Impact of Contextual-Level Social Determinants of Health on Newer Antidiabetic Drug Adoption in Patients with Type 2 Diabetes
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Source and Study Population
2.2. Study Outcome and Covariates
2.3. Contextual-Level SDoH
2.4. Statistical Analysis
3. Results
3.1. Descriptive Analysis
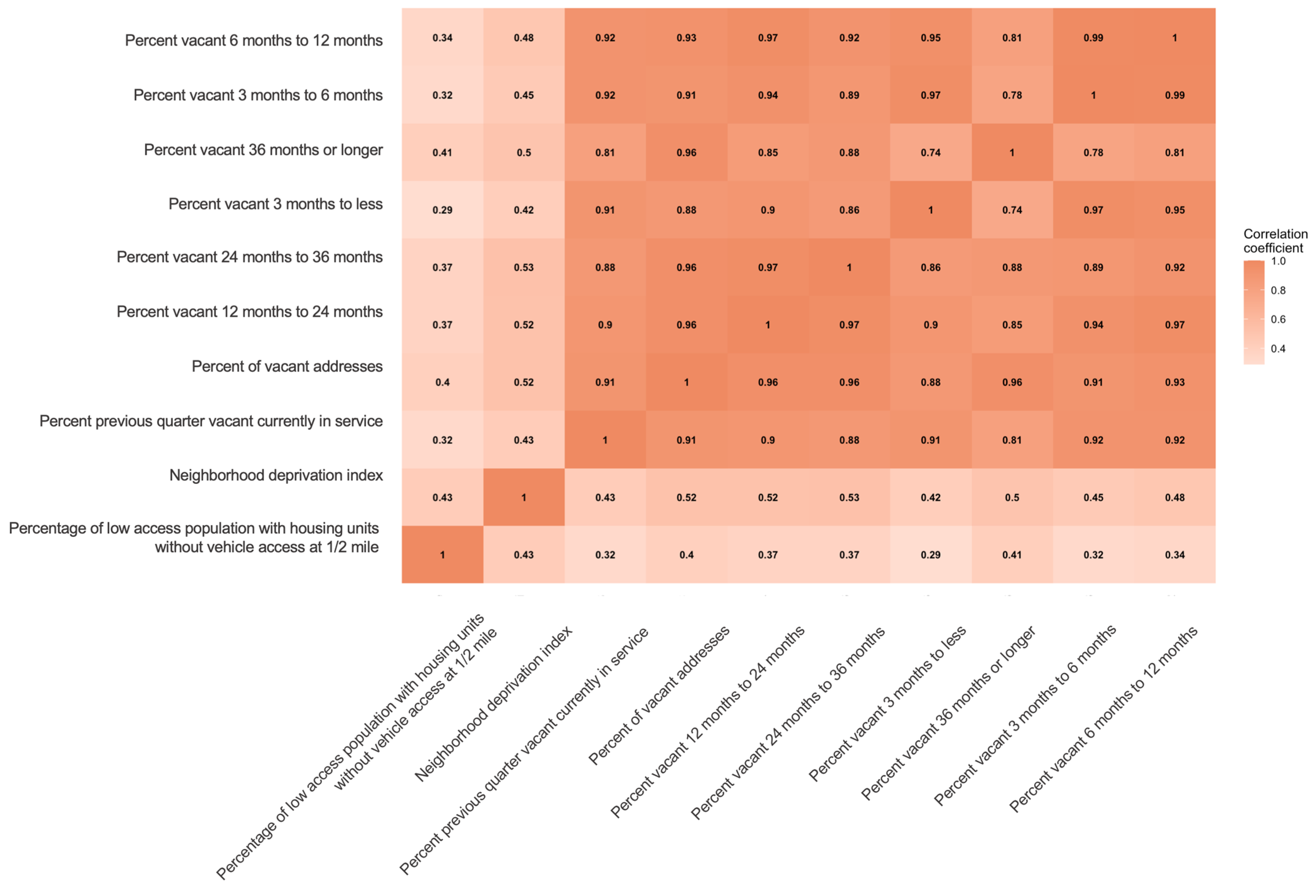
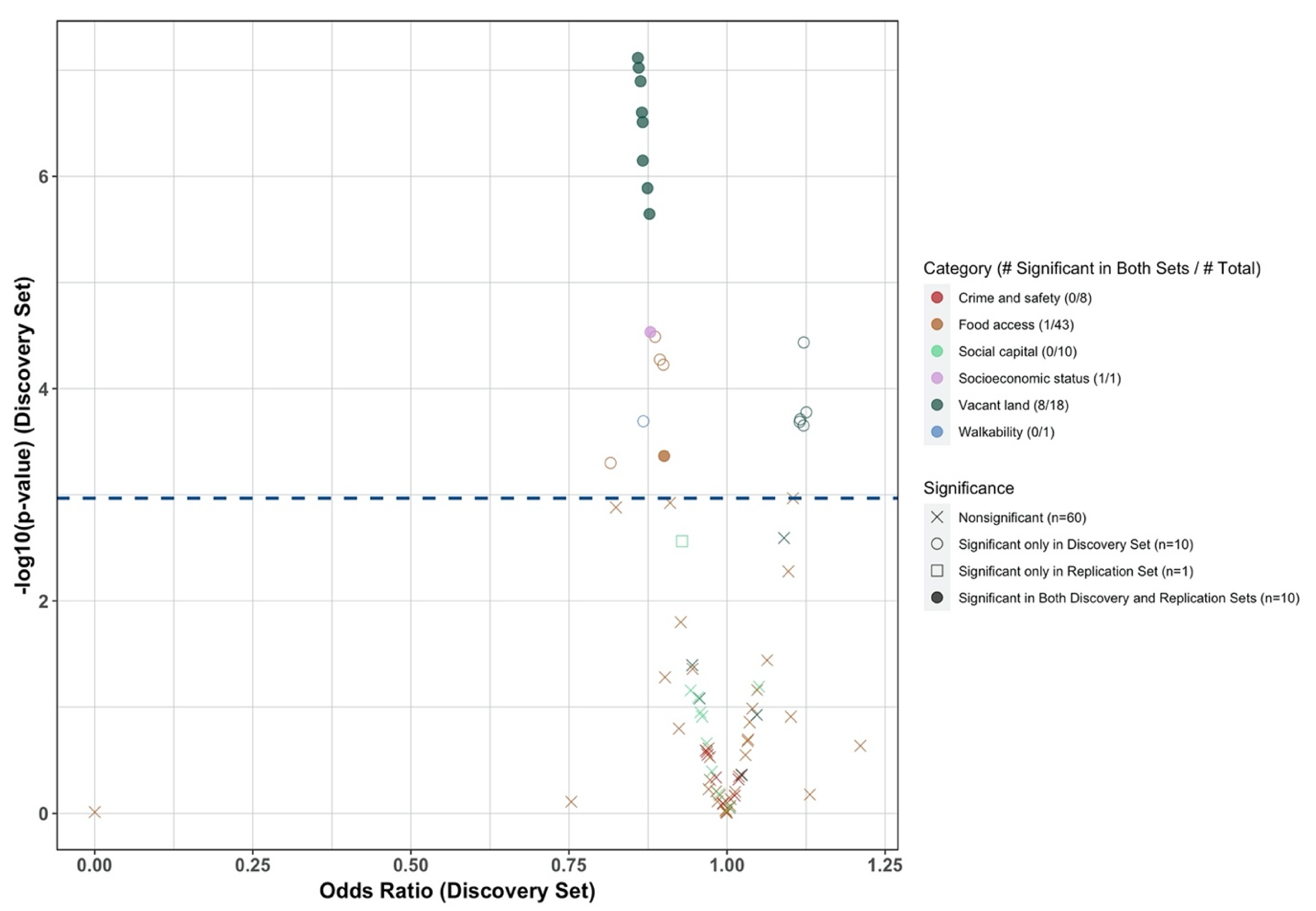
3.2. Selection of Contextual-Level SDoH
3.3. Association of Contextual-Level SDoH and New ADD Initiation across Racial and Ethnic Groups
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
References
- Murphy, S.L.; Kochanek, K.D.; Xu, J.; Arias, E. Mortality in the United States, 2020; NCHS Data Briefs; National Center for Health Statistics: Hyattsville, MD, USA, 2021. [Google Scholar]
- Virani, S.S.; Alonso, A.; Benjamin, E.J.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Delling, F.N.; et al. Heart Disease and Stroke Statistics—2020 Update: A Report From the American Heart Association. Circulation 2020, 141, e139–e596. [Google Scholar] [CrossRef] [PubMed]
- Palmer, S.C.; Tendal, B.; Mustafa, R.A.; Vandvik, P.O.; Li, S.; Hao, Q.; Tunnicliffe, D.; Ruospo, M.; Natale, P.; Saglimbene, V.; et al. Sodium-glucose cotransporter protein-2 (SGLT-2) inhibitors and glucagon-like peptide-1 (GLP-1) receptor agonists for type 2 diabetes: Systematic review and network meta-analysis of randomised controlled trials. BMJ 2021, 372, m4573. [Google Scholar] [CrossRef]
- American Diabetes Association. 9. Pharmacologic Approaches to Glycemic Treatment: Standards of Medical Care in Diabetes—2021. Diabetes Care 2021, 44, S111–S124. [Google Scholar] [CrossRef]
- Writing Committee; Das, S.R.; Everett, B.M.; Birtcher, K.K.; Brown, J.M.; Januzzi, J.L.; Kalyani, R.R.; Kosiborod, M.; Magwire, M.; Morris, P.B.; et al. 2020 Expert Consensus Decision Pathway on Novel Therapies for Cardiovascular Risk Reduction in Patients with Type 2 Diabetes a Report of the American College of Cardiology Solution Set Oversight Committee. J. Am. Coll. Cardiol. 2020, 76, 1117–1145. [Google Scholar] [CrossRef]
- Mahtta, D.; Ramsey, D.J.; Lee, M.T.; Chen, L.; Al Rifai, M.; Akeroyd, J.M.; Vaughan, E.M.; Matheny, M.E.; Santo, K.R.D.E.; Navaneethan, S.D.; et al. Utilization Rates of SGLT2 Inhibitors and GLP-1 Receptor Agonists and Their Facility-Level Variation Among Patients with Atherosclerotic Cardiovascular Disease and Type 2 Diabetes: Insights from the Department of Veterans Affairs. Diabetes Care 2022, 45, 372–380. [Google Scholar] [CrossRef]
- Eberly, L.A.; Yang, L.; Eneanya, N.D.; Essien, U.; Julien, H.; Nathan, A.S.; Khatana, S.A.M.; Dayoub, E.J.; Fanaroff, A.C.; Giri, J.; et al. Association of Race/Ethnicity, Gender, and Socioeconomic Status With Sodium-Glucose Cotransporter 2 Inhibitor Use Among Patients With Diabetes in the US. JAMA Netw. Open 2021, 4, e216139. [Google Scholar] [CrossRef] [PubMed]
- McCoy, R.G.; Dykhoff, H.J.; Sangaralingham, L.; Ross, J.S.; Karaca-Mandic, P.; Montori, V.M.; Shah, N.D. Adoption of New Glucose-Lowering Medications in the U.S.—The Case of SGLT2 Inhibitors: Nationwide Cohort Study. Diabetes Technol. Ther. 2019, 21, 702–712. [Google Scholar] [CrossRef] [PubMed]
- Eberly, L.A.; Yang, L.; Essien, U.R.; Eneanya, N.D.; Julien, H.M.; Luo, J.; Nathan, A.S.; Khatana, S.A.M.; Dayoub, E.J.; Fanaroff, A.C.; et al. Racial, Ethnic, and Socioeconomic Inequities in Glucagon-Like Peptide-1 Receptor Agonist Use Among Patients With Diabetes in the US. JAMA Health Forum 2021, 2, e214182. [Google Scholar] [CrossRef]
- Elhussein, A.; Anderson, A.; Bancks, M.P.; Coday, M.; Knowler, W.C.; Peters, A.; Vaughan, E.M.; Maruthur, N.M.; Clark, J.M.; Pilla, S.; et al. Racial/ethnic and socioeconomic disparities in the use of newer diabetes medications in the Look AHEAD study. Lancet Reg. Health. Am. 2022, 6, 100111. [Google Scholar] [CrossRef]
- Davis, J.; Fischl, A.H.; Beck, J.; Browning, L.; Carter, A.; Condon, J.E.; Dennison, M.; Francis, T.; Hughes, P.J.; Jaime, S.; et al. 2022 National Standards for Diabetes Self-Management Education and Support. Diabetes Spectr. 2022, 35, 137–149. [Google Scholar] [CrossRef]
- Walker, R.J.; Smalls, B.L.; Campbell, J.A.; Strom Williams, J.L.; Egede, L.E. Impact of social determinants of health on outcomes for type 2 diabetes: A systematic review. Endocrine 2014, 47, 29–48. [Google Scholar] [CrossRef] [PubMed]
- U.S. Department of Health and Human Services. Social Determinants of Health—Healthy People 2030. Available online: https://health.gov/healthypeople/priority-areas/social-determinants-health/ (accessed on 14 February 2023).
- Zinman, B.; Wanner, C.; Lachin, J.M.; Fitchett, D.; Bluhmki, E.; Hantel, S.; Mattheus, M.; Devins, T.; Johansen, O.E.; Woerle, H.J.; et al. Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. N. Engl. J. Med. 2015, 373, 2117–2128. [Google Scholar] [CrossRef] [PubMed]
- Inzucchi, S.E.; Zinman, B.; Fitchett, D.; Wanner, C.; Ferrannini, E.; Schumacher, M.; Schmoor, C.; Ohneberg, K.; Johansen, O.E.; George, J.T.; et al. How Does Empagliflozin Reduce Cardiovascular Mortality? Insights From a Mediation Analysis of the EMPA-REG OUTCOME Trial. Diabetes Care 2017, 41, 356–363. [Google Scholar] [CrossRef]
- Kauhl, B.; Schweikart, J.; Krafft, T.; Keste, A.; Moskwyn, M. Do the risk factors for type 2 diabetes mellitus vary by location? A spatial analysis of health insurance claims in Northeastern Germany using kernel density estimation and geographically weighted regression. Int. J. Health Geogr. 2016, 15, 38. [Google Scholar] [CrossRef] [PubMed]
- Dijkstra, A.; Janssen, F.; Bakker, M.D.; Bos, J.; Lub, R.; Wissen, L.J.G.V.; Hak, E. Using Spatial Analysis to Predict Health Care Use at the Local Level: A Case Study of Type 2 Diabetes Medication Use and Its Association with Demographic Change and Socioeconomic Status. PLoS ONE 2013, 8, e72730. [Google Scholar] [CrossRef]
- Bazemore, A.W.; Cottrell, E.K.; Gold, R.; Hughes, L.S.; Phillips, R.L.; Angier, H.; Burdick, T.E.; Carrozza, M.A.; DeVoe, J.E. “Community vital signs”: Incorporating geocoded social determinants into electronic records to promote patient and population health. J. Am. Med. Inform. Assoc. 2016, 23, 407–412. [Google Scholar] [CrossRef]
- Tinetti, M.E.; Naik, A.D.; Dodson, J.A. Moving From Disease-Centered to Patient Goals–Directed Care for Patients with Multiple Chronic Conditions: Patient Value-Based Care. JAMA Cardiol. 2016, 1, 9. [Google Scholar] [CrossRef]
- OneFlorida+ Clinical Research Network. Data Summary—OneFlorida. Available online: https://onefloridaconsortium.org/data/ (accessed on 5 May 2022).
- Wiese, A.D.; Roumie, C.L.; Buse, J.B.; Guzman, H.; Bradford, R.; Zalimeni, E.; Knoepp, P.; Morris, H.L.; Donahoo, W.T.; Fanous, N.; et al. Performance of a computable phenotype for identification of patients with diabetes within PCORnet: The Patient-Centered Clinical Research Network. Pharmacoepidemiol. Drug Saf. 2019, 28, 632–639. [Google Scholar] [CrossRef]
- Shivade, C.; Raghavan, P.; Fosler-Lussier, E.; Embi, P.J.; Elhadad, N.; Johnson, S.B.; Lai, A.M. A review of approaches to identifying patient phenotype cohorts using electronic health records. J. Am. Med. Inform. Assoc. 2014, 21, 221–230. [Google Scholar] [CrossRef]
- U.S. Department of Agriculture Economic Research Service. USDA ERS—Food Access Research Atlas. Available online: https://www.ers.usda.gov/data-products/food-access-research-atlas/ (accessed on 2 December 2022).
- Thomas, J.; Zeller, L. National Walkability Index User Guide and Methodology; Prot. Agency: Washington, DC, USA, 2017. [Google Scholar]
- Garvin, E.; Branas, C.; Keddem, S.; Sellman, J.; Cannuscio, C. More Than Just An Eyesore: Local Insights and Solutions on Vacant Land and Urban Health. J. Urban Health 2013, 90, 412–426. [Google Scholar] [CrossRef]
- Messer, L.C.; Laraia, B.A.; Kaufman, J.S.; Eyster, J.; Holzman, C.; Culhane, J.; Elo, I.; Burke, J.G.; O’Campo, P. The development of a standardized neighborhood deprivation index. J. Urban Health 2006, 83, 1041–1062. [Google Scholar] [CrossRef] [PubMed]
- Rupasingha, A.; Goetz, S.J.; Freshwater, D. The production of social capital in US counties. J. Socio-Econ. 2006, 35, 83–101. [Google Scholar] [CrossRef]
- Barnett-Ryan, C. Introduction to the uniform crime reporting program. In Understanding Crime Statistics: Revisiting the Divergence of the NCVS and the UCR; Cambridge University Press: Cambridge, UK, 2007; pp. 55–89. [Google Scholar]
- Peterson, R.A. Finding Optimal Normalizing Transformations via best Normalize. R J. 2021, 13, 310–329. [Google Scholar] [CrossRef]
- Hu, H.; Zhao, J.; Savitz, D.A.; Prosperi, M.; Zheng, Y.; Pearson, T.A. An external exposome-wide association study of hypertensive disorders of pregnancy. Environ. Int. 2020, 141, 105797. [Google Scholar] [CrossRef]
- Agier, L.; Portengen, L.; Chadeau-Hyam, M.; Basagaña, X.; Giorgis-Allemand, L.; Siroux, V.; Robinson, O.; Vlaanderen, J.; González Juan, R.; Nieuwenhuijsen Mark, J.; et al. A Systematic Comparison of Linear Regression–Based Statistical Methods to Assess Exposome-Health Associations. Environ. Health Perspect. 2016, 124, 1848–1856. [Google Scholar] [CrossRef]
- Benjamini, Y.; Hochberg, Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J. R. Stat. Soc. Ser. B (Methodol.) 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Vatcheva, K.P.; Lee, M.; McCormick, J.B.; Rahbar, M.H. Multicollinearity in Regression Analyses Conducted in Epidemiologic Studies. Epidemiology 2016, 6, 227. [Google Scholar] [CrossRef] [PubMed]
- Christine, P.J.; Auchincloss, A.H.; Bertoni, A.G.; Carnethon, M.R.; Sánchez, B.N.; Moore, K.; Adar, S.D.; Horwich, T.B.; Watson, K.E.; Diez Roux, A.V. Longitudinal Associations Between Neighborhood Physical and Social Environments and Incident Type 2 Diabetes Mellitus. JAMA Intern. Med. 2015, 175, 1311. [Google Scholar] [CrossRef]
- Kanchi, R.; Lopez, P.; Rummo, P.E.; Lee, D.C.; Adhikari, S.; Schwartz, M.D.; Avramovic, S.; Siegel, K.R.; Rolka, D.B.; Imperatore, G.; et al. Longitudinal Analysis of Neighborhood Food Environment and Diabetes Risk in the Veterans Administration Diabetes Risk Cohort. JAMA Netw. Open 2021, 4, e2130789. [Google Scholar] [CrossRef]
- Mcdoom, M.M.; Cooper, L.A.; Hsu, Y.-J.; Singh, A.; Perin, J.; Thornton, R.L.J. Neighborhood Environment Characteristics and Control of Hypertension and Diabetes in a Primary Care Patient Sample. J. Gen. Intern. Med. 2020, 35, 1189–1198. [Google Scholar] [CrossRef]
- Gebreab, S.Y.; Hickson, D.A.; Sims, M.; Wyatt, S.B.; Davis, S.K.; Correa, A.; Diez-Roux, A.V. Neighborhood social and physical environments and type 2 diabetes mellitus in African Americans: The Jackson Heart Study. Health Place 2017, 43, 128–137. [Google Scholar] [CrossRef] [PubMed]
- Tabaei, B.P.; Rundle, A.G.; Wu, W.Y.; Horowitz, C.R.; Mayer, V.; Sheehan, D.M.; Chamany, S. Associations of Residential Socioeconomic, Food, and Built Environments with Glycemic Control in Persons with Diabetes in New York City From 2007–2013. Am. J. Epidemiol. 2018, 187, 736–745. [Google Scholar] [CrossRef] [PubMed]
- Gary-Webb, T.L.; Baptiste-Roberts, K.; Pham, L.; Wesche-Thobaben, J.; Patricio, J.; Pi-Sunyer, F.X.; Brown, A.F.; Jones-Corneille, L.; Brancati, F.L. Neighborhood Socioeconomic Status, Depression, and Health Status in the Look AHEAD (Action for Health in Diabetes) Study. BMC Public Health 2011, 11, 349. [Google Scholar] [CrossRef]
- Donneyong, M.M.; Chang, T.-J.; Jackson, J.W.; Langston, M.A.; Juarez, P.D.; Sealy-Jefferson, S.; Lu, B.; Im, W.; Valdez, R.B.; Way, B.M.; et al. Structural and Social Determinants of Health Factors Associated with County-Level Variation in Non-Adherence to Antihypertensive Medication Treatment. Int. J. Environ. Res. Public Health 2020, 17, 6684. [Google Scholar] [CrossRef] [PubMed]
- Billimek, J.; August, K.J. Costs and beliefs: Understanding individual- and neighborhood-level correlates of medication nonadherence among Mexican Americans with type 2 diabetes. Health Psychol. 2014, 33, 1602–1605. [Google Scholar] [CrossRef]
- McClintock, H.F.d.V.; Wiebe, D.J.; O’Donnell, A.J.; Morales, K.H.; Small, D.S.; Bogner, H.R. Neighborhood Social Environment and Patterns of Adherence to Oral Hypoglycemic Agents Among Patients With Type 2 Diabetes Mellitus. Fam. Community Health 2015, 38, 169–179. [Google Scholar] [CrossRef]
- Schillinger, D.; Grumbach, K.; Piette, J.; Wang, F.; Osmond, D.; Daher, C.; Palacios, J.; Sullivan, G.D.; Bindman, A.B. Association of health literacy with diabetes outcomes. JAMA 2002, 288, 475–482. [Google Scholar] [CrossRef]
- Diez Roux, A.V.; Mair, C. Neighborhoods and health. Ann. N. Y. Acad. Sci. 2010, 1186, 125–145. [Google Scholar] [CrossRef]
- Hohl, B.C.; Kondo, M.C.; Kajeepeta, S.; MacDonald, J.M.; Theall, K.P.; Zimmerman, M.A.; Branas, C.C. Creating Safe and Healthy Neighborhoods with Place-Based Violence Interventions. Health Aff. 2019, 38, 1687–1694. [Google Scholar] [CrossRef]
- Cole, M.B.; Nguyen, K.H. Unmet social needs among low-income adults in the United States: Associations with health care access and quality. Health Serv. Res. 2020, 55, 873–882. [Google Scholar] [CrossRef]
- Titus, S.K.; Kataoka-Yahiro, M. A Systematic Review of Barriers to Access-to-Care in Hispanics With Type 2 Diabetes. J. Transcult. Nurs. 2019, 30, 280–290. [Google Scholar] [CrossRef] [PubMed]
- Daiski, I. The Health Bus: Healthcare for Marginalized Populations. Policy Politics Nurs. Pract. 2005, 6, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Whelan, C.; Chambers, C.; Chan, M.; Thomas, S.; Ramos, G.; Hwang, S.W. Why Do Homeless People Use a Mobile Health Unit in a Country With Universal Health Care? J. Prim. Care Community Health 2010, 1, 78–82. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Gabriel, N.; Korytkowski, M.; Hernandez, I.; Gellad, W.F. Association of formulary restrictions and initiation of an SGLT2i or GLP1-RA among Medicare beneficiaries with type 2 diabetes. Diabetes Res. Clin. Pract. 2022, 187, 109855. [Google Scholar] [CrossRef] [PubMed]
| Category | Data Source | Time Period | Spatial Scale | Temporal Scale | Number of Variables |
|---|---|---|---|---|---|
| Food access | Food Access Research Atlas, USDA | 2015, 2019 | Census tract | 1-year | 43 |
| Walkability | Walkability Index, EPA | 2006–2013 | Census block group | Cross-sectional | 1 |
| Vacant land | Aggregated USPS Administrative Data on Address Vacancies, HUD | 2015–2019 | Census tract | 3-month | 18 |
| Socioeconomic status | Neighborhood Deprivation Index, ACS | 2015–2019 | Census block group | 5-year | 1 |
| Social capital | Census Business Pattern | 2015–2019 | ZCTA5 | 1-year | 10 |
| Crime and safety | Uniform Crime Reporting Program, FBI | 2015–2019 | County | 1-year | 8 |
| Overall (n = 28,874) | NHW (n = 11,892) | NHB (n = 10,427) | Hispanics (n = 5458) | Others (n = 818) | p-Value | |
|---|---|---|---|---|---|---|
| Age, mean (SD) | 57.65 (15.19) | 58.65 (14.44) | 54.60 (14.96) | 60.84 (16.17) | 60.40 (15.06) | <0.0001 |
| Age group, %(n) | <0.0001 | |||||
| <25 | 2.01 (580) | 1.54 (183) | 2.61 (272) | 1.89 (103) | 1.71 (14) | |
| 25–34 | 5.69 (1644) | 4.40 (523) | 8.00 (834) | 4.53 (247) | 3.06 (25) | |
| 35–44 | 11.81 (3410) | 10.43 (1240) | 14.75 (1538) | 9.60 (524) | 9.66 (79) | |
| 45–54 | 19.65 (5674) | 19.44 (2312) | 21.24 (2215) | 17.09 (933) | 20.66 (169) | |
| 55–64 | 28.33 (8180) | 29.57 (3516) | 28.93 (3017) | 25.30 (1381) | 22.62 (185) | |
| 65–74 | 18.68 (5393) | 21.21 (2522) | 15.14 (1579) | 19.13 (1044) | 22.62 (185) | |
| ≥75 | 13.83 (3993) | 13.42 (1596) | 9.32 (972) | 22.46 (1226) | 19.68 (161) | |
| Female, %(n) | 61.18 (17,666) | 55.26 (6571) | 67.90 (7080) | 62.48 (3410) | 55.38 (453) | <0.0001 |
| Primary payer, %(n) | <0.0001 | |||||
| Medicare | 37.57 (10,847) | 38.73 (4606) | 33.36 (3478) | 44.10 (2407) | 31.66 (259) | |
| Medicaid | 34.94 (10,090) | 28.85 (3431) | 41.44 (4321) | 37.38 (2040) | 25.43 (208) | |
| Private insurance | 19.96 (5764) | 23.74 (2823) | 18.64 (1944) | 12.42 (678) | 32.52 (266) | |
| No insurance | 2.26 (652) | 1.95 (232) | 3.19 (333) | 1.14 (62) | 2.81 (23) | |
| Others | 5.27 (1521) | 6.73 (800) | 3.37 (351) | 4.97 (271) | 7.58 (62) | |
| BMI categories, %(n) | <0.0001 | |||||
| ≤25 | 9.51 (2745) | 9.41 (1119) | 9.03 (942) | 8.94 (488) | 20.78 (170) | |
| 25–30 | 18.16 (5244) | 18.05 (2146) | 16.74 (1746) | 19.77 (1079) | 27.87 (228) | |
| 30–100 | 54.37 (15,699) | 57.69 (6861) | 58.22 (6071) | 42.84 (2338) | 36.92 (302) | |
| missing | 17.96 (5186) | 14.85 (1766) | 16.00 (1668) | 28.45 (1553) | 14.43 (118) | |
| HbA1c categories, %(n) | <0.0001 | |||||
| ≤7 mmHg | 18.43 (5322) | 21.72 (2583) | 19.37 (2020) | 8.67 (473) | 25.18 (206) | |
| 7–10 mmHg | 23.45 (6772) | 28.33 (3369) | 23.60 (2461) | 10.90 (595) | 32.03 (262) | |
| 10–21 mmHg | 10.44 (3014) | 9.59 (1141) | 13.91 (1450) | 5.79 (316) | 10.27 (84) | |
| missing | 47.68 (13,766) | 40.35 (4799) | 43.12 (4496) | 74.64 (4074) | 32.52 (266) | |
| Type of residence, %(n) | <0.0001 | |||||
| Metro Areas | 91.99 (26,553) | 86.65 (10,300) | 94.66 (9868) | 97.97 (5345) | 95.96 (784) | |
| Urban or suburban Areas | 7.79 (2248) | 12.91 (1535) | 5.29 (551) | 2.00 (109) | 3.79 (31) | |
| Rural Areas | 0.22 (63) | 0.44 (52) | 0.06 (6) | 0.04 (2) | 0.24 (2) | |
| OneFlorida network site, %(n) | <0.0001 | |||||
| A | 17.76 (5127) | 18.31 (2177) | 14.17 (1478) | 23.10 (1261) | 20.90 (171) | |
| B | 0.27 (78) | 0.45 (53) | 0.19 (20) | 0 | 0.12 (1) | |
| C | 3.88 (1121) | 3.14 (374) | 4.99 (520) | 2.91 (159) | 7.70 (63) | |
| D | 5.94 (1716) | 6.23 (741) | 8.47 (883) | 0.77 (42) | 2.57 (21) | |
| E | 52.11 (15,045) | 64.96 (7725) | 56.09 (5849) | 14.40 (786) | 61.25 (501) | |
| F | 20.04 (5787) | 6.91 (822) | 16.08 (1677) | 58.81 (3210) | 7.46 (61) | |
| CVD, %(n) | 41.26 (11,797) | 40.87 (4860) | 41.09 (4824) | 43.64 (2382) | 33.13 (271) | <0.0001 |
| CKD, %(n) | 33.24 (33.24) | 30.21 (3592) | 36.55 (3811) | 33.86 (1848) | 31.17 (255) | <0.0001 |
| Insulin use, %(n) | 48.59 (14,029) | 45.63 (5426) | 53.28 (5556) | 47.45 (2590) | 41.20 (337) | <0.0001 |
| Any non-insulin antidiabetics use, %(n) | 67.30 (19,432) | 66.25 (7878) | 67.33 (7021) | 69.02 (3767) | 70.29 (575) | 0.0008 |
| Metformin, %(n) | 45.68 (13,190) | 45.01 (5353) | 45.98 (4794) | 46.01 (2511) | 48.78 (399) | 0.117 |
| DPP-4 inhibitors, %(n) | 21.27 (6141) | 21.41 (2546) | 19.62 (2046) | 23.43 (1279) | 23.59 (193) | <0.0001 |
| Sulfonylureas, %(n) | 31.49 (9092) | 29.09 (3459) | 31.77 (3313) | 35.87 (1958) | 35.21 (288) | <0.0001 |
| Thiazolidinediones, %(n) | 4.68 (1351) | 5.05 (601) | 3.86 (403) | 5.15 (281) | 6.60 (54) | <0.0001 |
| Exposure | Transformation | Standard Deviation | Phase 1 | Phase 2 | |||||
|---|---|---|---|---|---|---|---|---|---|
| Discovery Set | Replication Set | ||||||||
| Variable | Category | OR (95% CI) | q-Value | OR (95% CI) | q-Value | OR (95% CI) | p-Value | ||
| Percentage of low access population with housing units without vehicle access at 1/2 mile | Food access | log_x | 0.165 | 0.90 (0.85, 0.95) | 0.0349 | 0.86 (0.81, 0.91) | <0.0001 | 0.96 (0.91, 1.01) | 0.0905 |
| Neighborhood deprivation index | Socioeconomic status | Yeo-Johnson (lamda = −0.49) | 2.052 | 0.88 (0.83, 0.93) | 0.0024 | 0.84 (0.79, 0.89) | <0.0001 | 0.92 (0.88, 0.97) | 0.0031 |
| Percent of vacant addresses | Vacant land | Square Root | 0.089 | 0.86 (0.81, 0.91) | <0.0001 | 0.86 (0.82, 0.91) | <0.0001 | 0.91 (0.87, 0.95) | <0.0001 |
| Variable | Overall (n = 28,874) | NHW Subgroup (n = 11,892) | NHB Subgroup (n = 10,427) | Hispanic Subgroup (n = 5458) | Other Race Subgroup (n = 818) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Category | aOR (95% CI) | p-Value | aOR (95% CI) | p-Value | aOR (95% CI) | p-Value | aOR (95% CI) | p-Value | aOR (95% CI) | p-Value | |
| Neighborhood deprivation index | Socioeconomic Status | 0.87 (0.81, 0.94) | 0.0003 | 0.80 (0.67, 0.95) | 0.0121 | 0.91 (0.82, 1.00) | 0.0517 | 0.83 (0.70, 0.97) | 0.0215 | 0.81 (0.40, 1.66) | 0.5653 |
| Percent of vacant addresses | Vacant Land | 0.91 (0.85, 0.98) | 0.0087 | 0.95 (0.84, 1.08) | 0.46 | 0.87 (0.79, 0.96) | 0.0073 | 0.96 (0.77, 1.18) | 0.6899 | 0.92 (0.54, 1.57) | 0.7645 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Hu, H.; Zheng, Y.; Donahoo, W.T.; Guo, Y.; Xu, J.; Chen, W.-H.; Liu, N.; Shenkman, E.A.; Bian, J.; et al. Impact of Contextual-Level Social Determinants of Health on Newer Antidiabetic Drug Adoption in Patients with Type 2 Diabetes. Int. J. Environ. Res. Public Health 2023, 20, 4036. https://doi.org/10.3390/ijerph20054036
Li Y, Hu H, Zheng Y, Donahoo WT, Guo Y, Xu J, Chen W-H, Liu N, Shenkman EA, Bian J, et al. Impact of Contextual-Level Social Determinants of Health on Newer Antidiabetic Drug Adoption in Patients with Type 2 Diabetes. International Journal of Environmental Research and Public Health. 2023; 20(5):4036. https://doi.org/10.3390/ijerph20054036
Chicago/Turabian StyleLi, Yujia, Hui Hu, Yi Zheng, William Troy Donahoo, Yi Guo, Jie Xu, Wei-Han Chen, Ning Liu, Elisabeth A. Shenkman, Jiang Bian, and et al. 2023. "Impact of Contextual-Level Social Determinants of Health on Newer Antidiabetic Drug Adoption in Patients with Type 2 Diabetes" International Journal of Environmental Research and Public Health 20, no. 5: 4036. https://doi.org/10.3390/ijerph20054036
APA StyleLi, Y., Hu, H., Zheng, Y., Donahoo, W. T., Guo, Y., Xu, J., Chen, W.-H., Liu, N., Shenkman, E. A., Bian, J., & Guo, J. (2023). Impact of Contextual-Level Social Determinants of Health on Newer Antidiabetic Drug Adoption in Patients with Type 2 Diabetes. International Journal of Environmental Research and Public Health, 20(5), 4036. https://doi.org/10.3390/ijerph20054036









