Risk Factors of Microalbuminuria among Patients with Type 2 Diabetes Mellitus in Korea: A Cross-Sectional Study Based on 2019–2020 Korea National Health and Nutrition Examination Survey Data
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Collection and Study Population
2.2. Assessment of Microalbuminuria Using the ACR Index
2.3. Anthropometric and Biochemical Data
2.4. Demographic Characteristics
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- de Boer, I.H.; Caramori, M.L.; Chan, J.C.; Heerspink, H.J.; Hurst, C.; Khunti, K.; Liew, A.; Michos, E.D.; Navaneethan, S.D.; Olowu, W.A.; et al. KDIGO 2020 clinical practice guideline for diabetes management in chronic kidney disease. Kidney Int. 2020, 98, S1–S115. [Google Scholar] [CrossRef] [PubMed]
- Gluhovschi, C.; Gluhovschi, G.; Petrica, L.; Timar, R.; Velciov, S.; Ionita, I.; Kaycsa, A.; Timar, B. Urinary biomarkers in the assessment of early diabetic nephropathy. J. Diabetes Res. 2016, 2016, 46261253. [Google Scholar] [CrossRef] [PubMed]
- Verma, M.K.; Kumar, P.; Sharma, P.; Singh, V.K.; Singh, S.P. Study of microalbuminuria as early risk marker of nephropathy in type 2 diabetic subjects. Int. J. Res. Med. Sci. 2017, 5, 3161–3166. [Google Scholar] [CrossRef]
- Ahmed, S.S.; Rizwan, M.A.; Khan, M.A.; Laila, T.R.; Hafez, M.A. Early recognition and treatment of diabetic kidney disease. Med. Today 2014, 26, 56–62. [Google Scholar] [CrossRef]
- Jatoi, N.A.; Said, A.H.; Al-Ghamdi, M.S.; Al-Abdulmhsin, M.F.; Bin-Jaban, R.A.; Al-Tayeb, J.A.; Aljarri, S.A.; Saeed, I. Prevalence of microalbuminuria and cardiovascular risk factors in patients with diabetes mellitus type-II in Al-Khobar, Kingdom of Saudi Arabia. Cureus 2022, 14, e29808. [Google Scholar] [CrossRef]
- Samsu, N. Diabetic nephropathy: Challenges in pathogenesis, diagnosis, and treatment. Biomed. Res. Int. 2021, 2021, 1497449. [Google Scholar] [CrossRef] [PubMed]
- Bilous, R. Microvascular disease: What does the UKPDS tell us about diabetic nephropathy. Diabet. Med. 2008, 25 (Suppl. 2), 25–29. [Google Scholar] [CrossRef]
- Eriguchi, M.; Lin, M.; Yamashita, M.; Zhao, T.V.; Khan, Z.; Bernstein, E.A.; Gurley, S.B.; Gonzalez-Villalobos, R.A.; Bernstein, K.E.; Giani, J.F. Renal tubular ACE-mediated tubular injury is the major contributor to microalbuminuria in early diabetic nephropathy. Am. J. Physiol. Renal. Physiol. 2018, 314, F531–F542. [Google Scholar] [CrossRef]
- Roshan, B.; Stanton, R.C. A story of microalbuminuria and diabetic nephropathy. J. Nephropathol. 2013, 2, 234–240. [Google Scholar]
- Ahn, J.H.; Yu, J.H.; Ko, S.H.; Kwon, H.S.; Kim, D.J.; Kim, J.H.; Kim, C.S.; Song, K.H.; Won, J.C.; Lim, S.; et al. Prevalence and determinants of diabetic nephropathy in Korea: Korea National Health and Nutrition Examination Survey. Diabetes Metab. J. 2014, 38, 109–119. [Google Scholar] [CrossRef]
- Dounousi, E.; Duni, A.; Leivaditis, K.; Vaios, V.; Eleftheriadis, T.; Liakopoulos, V. Improvements in the management of diabetic nephropathy. Rev. Diabet. Stud. 2015, 12, 119–133. [Google Scholar] [CrossRef] [PubMed]
- KDOQI. KDOQI clinical practice guidelines and clinical practice recommendations for diabetes and chronic kidney disease. Am. J. Kidney Dis. 2007, 49, 12–154. [Google Scholar] [CrossRef] [PubMed]
- Jitraknatee, J.; Ruengorn, C.; Nochaiwong, S. Prevalence and risk factors of chronic kidney disease among type 2 diabetes patients: A cross-sectional study in primary care practice. Sci. Rep. 2020, 10, 6205. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association. Standards of medical care in diabetes—2013. Diabetes Care 2013, 36 (Suppl. 1), S11–S66. [Google Scholar] [CrossRef]
- Filla, L.A.; Edwards, J.L. Metabolomics in diabetic complications. Mol. Biosyst. 2016, 12, 1090–1105. [Google Scholar] [CrossRef]
- Nathan, D.M. The diabetes control and complications trial/epidemiology of diabetes interventions and complications study at 30 years: Overview. Diabetes Care 2014, 37, 9–16. [Google Scholar] [CrossRef]
- Shi, Y.; Vanhoutte, P.M. Macro- and microvascular endothelial dysfunction in diabetes. J. Diabetes 2017, 9, 434–449. [Google Scholar] [CrossRef]
- Ahmed, A.; Sattar, N.; Yaghootkar, H. Advancing a causal role of type 2 diabetes and its components in developing macro- and microvascular complications via genetic studies. Diabet. Med. 2022, 39, e14982. [Google Scholar] [CrossRef]
- Alwakeel, J.S.; Al-Suwaida, A.; Isnani, A.C.; Al-Harbi, A.; Alam, A. Concomitant macro and microvascular complications in diabetic nephropathy. Saudi. J. Kidney Dis. Transplant. 2009, 20, 402–409. [Google Scholar]
- Narres, M.; Claessen, H.; Droste, S.; Kvitkina, T.; Koch, M.; Kuss, O.; Icks, A. The incidence of end-stage renal disease in the diabetic (compared to the non-diabetic) population: A systematic review. PLoS ONE 2016, 11, e0147329. [Google Scholar] [CrossRef]
- Packham, D.K.; Alves, T.P.; Dwyer, J.P.; Atkins, R.; de Zeeuw, D.; Cooper, M.; Shahinfar, S.; Lewis, J.B.; Lambers Heerspink, H.J. Relative incidence of ESRD versus cardiovascular mortality in proteinuric type 2 diabetes and nephropathy: Results from the DIAMETRIC (Diabetes Mellitus Treatment for Renal Insufficiency Consortium) database. Am. J. Kidney Dis. 2012, 59, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Idowu, A.A.; Ajose, A.O.; Adedeji, A.T.; Adegoke, A.O.; Jimoh, K.A. Microalbuminuria, other markers of nephropathy and biochemical derangements in type 2 diabetes mellitus: Relationships and determinants. Ghana Med. J. 2017, 51, 56–63. [Google Scholar] [PubMed]
- Thakur, S.K.; Dhakal, S.P.; Parajuli, S.; Sah, A.K.; Nepal, S.P.; Paudel, B.D. Microalbuminuria and its risk factors in type 2 diabetic patients. J. Nepal. Health Res. Counc. 2019, 17, 61–65. [Google Scholar] [CrossRef] [PubMed]
- Araki, S.; Haneda, M.; Koya, D.; Hidaka, H.; Sugimoto, T.; Isono, M.; Isshiki, K.; Chin-Kanasaki, M.; Uzu, T.; Kashiwagi, A. Reduction in microalbuminuria as an integrated indicator for renal and cardiovascular risk reduction in patients with type 2 diabetes. Diabetes 2007, 56, 1727–1730. [Google Scholar] [CrossRef]
- Yang, C.W.; Park, J.T.; Kim, Y.S.; Kim, Y.L.; Lee, Y.S.; Oh, Y.S.; Kang, S.W. Prevalence of diabetic nephropathy in primary care type 2 diabetic patients with hypertension: Data from the Korean Epidemiology Study on Hypertension III (KEY III study). Nephrol. Dial. Transplant. 2011, 26, 3249–3255. [Google Scholar] [CrossRef]
- Sukhram, S.D.; Zarini, G.G.; Shaban, L.H.; Vaccaro, J.A.; Huffman, F.G. Microalbuminuria and hypertension among immigrants with type 2 diabetes: A community-based cross-sectional study. J. Pers. Med. 2022, 12, 1777. [Google Scholar] [CrossRef]
- Poudel, B.; Yadav, B.K.; Nepal, A.K.; Jha, B.; Raut, K.B. Prevalence and association of microalbuminuria in essential hypertensive patients. N. Am. J. Med. Sci. 2012, 4, 331–335. [Google Scholar] [CrossRef]
- Molefe-Baikai, O.J.; Molefi, M.; Cainelli, F.; Rwegerera, G.M. The prevalence of microalbuminuria and associated factors among patients with type 2 diabetes mellitus in Botswana. Niger. J. Clin. Pract. 2018, 21, 1430–1437. [Google Scholar]
- Maiti, A.; Dey, S.K.; Raychaudhuri, P.; Sinha, P.K.; De, J.; Chatterjee, A.; Mukhopadhaya, S. Changes in microalbuminuria in relation to glycosylated haemoglobin (HbA1c) and duration in type 2 diabetes mellitus. Indian Med. Gazette 2012, 145, 394–399. [Google Scholar]
- Hieshima, K.; Suzuki, T.; Sugiyama, S.; Kurinami, N.; Yoshida, A.; Miyamoto, F.; Kajiwara, K.; Jinnouchi, T.; Jinnouchi, H. Smoking cessation ameliorates microalbuminuria with reduction of blood pressure and pulse rate in patients with already diagnosed diabetes mellitus. J. Clin. Med. Res. 2018, 10, 478–485. [Google Scholar] [CrossRef]
- Chu, J.; Cho, S.; Kim, S.; Kwon, E.; Nah, E.H. Relationship between hypertension and microalbuminuria according to obesity status in prediabetes. Korean J. Health Promot. 2019, 19, 202–209. [Google Scholar] [CrossRef]
- Liu, X.; Liu, Y.; Chen, Y.; Li, Y.; Shao, X.; Liang, Y.; Li, B.; Holthöfer, H.; Zhang, G.; Zou, H. Body mass index (BMI) is associated with microalbuminuria in Chinese hypertensive patients. Int. J. Environ. Res. Public Health 2015, 12, 1998–2008. [Google Scholar] [CrossRef] [PubMed]
- Korea Disease Control and Prevention Agency. Korea National Health and Nutrition Examination Survey, KNHANES 2022. Available online: https://knhanes.kdca.go.kr/knhanes/sub03/sub03_02_05.do (accessed on 25 November 2022).
- Kweon, S.; Kim, Y.; Jang, M.J.; Kim, Y.; Kim, K.; Choi, S.; Chun, C.; Khang, Y.H.; Oh, K. Data resource profile: The Korea National Health and Nutrition Examination Survey (KNHANES). Int. J. Epidemiol. 2014, 43, 69–77. [Google Scholar] [CrossRef]
- Gawandi, S.; Gangawane, S.; Chakrabarti, A.; Kedare, S.; Bantwal, K.; Wadhe, V.; Kulkarni, A.; Kulkarni, S.; Rajan, M.G.R. A study of microalbuminuria (MAU) and advanced glycation end products (AGEs) levels in diabetic and hypertensive subjects. Indian J. Clin. Biochem. 2018, 33, 81–85. [Google Scholar] [CrossRef] [PubMed]
- Vaziri, N.D. Lipotoxicity and impaired high-density lipoprotein-mediated reverse cholesterol transport in chronic kidney disease. J. Ren. Nutr. 2010, 20, S35–S43. [Google Scholar] [CrossRef]
- Sun, X.; Xiao, Y.; Li, P.M.; Ma, X.Y.; Sun, X.J.; Lv, W.S.; Wu, Y.L.; Liu, P.; Wang, Y.G. Association of serum high-density lipoprotein cholesterol with microalbuminuria in type 2 diabetes patients. Lipids Health Dis. 2018, 17, 229. [Google Scholar] [CrossRef]
- Chang, Y.H.; Chang, D.M.; Lin, K.C.; Hsieh, C.H.; Lee, Y.J. High-density lipoprotein cholesterol and the risk of nephropathy in type 2 diabetic patients. Nutr. Metab. Cardiovasc. Dis. 2013, 23, 751–757. [Google Scholar] [CrossRef]
- Li, C.; Gu, Q. Protective effect of paraoxonase 1 of high-density lipoprotein in type 2 diabetic patients with nephropathy. Nephrology 2009, 14, 514–520. [Google Scholar] [CrossRef]
- Drew, B.G.; Duffy, S.J.; Formosa, M.F.; Natoli, A.K.; Henstridge, D.C.; Penfold, S.A.; Thomas, W.G.; Mukhamedova, N.; de Courten, B.; Forbes, J.M.; et al. High-density lipoprotein modulates glucose metabolism in patients with type 2 diabetes mellitus. Circulation 2009, 119, 2103–2111. [Google Scholar] [CrossRef]
- Tu, S.T.; Chang, S.J.; Chen, J.F.; Tien, K.J.; Hsiao, J.Y.; Chen, H.C.; Hsieh, M.C. Prevention of diabetic nephropathy by tight target control in an Asian population with type 2 diabetes mellitus: A 4-year prospective analysis. Arch. Intern. Med. 2010, 170, 155–161. [Google Scholar] [CrossRef]
- American Diabetes Association. Standards of Medical Care in Diabetes—2019 abridged for primary care providers. Clin. Diabetes 2019, 37, 11–34. [Google Scholar] [CrossRef] [PubMed]
- Kee, Y.K.; Han, S.H. Recent updates on diabetic nephropathy. J. Korean Diabetes 2017, 18, 214–228. [Google Scholar] [CrossRef]
- Sahay, M.; Kalra, S.; Badani, R.; Bantwal, G.; Bhoraskar, A.; Das, A.K.; Dhorepatil, B.; Ghosh, S.; Jeloka, T.; Khandelwal, D.; et al. Diabetes and Anemia: International Diabetes Federation (IDF)—Southeast Asian Region (SEAR) position statement. Diabetes Metab. Syndr. 2017, 11, S685–S695. [Google Scholar] [CrossRef] [PubMed]
- Adetunji, O.R.; Mani, H.; Olujohungbe, A.; Abraham, K.A.; Gill, G.V. ‘Microalbuminuric anaemia’—The relationship between haemoglobin levels and albuminuria in diabetes. Diabetes Res. Clin. Pract. 2009, 85, 179–182. [Google Scholar] [CrossRef] [PubMed]
- Bonakdaran, S.; Gharebaghi, M.; Vahedian, M. Prevalence of anemia in type 2 diabetes and role of renal involvement. Saudi J. Kidney Dis. Transpl. 2011, 22, 286–290. [Google Scholar]
- Han, J.S.; Lee, M.J.; Park, K.S.; Han, S.H.; Yoo, T.H.; Oh, K.H.; Park, S.K.; Lee, J.; Hyun, Y.Y.; Chung, W.; et al. Albuminuria as a risk factor for anemia in chronic kidney disease: Result from the KoreaN Cohort Study for Outcomes in Patients with Chronic Kidney Disease (KNOW-CKD). PLoS ONE 2015, 10, e0139747. [Google Scholar] [CrossRef]
- Ishigami, J.; Grams, M.E.; Naik, R.P.; Caughey, M.C.; Loehr, L.R.; Uchida, S.; Coresh, J.; Matsushita, K. Hemoglobin, albuminuria, and kidney function in cardiovascular risk: The ARIC (Atherosclerosis Risk in Communities) study. J. Am. Heart Assoc. 2018, 7, e007209. [Google Scholar] [CrossRef]
- Shen, Q.; Jin, W.; Ji, S.; Chen, X.; Zhao, X.; Behera, T.R. The association between socioeconomic status and prevalence of chronic kidney disease: A cross-sectional study among rural residents in eastern China. Medicine 2019, 98, e14822. [Google Scholar] [CrossRef]
- Kim, D.J. The association of socioeconomic status with diabetes, and cardiovascular disease. Korean J. Med. 2008, 74, 349–357. [Google Scholar]
| Variables | Microalbuminuria | Normoalbuminuria | Chi-Square Test (p-Value) | |
|---|---|---|---|---|
| n (%) | n (%) | |||
| Age (years) | <50 | 9 (9.47%) | 48 (11.32%) | 2.43 (0.49) |
| 50~59 | 17 (17.89%) | 87 (20.52%) | ||
| 60~69 | 28 (29.47%) | 142 (33.49%) | ||
| ≥70 | 41 (43.16%) | 147 (34.67%) | ||
| Sex | Male | 47 (49.47%) | 208 (49.06%) | 0.01 (0.94) |
| Female | 48 (50.53%) | 216 (50.94%) | ||
| Economic status | High | 20 (21.05%) | 106 (25.00%) | 3.46 (0.33) |
| Middle–High | 23 (24.21%) | 110 (25.94%) | ||
| Middle–Low | 21 (22.11%) | 108 (25.47%) | ||
| Low | 31 (32.63%) | 100 (23.58%) | ||
| Duration of diabetes | <10 | 34 (35.79%) | 235 (55.43%) | 18.36 (<0.001) |
| 10~20 | 27 (28.42%) | 115 (27.12%) | ||
| >20 | 34 (35.79%) | 74 (17.45%) | ||
| Smoking status | Current smoker | 19 (3.66%) | 71 (13.68%) | 1.21 (0.54) |
| Ex-smoker | 23 (4.43%) | 124 (23.89%) | ||
| Never smoker | 53 (10.21%) | 229 (44.12%) | ||
| Medication for diabetes | Yes No | 90 (17.34%) 5 (0.96%) | 395 (76.11%) 29 (5.59%) | 0.32 (0.57) |
| Medication for hypertension | Yes No | 27 (5.20%) 68 (13.10%) | 68 (13.10%) 356 (68.59%) | 7.96 (0.00) |
| Variables | Microalbuminuria | Normoalbuminuria | t-Test (p-Value) |
|---|---|---|---|
| M ± SD | M ± SD | ||
| Glycosylated hemoglobin (%) | 7.78 ± 1.31 | 7.20 ± 1.20 | 13.95 (<0.001) |
| Fasting blood sugar (g/dL) | 155.60 ± 58.32 | 134.96 ± 38.45 | 9.46 (<0.001) |
| Hemoglobin (g/dL) | 13.52 ± 1.59 | 13.75 ± 1.59 | 7.98 (<0.001) |
| Body mass index (kg/m2) | 24.99 ± 3.09 | 24.42 ± 3.23 | 2.31 (0.10) |
| Triglycerides (mg/dL) | 145.75 ± 91.75 | 142.49 ± 111.84 | 8.72 (<0.001) |
| Total cholesterol (mg/dL) | 165.86 ± 45.00 | 165.34 ± 37.64 | 1.19 (0.31) |
| HDL-C (mg/dL) | 44.25 ± 10.53 | 47.89 ± 11.16 | 5.08 (<0.001) |
| LDL-C (mg/dL) | 92.46 ± 39.04 | 88.96 ± 34.01 | 0.60 (0.55) |
| Blood urea nitrogen (mg/dL) | 17.29 ± 6.08 | 16.96 ± 5.24 | 6.67 (<0.001) |
| Serum creatinine (mg/dL) | 0.86 ± 0.32 | 0.81 ± 0.21 | 7.85 (<0.001) |
| Waist circumference (cm) | 90.20 ± 8.15 | 87.83 ± 8.87 | 5.99 (<0.001) |
| Systolic blood pressure (mmHg) | 128.42 ± 16.56 | 120.91 ± 14.16 | 11.21 (<0.001) |
| Diastolic blood pressure (mmHg) | 71.69 ± 9.79 | 72.02 ± 9.68 | 0.07 (0.93) |
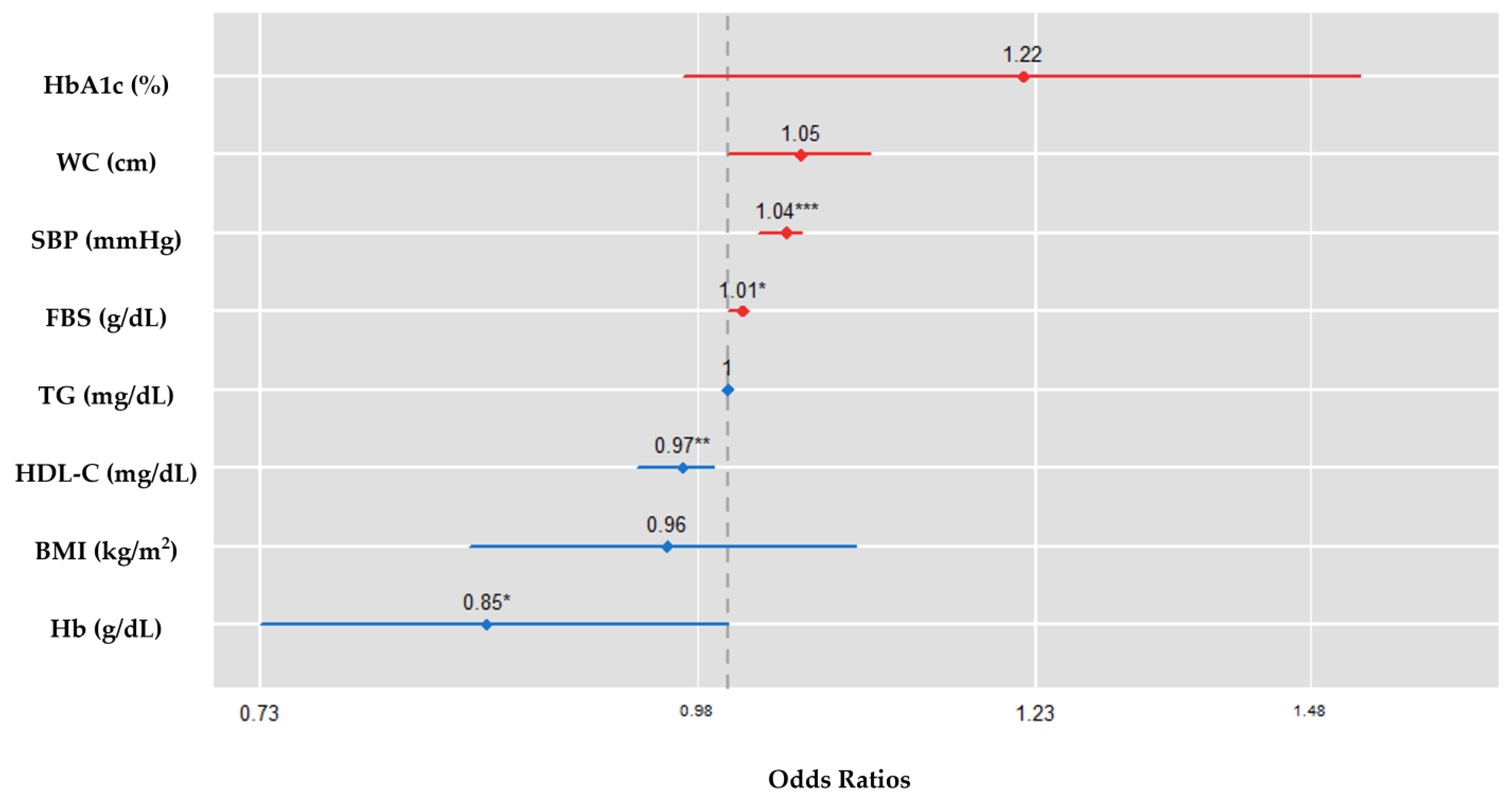
| Variable | Unadjusted | Adjusted | p-Value |
|---|---|---|---|
| OR (95% CI) | OR (95% CI) | ||
| Glycosylated hemoglobin (%) | 1.139 (0.851, 1.51) | 1.216 (0.965, 1.524) | 0.092 |
| Fasting blood sugar (g/dL) | 1.010 (1.005, 1.015) | 1.008 (1.002, 1.014) | 0.015 |
| Hemoglobin (g/dL) | 0.885 (0.749, 1.043) | 0.855 (0.729, 0.998) | 0.043 |
| Body mass index (kg/m2) | 0.971 (0.996, 1.001) | 0.956 (0.84, 1.085) | 0.489 |
| Triglycerides (mg/dL) | 0.979 (0.929, 1.031) | 0.999 (0.996, 1.001) | 0.303 |
| HDL-C (mg/dL) | 0.973 (0.949, 0.995) | 0.966 (0.941, 0.989) | 0.007 |
| Waist circumference (cm) | 0.978 (0.951, 1.005) | 1.045 (0.995, 1.098) | 0.078 |
| Systolic blood pressure (mmHg) | 1.032 (1.015, 1.060) | 1.036 (1.019, 1.053) | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bae, E.S.; Hur, J.Y.; Jang, H.S.; Kim, J.S.; Kang, H.S. Risk Factors of Microalbuminuria among Patients with Type 2 Diabetes Mellitus in Korea: A Cross-Sectional Study Based on 2019–2020 Korea National Health and Nutrition Examination Survey Data. Int. J. Environ. Res. Public Health 2023, 20, 4169. https://doi.org/10.3390/ijerph20054169
Bae ES, Hur JY, Jang HS, Kim JS, Kang HS. Risk Factors of Microalbuminuria among Patients with Type 2 Diabetes Mellitus in Korea: A Cross-Sectional Study Based on 2019–2020 Korea National Health and Nutrition Examination Survey Data. International Journal of Environmental Research and Public Health. 2023; 20(5):4169. https://doi.org/10.3390/ijerph20054169
Chicago/Turabian StyleBae, Eun Sook, Jung Yi Hur, Hyung Soon Jang, Jeong Suk Kim, and Hye Seung Kang. 2023. "Risk Factors of Microalbuminuria among Patients with Type 2 Diabetes Mellitus in Korea: A Cross-Sectional Study Based on 2019–2020 Korea National Health and Nutrition Examination Survey Data" International Journal of Environmental Research and Public Health 20, no. 5: 4169. https://doi.org/10.3390/ijerph20054169
APA StyleBae, E. S., Hur, J. Y., Jang, H. S., Kim, J. S., & Kang, H. S. (2023). Risk Factors of Microalbuminuria among Patients with Type 2 Diabetes Mellitus in Korea: A Cross-Sectional Study Based on 2019–2020 Korea National Health and Nutrition Examination Survey Data. International Journal of Environmental Research and Public Health, 20(5), 4169. https://doi.org/10.3390/ijerph20054169






