. Atmos Environ. 2001, 35, 5223–5233. [Google Scholar] [CrossRef]
- Yoshikawa, M.; Aoki, K.; Ebine, N.; Kusunoki, M.; Okamoto, A. Correlation between the arsenic concentrations in the air and the SMR of lung cancer. Environ. Health Prev. Med. 2008, 13, 207–218. [Google Scholar] [CrossRef]
- Hettick, B.E.; Cañas-Carrell, J.E.; French, A.D.; Klein, D.M. Arsenic: A Review of the Element’s Toxicity, Plant Interactions, and Potential Methods of Remediation. J. Agric. Food Chem. 2015, 63, 7097–7107. [Google Scholar] [CrossRef]
- Barra, C.M.; Santelli, R.E.; Abrão, J.J.; Guardia, M.D.L. Especiação de Arsênio—Uma Revisão. Química Nova 2000, 23, 58–70. [Google Scholar] [CrossRef]
- Wang, S.; Mulligan, C.N. Occurrence of arsenic contamination in Canada: Sources, behavior and distribution. Sci. Total Environ. 2006, 366, 701–721. [Google Scholar] [CrossRef] [PubMed]
- Mertens, F.; Távora, R.; Nakano, E.Y.; Castilhos, Z.C. Information sources, awareness and preventive health behaviors in a population at risk of Arsenic exposure: The role of gender and social networks. PLoS ONE 2017, 12, e0186130. [Google Scholar] [CrossRef] [PubMed]
- CETESB. Guia Nacional de Coleta e Preservação de Amostras—Água, Sedimento, Comunidades Aquáticas e Efluentes Líquidos. Cia Ambient. Do Estado São Paulo 2011, 11, 85–90. [Google Scholar]
- Pruszkowski, E.; Bosnak, C. The Analysis of Drinking Waters by U.S. EPA Method 200.8 Using the NexION 300Q/350Q ICP-MS in Standard Mode; PerkinElmer Application Note; United States Environmental Protection Agency: Washington, DC, USA, 2012.
- USEPA. Method 3051A: Microwave assisted digestion of sediments, sludges, soils and oils. Z. Für Anal. Chem. 2007, 111, 362–366. [Google Scholar]
- Siciliano, B.; Dantas, G.; da Silva, C.M.; Arbilla, G. The updated Brazilian national air quality standards: A critical review. J. Braz. Chem. Soc. 2020, 31, 523–535. [Google Scholar] [CrossRef]
- States, U.; Protection, E. Compendium of Methods for the Determination of Toxic Organic Compounds in Ambient Air—Second Edition Compendium of Methods Determination of Toxic Organic Compounds in Ambient Air. Environ. Res. 1999. [Google Scholar]
- Standard Test Method for Determination of Volatile Organic Chemicals in Atmospheres (Canister Sampling Methodology); USEPA—United States Environmental Protection Agency: Washington, DC, USA, 2011.
- APHA; Awwa, W. Standard Methods for Examination of Water and Wastewater. Wash. Am. Public Health Assoc. 2012, 1, 185. [Google Scholar]
- Rocha, B.C.; Almeida, E.A.; Moreira, A.B.; Bisinoti, M.C. Relação entre acúmulo de metais em tecido muscular de peixes com diferentes hábitos alimentares oriundos da bacia hidrográfica do Turvo/Grande. Periódico Eletrônico Fórum Ambiental da Alta Paulista 2015, 11. [Google Scholar] [CrossRef]
- Batista, B.L.; de Oliveira Souza, V.C.; da Silva, F.G.; Barbosa, F. Survey of 13 trace elements of toxic and nutritional significance in rice from Brazil and exposure assessment. Food Addit. Contam. Part B Surveill. 2010, 3, 253–262. [Google Scholar] [CrossRef]
- Hott, R.C.; Magalhães, T.S.; Maia, L.F.O.; Santos, K.S.F.; Rodrigues, G.L.; Oliveira, L.C.A.; Pereira, M.C.; Faria, M.C.S.; Carli, A.P.; Souza Alves, C.C.; et al. Purification of arsenic-contaminated water using iron molybdate filters and monitoring of their genotoxic, mutagenic, and cytotoxic effects through bioassays. Environ. Sci. Pollut. Res. Int. 2021, 28, 5714–5730. [Google Scholar] [CrossRef]
- Conama CN do, M.A. RESOLUÇÃO CONAMA n. 357, de 17 de março de 2005 Publicada no DOU n. Diário da União 2005, 4, 7259–7297. [Google Scholar]
- Portaria Ministério da Saúde/Gabinete do Ministro GM/MS n. 888, 4 May 2021. Para Dispor Sobre os Proced-imentos de Controle e de Vigilância da Qualidade da Água para Consumo Humano e Seu Padrão de Potabilidade. Diário Oficial da União. Brasília, Brazil, n. 85, p. 127, 7 May 2021, Section 1. 2021. Available online: https://bvsms.saude.gov.br/bvs/saudelegis/gm/2021/prt0888_07_05_2021.html (accessed on 12 October 2022).
- Rizzatti, I.M.; Barbosa, M.T.; Fernandes, F.D.S.; Silva, I.O.; De Moura, R.C. Avaliação de Parâmetros Físicos, Químicos E Microbiológios de Água de Alguns Poços do Bairro Jardim das Copaíbas, Boa Vista, Roraima. Ambient. Gestão e Desenvolv. 2018, 11, 17–32. [Google Scholar] [CrossRef]
- Parron, L.M.; Muniz, D.H.; Pereira, C.M. Manual de procedimentos de amostragem e análise físico-química de água. EMBRAPA—Empresa Brasileira de Pesquisa Agropecuária, Embrapa Florestas. Ministério da Agricultura, Pecuária e Abastecimento. 2011. Available online: https://www.embrapa.br/florestas/busca-de-publicacoes/-/publicacao/921050/manual-de-procedimentos-de-amostragem-e-analise-fisico-quimica-de-agua (accessed on 12 December 2022).
- WHO. Guidelines for Drinking-Water Quality, 4th ed.; WHO: Geneva, Switzerland, 2011; p. 340.
- Ministério da Saúde. Portaria MS No 518/2004. 2004. Available online: https://bvsms.saude.gov.br/bvs/publicacoes/portaria_518_2004.pdf (accessed on 11 February 2023).
- Conselho Nacional do Meio Ambiente (CONAMA). Resolução n° 344, de 25 de Março de 2004. Estabelece as Diretrizes Gerais e os Procedimentos Mínimos para a Avaliação do Material a ser Dragado em Águas Jurisdicionais Brasileiras, e dá Outras Providências. 2004. Available online: http://www.mma.gov.br/port/conama/legiabre.cfm?codlegi=445 (accessed on 5 February 2023).
- Nacional do Meio Ambiente (CONAMA). Resolução n° 420/2009: Dispõe Sobre os Critérios e Valores Orientadores de Qualidade do solo Quanto à Presença de Substâncias Químicas e Estabelece Diretrizes para o Gerenciamento Ambiental de Áreas Contaminadas por Essas Substâncias em Decorrência de Atividades Antrópica. 2009. Available online: https://cetesb.sp.gov.br/areas-contaminadas/wp-content/uploads/sites/17/2017/09/resolucao-conama-420-2009-gerenciamento-de-acs.pdf (accessed on 5 February 2023).
- Silva, S.R.; Procópio, S.O.; Queiroz, T.F.N.; Dias, L.E. Caracterização de rejeito de mineração de ouro para avaliação de solubilização de metais pesados e arsênio e revegetação local. Rev. Bras. Ciência Do Solo 2004, 28, 189–196. [Google Scholar] [CrossRef]
- Instituto Mineiro de Gestão das Águas. Portaria n° 48, de 04 de outubro de 2019: Estabelece Normas Suplementares para a Regularização dos Recursos Hídricos de Domínio do Estado de Minas Gerais e dá Outras Providências. 2019. Available online: http://www.siam.mg.gov.br/sla/download.pdf?idNorma=49719 (accessed on 16 October 2022).
- Huang, G.; London, J.K. Cumulative environmental vulnerability and environmental justice in California’s San Joaquin Valley. Int. J. Environ. Res. Public Health 2012, 9, 1593. [Google Scholar] [CrossRef] [PubMed]
- ANVISA. Resolução—RDC N° 42, de 29 de agosto de 2013. Dispõe sobre o Regulamento Técnico MERCOSUL Sobre Limites Máximos de Contaminantes Inorgânicos em Alimentos, Brasil, DOU p. 1. 2013. Available online: https://bvsms.saude.gov.br/bvs/saudelegis/anvisa/2013/rdc0042_29_08_2013.html (accessed on 11 February 2023).
- Soares, J.L.F.; Goch, Y.G.F.; Peleja, J.R.P.; Forsberg, B.; Lemos, E.; Sousa, O. Bioacumulação de Mercúrio Total (HgT) e Hábitos Alimentares de Peixes da Bacia do Rio Negro, Amazônia, Brasil. Biota Amaz. 2016, 6, 102–106. [Google Scholar] [CrossRef]
- Kalantzi, I.; Mylona, K.; Sofoulaki, K.; Tsapakis, M.; Pergantis, S.A. Arsenic speciation in fish from Greek coastal areas. J. Environ. Sci. 2017, 56, 300–312. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Guo, Z.; Zhou, Y.; Chen, L.; Zhang, L. Comparative contribution of trophic transfer and biotransformation on arsenobetaine bioaccumulation in two marine fish. Aquat. Toxicol. 2016, 179, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Thomas, D.J. Molecular processes in cellular arsenic metabolism. Toxicol. Appl. Pharmacol. 2007, 222, 365–373. [Google Scholar] [CrossRef] [PubMed]
- Munera-Picazo, S.; Burló, F.; Carbonell-Barrachina, Á.A. Arsenic speciation in rice-based food for adults with celiac disease. Food Addit. Contam.—Part A Chem. Anal. Control Expo Risk Assess. 2014, 31, 1358–1366. [Google Scholar] [CrossRef]
- Sharples, J.M.; Meharg, A.A.; Chambers, S.M.; Cairney, J.W.G. Mechanism of arsenate resistance in the ericoid mycorrhizal fungus Hymenoscyphus ericae. Plant Physiol. 2000, 124, 1327–1334. [Google Scholar] [CrossRef] [PubMed]
- Kabata-Pendias, A. Trace Elements in Soils and Plants, 4th ed.; CRC Press: Boca Raton, FL, USA, 2010. [Google Scholar]
- Bianchi, J.; Espindola, E.L.G.; Marin-Morales, M.A. Genotoxicity and mutagenicity of water samples from the Monjolinho River (Brazil) after receiving untreated effluents. Ecotoxicol. Environ. Saf. 2011, 74, 826–833. [Google Scholar] [CrossRef] [PubMed]
- Kumari, M.; Mukherjee, A.; Chandrasekaran, N. Genotoxicity of silver nanoparticles in Allium cepa. Sci. Total Environ. 2009, 407, 5243–5246. [Google Scholar] [CrossRef] [PubMed]
- Darlington, C.D.; McLeish, J. Action of maleic hydrazide on the cell. Nature 1951, 167, 407–408. [Google Scholar] [CrossRef] [PubMed]
- Faita, F.; Cori, L.; Bianchi, F.; Andreassi, M.G. Arsenic-induced genotoxicity and genetic susceptibility to arsenic-related pathologies. Int. J. Environ. Res. Public Health 2013, 10, 1527–1546. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, S.T.; Mantovani, M.S.; Malaguttii, M.I.A.; Dias, A.L.; Fonseca, I.C.; Marin-Morales, M.A. Genotoxicity and mutagenicity of water contaminated with tannery effluents as evaluated by the micronucleus test and comet assay using the fish Oreochromis niloticus and chromosome aberrations in onion root-tips. Genet. Mol. Biol. 2006, 29, 148–158. [Google Scholar] [CrossRef]
| Analysis References | Collection Points | |||||
|---|---|---|---|---|---|---|
| P1 | P2 | P3 | P4 | P5 | ||
| pH Summer | 6.0–9.0 | 6.0 | 6.5 | 6.9 | 7.0 | 7.0 |
| pH Winter | 6.4 | 6.8 | 7.1 | 7.1 | 7.1 | |
| Ca2+ and MgCa2+ (mg L−1) Summer | - | 94.0 | 88.0 | 29.0 | 40.0 | 47.0 |
| CaCa2+ and MgCa2+ (mg L−1) Winter | 49.0 | 41.0 | 19.0 | 35.0 | 48.0 | |
| Turbidity (UNT) Summer | <40 | 1.7 | 0.87 | 2.1 | 1.0 | 3.0 |
| Turbidity (UNT) Winter | 3.5 | 8.3 | 1.6 | 0.71 | 2.1 | |
| Samples | P1 | P2 | P3 | P4 | P5 | NC |
|---|---|---|---|---|---|---|
| ALR (cm) | 0.25 ± 0.3 a | 0.27 ± 0.2 a | 0.18 ± 0.2 b | 0.37 ± 0.2 c | 0.12 ± 0.1 d | 0.42 ± 0.2 e |
| Inhibition rate | 41.00% | 37.00% | 55.30% | 11.31% | 66.22% | - |
| Samples | P1 | P2 | P3 | P4 | P5 | NC |
|---|---|---|---|---|---|---|
| MI | 2.66% a | 2.03% b | 1.75% c | 1.93% c | 2.20% b | 5.96% d |
| Samples | NCA | Bridges | Losses | Breaks | Sticky Chromosomes | C-Metaphase | Spindle Anomalies | CA |
|---|---|---|---|---|---|---|---|---|
| NC | 5000 | 2 | 2 | 19 | 0 | 4 | 0 | 27 a |
| Water P1 | 5000 | 9 | 3 | 28 | 20 | 24 | 2 | 86 b |
| Water P2 | 5000 | 7 | 1 | 22 | 20 | 17 | 1 | 68 c |
| Water P3 | 5000 | 5 | 0 | 17 | 18 | 12 | 0 | 52 d |
| Water P4 | 5000 | 4 | 3 | 16 | 5 | 12 | 0 | 40 e |
| Water P5 | 5000 | 7 | 4 | 23 | 22 | 21 | 3 | 80 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Faria, M.C.d.S.; Hott, R.d.C.; Santos, M.J.d.; Santos, M.S.; Andrade, T.G.; Bomfeti, C.A.; Rocha, B.A.; Barbosa, F., Jr.; Rodrigues, J.L. Arsenic in Mining Areas: Environmental Contamination Routes. Int. J. Environ. Res. Public Health 2023, 20, 4291. https://doi.org/10.3390/ijerph20054291
Faria MCdS, Hott RdC, Santos MJd, Santos MS, Andrade TG, Bomfeti CA, Rocha BA, Barbosa F Jr., Rodrigues JL. Arsenic in Mining Areas: Environmental Contamination Routes. International Journal of Environmental Research and Public Health. 2023; 20(5):4291. https://doi.org/10.3390/ijerph20054291
Chicago/Turabian StyleFaria, Márcia Cristina da Silva, Rodrigo de Carvalho Hott, Maicon Junior dos Santos, Mayra Soares Santos, Thainá Gusmão Andrade, Cleide Aparecida Bomfeti, Bruno Alves Rocha, Fernando Barbosa, Jr., and Jairo Lisboa Rodrigues. 2023. "Arsenic in Mining Areas: Environmental Contamination Routes" International Journal of Environmental Research and Public Health 20, no. 5: 4291. https://doi.org/10.3390/ijerph20054291
APA StyleFaria, M. C. d. S., Hott, R. d. C., Santos, M. J. d., Santos, M. S., Andrade, T. G., Bomfeti, C. A., Rocha, B. A., Barbosa, F., Jr., & Rodrigues, J. L. (2023). Arsenic in Mining Areas: Environmental Contamination Routes. International Journal of Environmental Research and Public Health, 20(5), 4291. https://doi.org/10.3390/ijerph20054291







