Effects of Magnetic Biochar Addition on Mesophilic Anaerobic Digestion of Sewage Sludge
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of MBC
2.2. Sewage Sludge Samples
2.3. Batch Experimental Setup
2.4. Analytical Methods
2.5. Kinetic Models
3. Results and Discussion
3.1. Characterization of MBC
3.2. Cumulative Biogas Yield
3.3. Effects of MBC on AD Performance
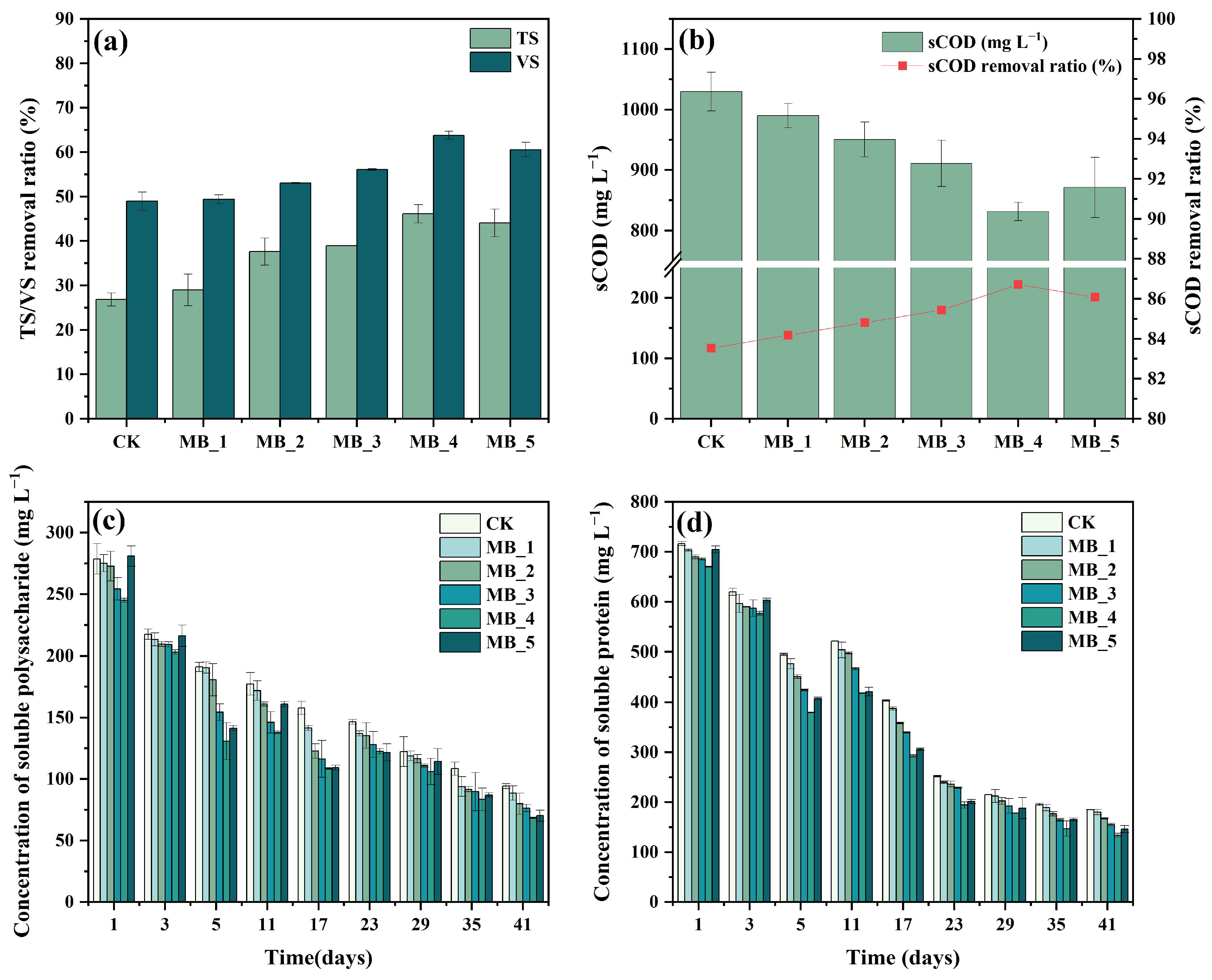
3.3.1. Removal of Organic Matter
3.3.2. Change of Soluble Protein and Soluble Polysaccharide
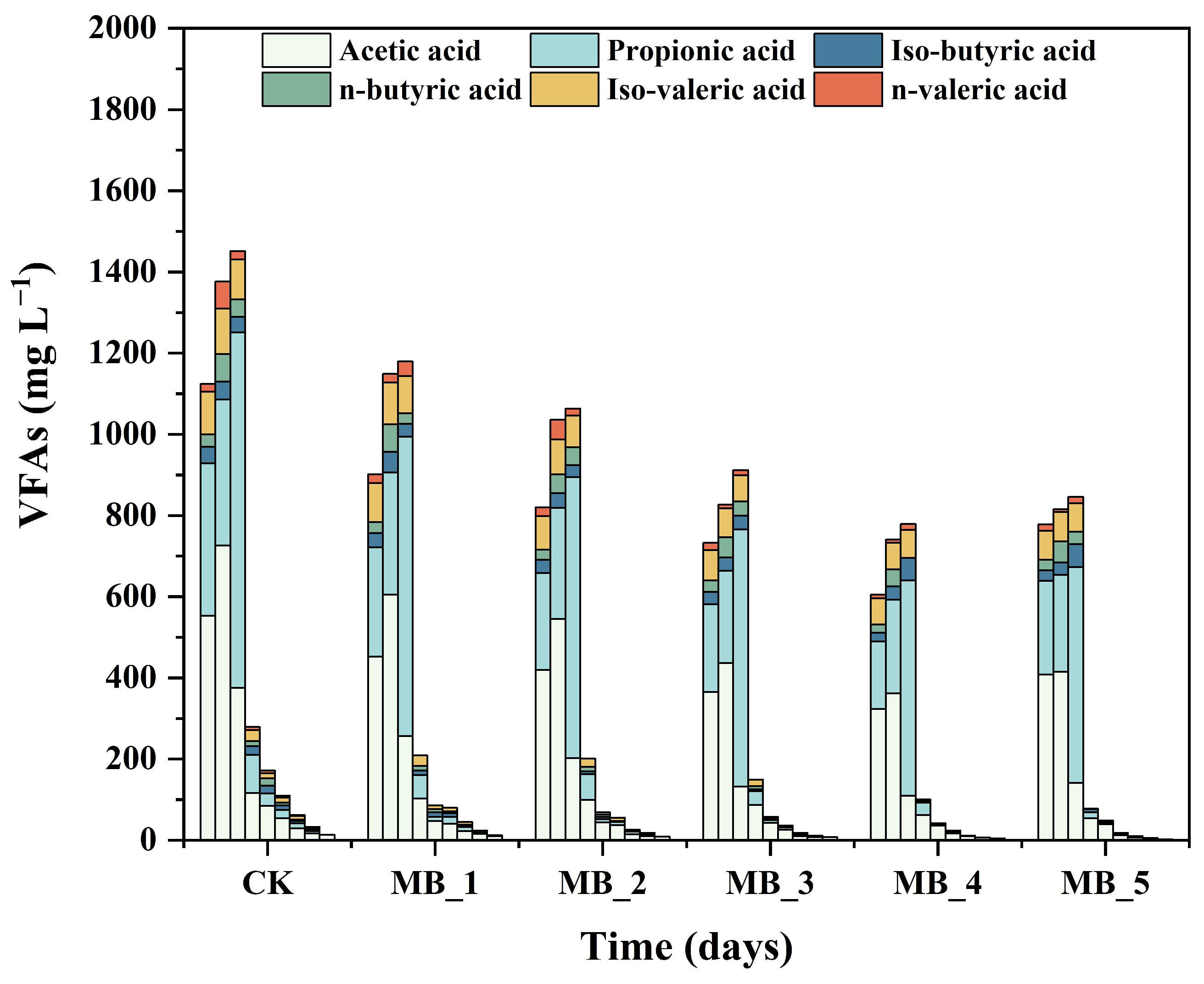
3.3.3. Change of VFAs and pH
3.4. Kinetic Analyses
3.5. Iron Concentration
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Wu, Q.; Zou, D.; Zheng, X.; Liu, F.; Li, L.; Xiao, Z. Effects of antibiotics on anaerobic digestion of sewage sludge: Performance of anaerobic digestion and structure of the microbial community. Sci. Total Environ. 2022, 845, 157384. [Google Scholar] [CrossRef] [PubMed]
- Tian, M.; Liu, F.; Guo, J.; Li, W.; Zhang, M.; Li, X. Effect of different acid and base potassium ferrate pretreatment on organic acid recovery by anaerobic digestion of sludge. Int. J. Environ. Res. Public Health 2022, 19, 15093. [Google Scholar] [CrossRef] [PubMed]
- Isha, A.; D’ Silva, T.C.; Subbarao, P.M.V.; Chandra, R.; Vijay, V.K. Stabilization of anaerobic digestion of kitchen wastes using protein-rich additives: Study of process performance, kinetic modelling and energy balance. Bioresour. Technol. 2021, 337, 125331. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Zhao, B.; Peng, M.; Shen, R.; Mao, L.; Zhang, W. Effects of inorganic passivators on gas production and heavy metal passivation performance during anaerobic digestion of pig manure and corn straw. Int. J. Environ. Res. Public Health 2022, 19, 14094. [Google Scholar] [CrossRef] [PubMed]
- Chandra, R.; Takeuchi, H.; Hasegawa, T. Methane production from lignocellulosic agricultural crop wastes: A review in context to second generation of biofuel production. Renew. Sustain. Energy Rev. 2012, 16, 1462–1476. [Google Scholar] [CrossRef]
- Rasapoor, M.; Young, B.; Brar, R.; Sarmah, A.; Zhuang, W.Q.; Baroutian, S. Recognizing the challenges of anaerobic digestion: Critical steps toward improving biogas generation. Fuel 2020, 261, 116497. [Google Scholar] [CrossRef]
- Xu, Y.; Gong, H.; Dai, X. High-solid anaerobic digestion of sewage sludge: Achievements and perspectives. Front. Environ. Sci. Eng. 2021, 15, 71. [Google Scholar] [CrossRef]
- Kazimierowicz, J.; Zieliński, M.; Bartkowska, I.; Dębowski, M. Effect of acid whey pretreatment using ultrasonic disintegration on the removal of organic compounds and anaerobic digestion efficiency. Int. J. Environ. Res. Public Health 2022, 19, 11362. [Google Scholar] [CrossRef]
- Romero-Güiza, M.S.; Vila, J.; Mata-Alvarez, J.; Chimenos, J.M.; Astals, S. The role of additives on anaerobic digestion: A review. Renew. Sustain. Energy Rev. 2016, 58, 1486–1499. [Google Scholar] [CrossRef]
- Bhuvaneshwari, S.; Hettiarachchi, H.; Meegoda, J.N. Crop residue burning in India: Policy challenges and potential solutions. Int. J. Environ. Res. Public Health 2019, 16, 832. [Google Scholar] [CrossRef] [PubMed]
- Yin, Q.; Wu, G. Advances in direct interspecies electron transfer and conductive materials: Electron flux, organic degradation and microbial interaction. Biotechnol. Adv. 2019, 37, 107443. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Monedero, M.A.; Cayuela, M.L.; Roig, A.; Jindo, K.; Mondini, C.; Bolan, N. Role of biochar as an additive in organic waste composting. Bioresour. Technol. 2018, 247, 1155–1164. [Google Scholar] [CrossRef] [PubMed]
- Kizito, S.; Jjagwe, J.; Mdondo, S.W.; Nagawa, C.B.; Bah, H.; Tumutegyereize, P. Synergetic effects of biochar addition on mesophilic and high total solids anaerobic digestion of chicken manure. J. Environ. Manag. 2022, 315, 115192. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Cao, L.; Fu, H.; Zhang, M.; Meng, J.; Althakafy, J.T.; Abo-Dief, H.M.; El-Bahy, S.M.; Zhang, Y.; Wei, H.; et al. Effect of sulfamethazine on anaerobic digestion of manure mediated by biochar. Chemosphere 2022, 306, 135567. [Google Scholar] [CrossRef] [PubMed]
- Baek, G.; Kim, J.; Cho, K.; Bae, H.; Lee, C. The biostimulation of anaerobic digestion with (semi) conductive ferric oxides: Their potential for enhanced biomethanation. Appl. Microbiol. Biotechnol. 2015, 99, 10355–10366. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Zhao, Z.; Zhang, Y. Magnetite-contained biochar derived from fenton sludge modulated electron transfer of microorganisms in anaerobic digestion. J. Hazard. Mater. 2021, 403, 123972. [Google Scholar] [CrossRef]
- Dong, Y.; Sanford, R.A.; Chang, Y.-j.; McInerney, M.J.; Fouke, B.W. Hematite reduction buffers acid generation and enhances nutrient uptake by a fermentative iron reducing bacterium, Orenia metallireducens Strain Z6. Environ. Sci. Technol. 2017, 51, 232–242. [Google Scholar] [CrossRef]
- Yu, B.; Zhang, D.; Shan, A.; Lou, Z.; Yuan, H.; Huang, X.; Yuan, W.; Dai, X.; Zhu, N. Methane-rich biogas production from waste-activated sludge with the addition of ferric chloride under a thermophilic anaerobic digestion system. RSC Adv. 2015, 5, 38538–38546. [Google Scholar] [CrossRef]
- Cieciura-Włoch, W.; Borowski, S.; Domański, J. Dark fermentative hydrogen production from hydrolyzed sugar beet pulp improved by iron addition. Bioresour. Technol. 2020, 314, 123713. [Google Scholar] [CrossRef]
- Tian, T.; Yu, H.-Q. Iron-assisted biological wastewater treatment: Synergistic effect between iron and microbes. Biotechnol. Adv. 2020, 44, 107610. [Google Scholar] [CrossRef]
- Rincón, B.; Banks, C.J.; Heaven, S. Biochemical methane potential of winter wheat (Triticum aestivum L.): Influence of growth stage and storage practice. Bioresour. Technol. 2010, 101, 8179–8184. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Liu, R.; Sun, C. Comparison of anaerobic digestion characteristics and kinetics of four livestock manures with different substrate concentrations. Bioresour. Technol. 2015, 198, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Shao, J.; Wang, X.; Deng, Y.; Yang, H.; Chen, H. Characterization of modified biochars derived from bamboo pyrolysis and their utilization for target component (Furfural) adsorption. Energy Fuels 2014, 28, 5119–5127. [Google Scholar] [CrossRef]
- Eaton, A. Standard Methods for the Examination of Water and Waste Water, 21st ed.; American Public Health Association: Washington, DC, USA, 2005. [Google Scholar]
- Xu, R.; Yang, Z.-H.; Zheng, Y.; Liu, J.-B.; Xiong, W.-P.; Zhang, Y.-R.; Lu, Y.; Xue, W.-J.; Fan, C.-Z. Organic loading rate and hydraulic retention time shape distinct ecological networks of anaerobic digestion related microbiome. Bioresour. Technol. 2018, 262, 184–193. [Google Scholar] [CrossRef]
- Yang, Z.-H.; Xu, R.; Zheng, Y.; Chen, T.; Zhao, L.-J.; Li, M. Characterization of extracellular polymeric substances and microbial diversity in anaerobic co-digestion reactor treated sewage sludge with fat, oil, grease. Bioresour. Technol. 2016, 212, 164–173. [Google Scholar] [CrossRef]
- Vargas-Muñoz, M.A.; Danchana, K.; Cerdà, V.; Palacio, E. Field-deployable method for iron analysis using a simple preconcentration procedure and a 3D portable spectrophotometric system. Microchem. J. 2021, 170, 106774. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, Z.; Xu, R.; Xiang, Y.P.; Jia, M.; Hu, J.H.; Zheng, Y.; Xiong, W.; Cao, J. Enhanced mesophilic anaerobic digestion of waste sludge with the iron nanoparticles addition and kinetic analysis. Sci. Total Environ. 2019, 683, 124–133. [Google Scholar] [CrossRef]
- Huang, J.-R.; Bu, J.; Cheng, J.-R.; Ming, J. Improving methane production from hydrogenogenic effluent with magnetic leaf biochar. Biomass Convers. Biorefinery 2022, 1–11. [Google Scholar] [CrossRef]
- Zhang, S.; Yang, X.; Liu, L.; Ju, M.; Zheng, K. Adsorption behavior of selective recognition functionalized biochar to Cd (II) in wastewater. Materials 2018, 11, 299. [Google Scholar] [CrossRef]
- Chen, T.; Zhou, Z.; Han, R.; Meng, R.; Wang, H.; Lu, W. Adsorption of cadmium by biochar derived from municipal sewage sludge: Impact factors and adsorption mechanism. Chemosphere 2015, 134, 286–293. [Google Scholar] [CrossRef]
- Badireddy, A.R.; Chellam, S.; Gassman, P.L.; Engelhard, M.H.; Lea, A.S.; Rosso, K.M. Role of extracellular polymeric substances in bioflocculation of activated sludge microorganisms under glucose-controlled conditions. Water Res. 2010, 44, 4505–4516. [Google Scholar] [CrossRef]
- Bakshi, S.; Banik, C.; Rathke, S.J.; Laird, D.A. Arsenic sorption on zero-valent iron-biochar complexes. Water Res. 2018, 137, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Kato, S.; Hashimoto, K.; Watanabe, K. Methanogenesis facilitated by electric syntrophy via (semi)conductive iron-oxide minerals. Environ. Microbiol. 2012, 14, 1646–1654. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Lesnik, K.L.; Liu, H. Stay connected: Electrical conductivity of microbial aggregates. Biotechnol. Adv. 2017, 35, 669–680. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Du, P.; Zhi, B.; Liu, L.; Song, Y.; Xiang, S.; Dai, Z.; Yang, G.; Wang, X.; Feng, Y. Magnetic biochar affects the metabolic pathway in methanogenesis of anaerobic digestion of food waste. GCB Bioenergy 2022, 14, 572–584. [Google Scholar] [CrossRef]
- Yu, B.; Zhang, D.; Dai, X.; Lou, Z.; Yuan, H.; Zhu, N. The synthetic effect on volatile fatty acid disinhibition and methane production enhancement by dosing FeCl3 in a sludge thermophilic anaerobic digestion system. RSC Adv. 2016, 6, 21090–21098. [Google Scholar] [CrossRef]
- Ali, M.M.; Mustafa, A.M.; Zhang, X.; Lin, H.; Zhang, X.; Abdulbaki Danhassan, U.; Zhou, X.; Sheng, K. Impacts of molybdate and ferric chloride on biohythane production through two-stage anaerobic digestion of sulfate-rich hydrolyzed tofu processing residue. Bioresour. Technol. 2022, 355, 127239. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Cao, Q.; Chen, Y.; Luo, Y.; Liu, X.; Li, D. The biological and abiotic effects of powdered activated carbon on the anaerobic digestion performance of cornstalk. Bioresour. Technol. 2022, 343, 126072. [Google Scholar] [CrossRef]
- Zhen, G.; Lu, X.; Kobayashi, T.; Kumar, G.; Xu, K. Anaerobic co-digestion on improving methane production from mixed microalgae (Scenedesmus sp., Chlorella sp.) and food waste: Kinetic modeling and synergistic impact evaluation. Chem. Eng. J. 2016, 299, 332–341. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, Z.; Zhang, Y.; Xiang, Y.; Xu, R.; Jia, M.; Cao, J.; Xiong, W. Effects of different conductive nanomaterials on anaerobic digestion process and microbial community of sludge. Bioresour. Technol. 2020, 304, 123016. [Google Scholar] [CrossRef]
- Sheng, G.-P.; Yu, H.-Q.; Li, X.-Y. Extracellular polymeric substances (EPS) of microbial aggregates in biological wastewater treatment systems: A review. Biotechnol. Adv. 2010, 28, 882–894. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Dong, B.; Dai, X. Mechanism analysis to improve sludge dewaterability during anaerobic digestion based on moisture distribution. Chemosphere 2019, 227, 247–255. [Google Scholar] [CrossRef] [PubMed]
- Mu, H.; Chen, Y. Long-term effect of ZnO nanoparticles on waste activated sludge anaerobic digestion. Water Res. 2011, 45, 5612–5620. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Y.; Yang, Z.; Zhang, Y.; Xu, R.; Zheng, Y.; Hu, J.; Li, X.; Jia, M.; Xiong, W.; Cao, J. Influence of nanoscale zero-valent iron and magnetite nanoparticles on anaerobic digestion performance and macrolide, aminoglycoside, β-lactam resistance genes reduction. Bioresour. Technol. 2019, 294, 122139. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Wang, D.; Liu, Y.; Ngo, H.H.; Guo, W.; Yang, Q.; Li, X. Novel stepwise pH control strategy to improve short chain fatty acid production from sludge anaerobic fermentation. Bioresour. Technol. 2018, 249, 431–438. [Google Scholar] [CrossRef]
- Kong, X.; Yu, S.; Xu, S.; Fang, W.; Liu, J.; Li, H. Effect of Fe0 addition on volatile fatty acids evolution on anaerobic digestion at high organic loading rates. Waste Manag. 2018, 71, 719–727. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, Z.; Wang, Y.; Zhong, H.; Sui, Q.; Zhang, C.; Wei, Y. Effects of graphene oxide on the performance, microbial community dynamics and antibiotic resistance genes reduction during anaerobic digestion of swine manure. Bioresour. Technol. 2017, 245, 850–859. [Google Scholar] [CrossRef]
- Yang, Q.; Luo, K.; Li, X.M.; Wang, D.B.; Zheng, W.; Zeng, G.M.; Liu, J.J. Enhanced efficiency of biological excess sludge hydrolysis under anaerobic digestion by additional enzymes. Bioresour. Technol. 2010, 101, 2924–2930. [Google Scholar] [CrossRef]
- Gong, L.; Yang, X.; You, X.; Wang, J.; Zhou, J.; Zhou, Y.; Yang, J. Explore the effect of Fe3O4 nanoparticles (NPs) on anaerobic digestion of sludge. Environ. Technol. 2021, 42, 1542–1551. [Google Scholar] [CrossRef]
- Zhang, W.; Wei, Q.; Wu, S.; Qi, D.; Li, W.; Zuo, Z.; Dong, R. Batch anaerobic co-digestion of pig manure with dewatered sewage sludge under mesophilic conditions. Appl. Energy 2014, 128, 175–183. [Google Scholar] [CrossRef]
- Shen, J.; Yan, H.; Zhang, R.; Liu, G.; Chen, C. Characterization and methane production of different nut residue wastes in anaerobic digestion. Renew. Energy 2018, 116, 835–841. [Google Scholar]
- Xu, H.; Chang, J.; Wang, H.; Liu, Y.; Zhang, X.; Liang, P.; Huang, X. Enhancing direct interspecies electron transfer in syntrophic-methanogenic associations with (semi) conductive iron oxides: Effects and mechanisms. Sci. Total Environ. 2019, 695, 133876. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Zhao, X.; Zhao, Y.; Wang, H.; Yuan, X.; Zhu, W.; Cui, Z.; Wang, X. Optimization of Fe2+ supplement in anaerobic digestion accounting for the Fe-bioavailability. Bioresour. Technol. 2018, 250, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, S.; Cheng, R.; Liu, R.; Liu, Z.; Yang, Q. Effects of nZVI and Fe3O4 NPs on anaerobic methanogenesis, microbial communities and metabolic pathways for treating domestic wastewater at ambient temperature. J. Water Process. Eng. 2022, 48, 102845. [Google Scholar] [CrossRef]
- Wang, S.; Xu, C.; Song, L.; Zhang, J. Anaerobic digestion of food waste and its microbial consortia: A historical review and future perspectives. Int. J. Environ. Res. Public Health 2022, 19, 9519. [Google Scholar] [CrossRef] [PubMed]
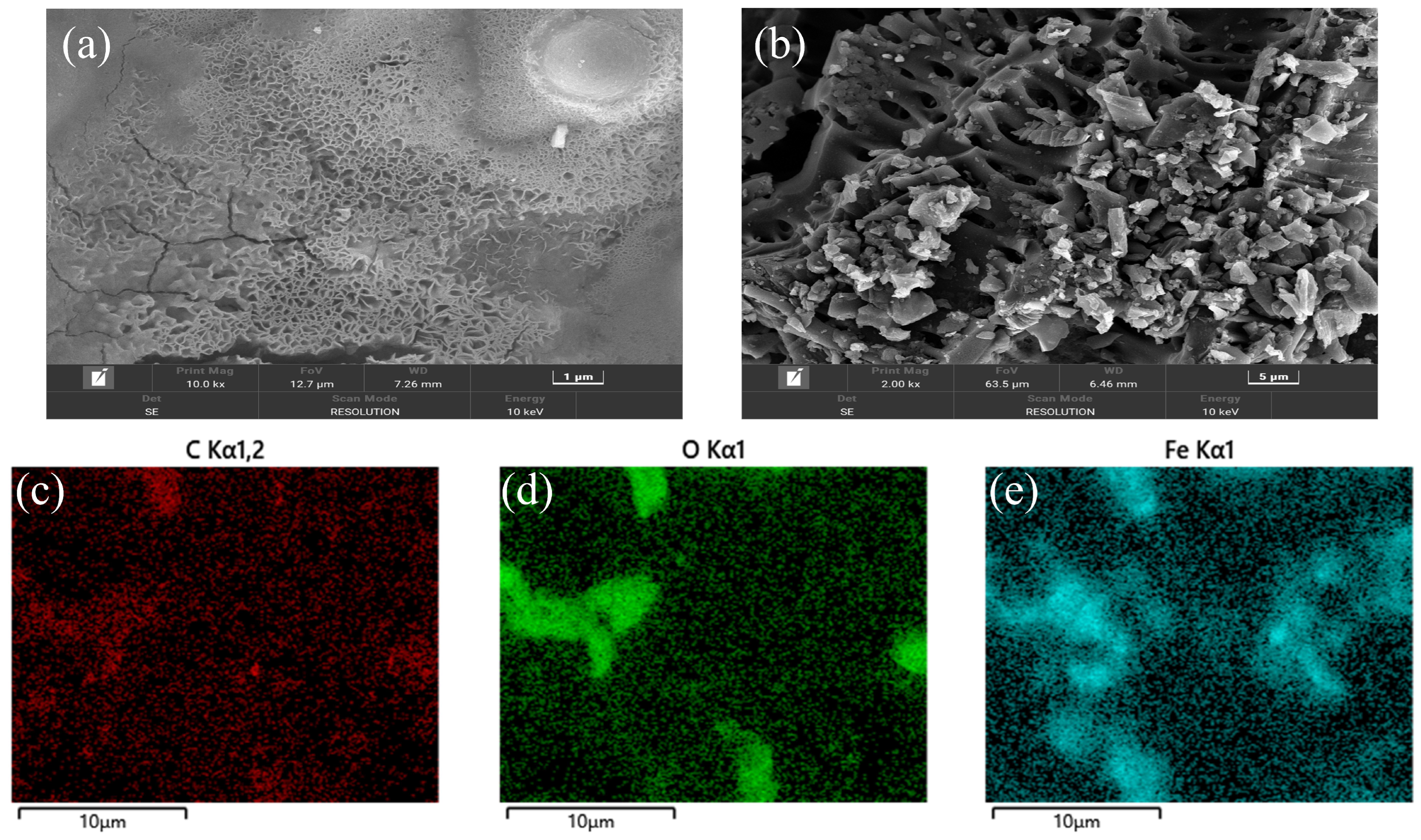
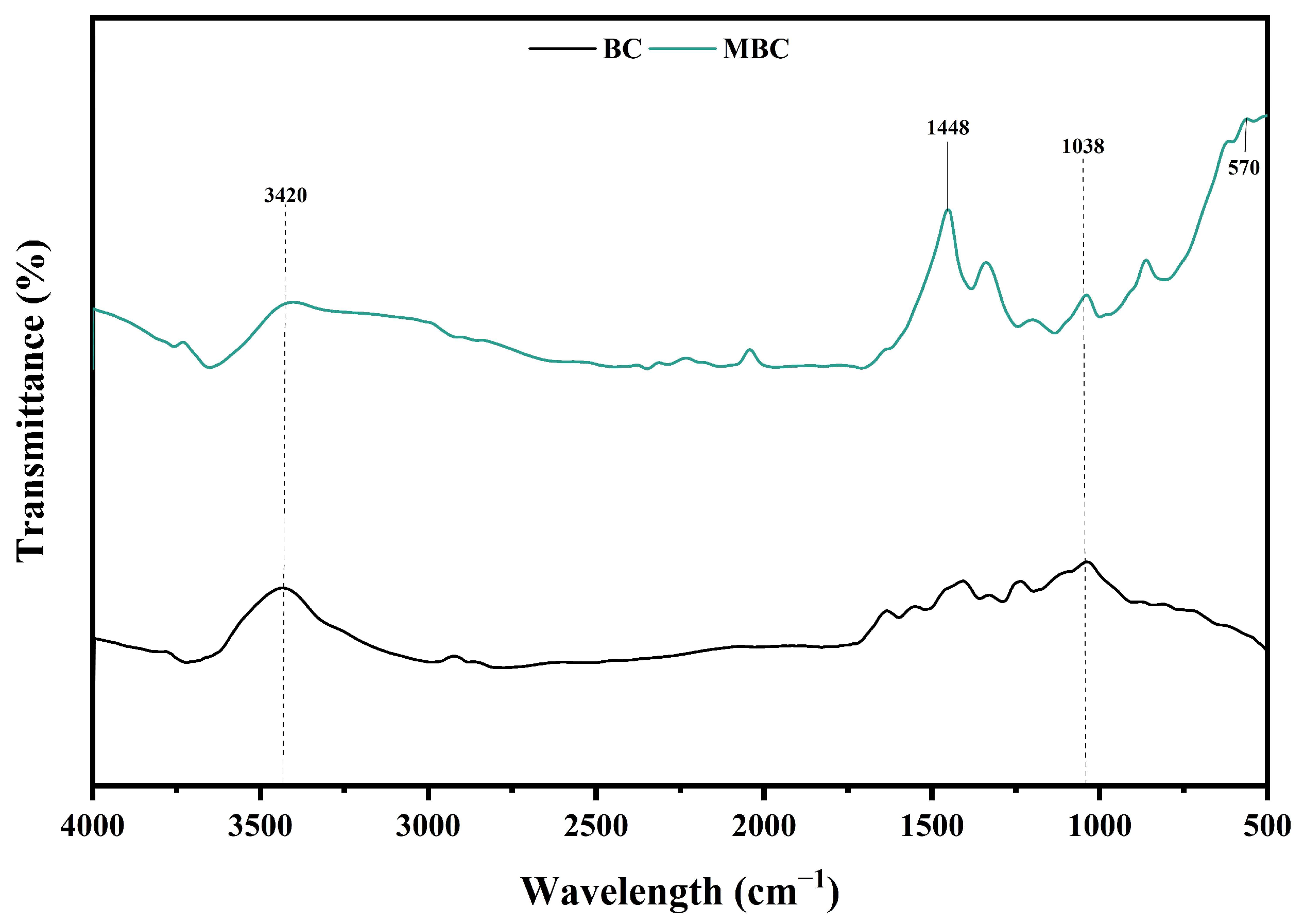
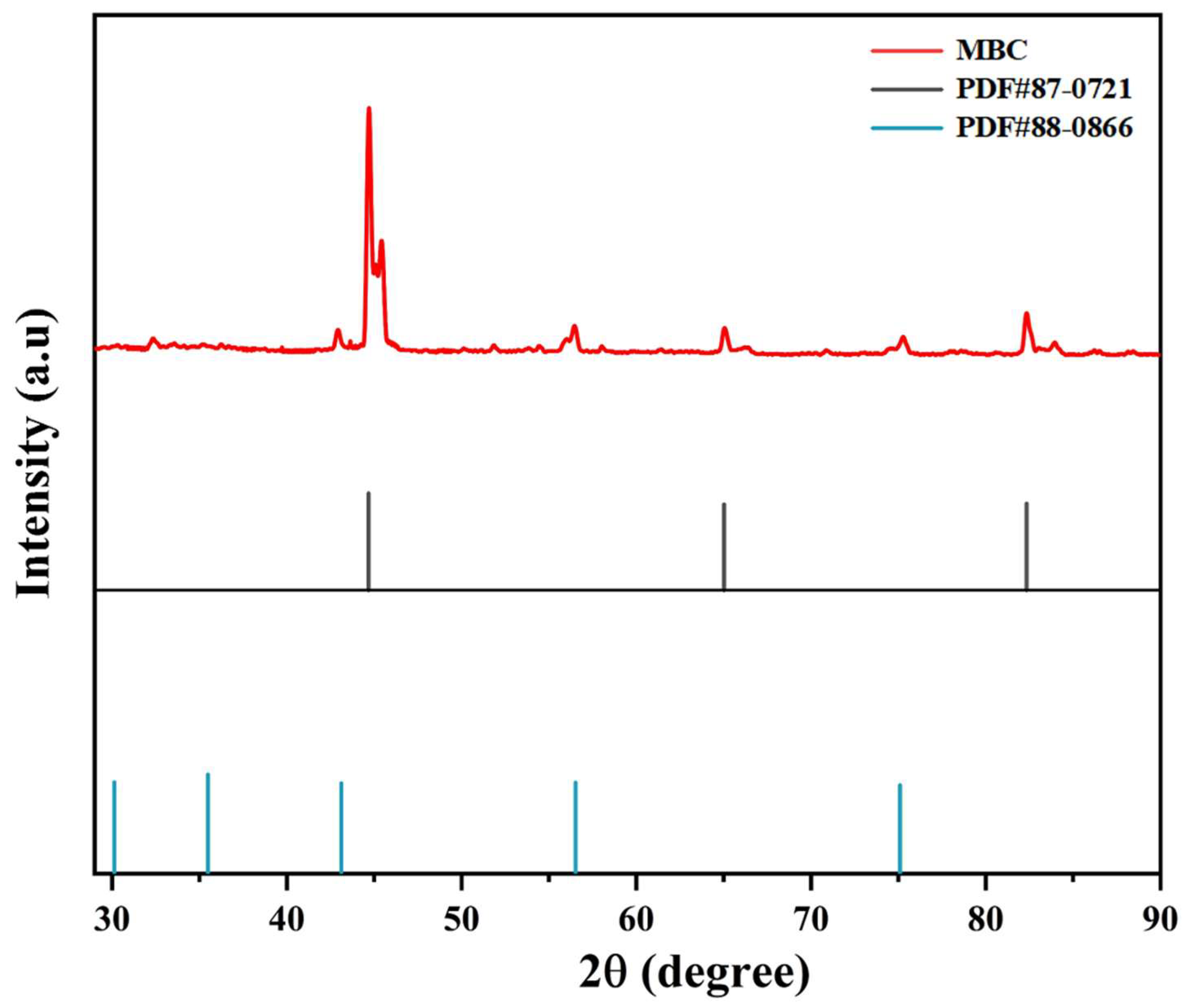


| Characteristics | Feed | Seed |
|---|---|---|
| Total solids (TS, g L−1 substrate) | 79.60 ± 1.36 | 19.60 ± 0.44 |
| Volatile solids (VS, g L−1 substrate) | 46.50 ± 0.61 | 7.93 ± 0.21 |
| Moisture (%) | 92.12 ± 0.13 | 98.07 ± 0.04 |
| pH | 7.14 ± 0.03 | 7.05 ± 0.01 |
| Soluble chemical oxygen demand (sCOD, mg L−1) | 6257.43 ± 101.38 | 463.37 ± 29.45 |
| Total polysaccharides (mg L−1) | 413.86 ± 16.28 | 46.62 ± 3.20 |
| Protein (mg L−1) | 452.70 ± 5.22 | 37.43 ± 8.11 |
| VFAs (mg L−1) | 1684.90 ± 15.81 | 38.39 ± 7.35 |
| NH4+-N (mg L−1) | 1000.31 ± 3.04 | 681.50 ± 9.62 |
| Model | Equation | Parameters |
|---|---|---|
| Modified Gompertz Model | , t ≥ 0 | Rm, B0, λ |
| Cone Model | , t > 0 | B0, k, n |
| Reactors | B0 (mL g−1 VSadded) | Rm | λ | R2 | Measured (mL g−1 VSadded) | Difference (%) |
|---|---|---|---|---|---|---|
| CK | 311.10 | 32.23 | 2.01 | 0.9988 | 309.20 ± 4.59 | 0.61 |
| MB_1 | 354.48 | 33.37 | 1.46 | 0.9997 | 353.58 ± 2.31 | 0.26 |
| MB_2 | 373.25 | 34.24 | 1.27 | 0.9995 | 372.28 ± 1.23 | 0.26 |
| MB_3 | 401.18 | 36.10 | 1.11 | 0.9994 | 400.16 ± 4.22 | 0.25 |
| MB_4 | 440.77 | 39.18 | 1.02 | 0.9995 | 439.71 ± 1.97 | 0.24 |
| MB_5 | 420.57 | 33.66 | 1.91 | 0.9993 | 418.54 ± 1.73 | 0.49 |
| Reactors | B0 (mL g−1 VSadded) | K | n | R2 | Measured (mL g−1 VSadded) | Difference (%) |
|---|---|---|---|---|---|---|
| CK | 316..47 | 0.1470 | 2.83 | 0.9941 | 309.20 ± 4.59 | 2.35 |
| MB_1 | 364.12 | 0.1491 | 2.45 | 0.9961 | 353.58 ± 2.31 | 2.98 |
| MB_2 | 384.55 | 0.1504 | 2.35 | 0.9956 | 372.28 ± 1.23 | 3.30 |
| MB_3 | 414.46 | 0.1517 | 2.27 | 0.9958 | 400.16 ± 4.22 | 3.57 |
| MB_4 | 456.13 | 0.1522 | 2.23 | 0.9957 | 439.71 ± 1.97 | 3.73 |
| MB_5 | 435.78 | 0.1227 | 2.43 | 0.9971 | 418.54 ± 1.73 | 4.12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, L.; Zhang, Y.; Zhu, Y.; Huang, Z.; Huang, J.; Wu, Z.; Zhang, X.; Qin, X.; Li, H. Effects of Magnetic Biochar Addition on Mesophilic Anaerobic Digestion of Sewage Sludge. Int. J. Environ. Res. Public Health 2023, 20, 4278. https://doi.org/10.3390/ijerph20054278
Jiang L, Zhang Y, Zhu Y, Huang Z, Huang J, Wu Z, Zhang X, Qin X, Li H. Effects of Magnetic Biochar Addition on Mesophilic Anaerobic Digestion of Sewage Sludge. International Journal of Environmental Research and Public Health. 2023; 20(5):4278. https://doi.org/10.3390/ijerph20054278
Chicago/Turabian StyleJiang, Li, Yanru Zhang, Yi Zhu, Zhongliang Huang, Jing Huang, Zijian Wu, Xuan Zhang, Xiaoli Qin, and Hui Li. 2023. "Effects of Magnetic Biochar Addition on Mesophilic Anaerobic Digestion of Sewage Sludge" International Journal of Environmental Research and Public Health 20, no. 5: 4278. https://doi.org/10.3390/ijerph20054278
APA StyleJiang, L., Zhang, Y., Zhu, Y., Huang, Z., Huang, J., Wu, Z., Zhang, X., Qin, X., & Li, H. (2023). Effects of Magnetic Biochar Addition on Mesophilic Anaerobic Digestion of Sewage Sludge. International Journal of Environmental Research and Public Health, 20(5), 4278. https://doi.org/10.3390/ijerph20054278






